Nonlethal Effects of Pesticides on Web-Building Spiders Might Account for Rapid Mosquito Population Rebound after Spray Application
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
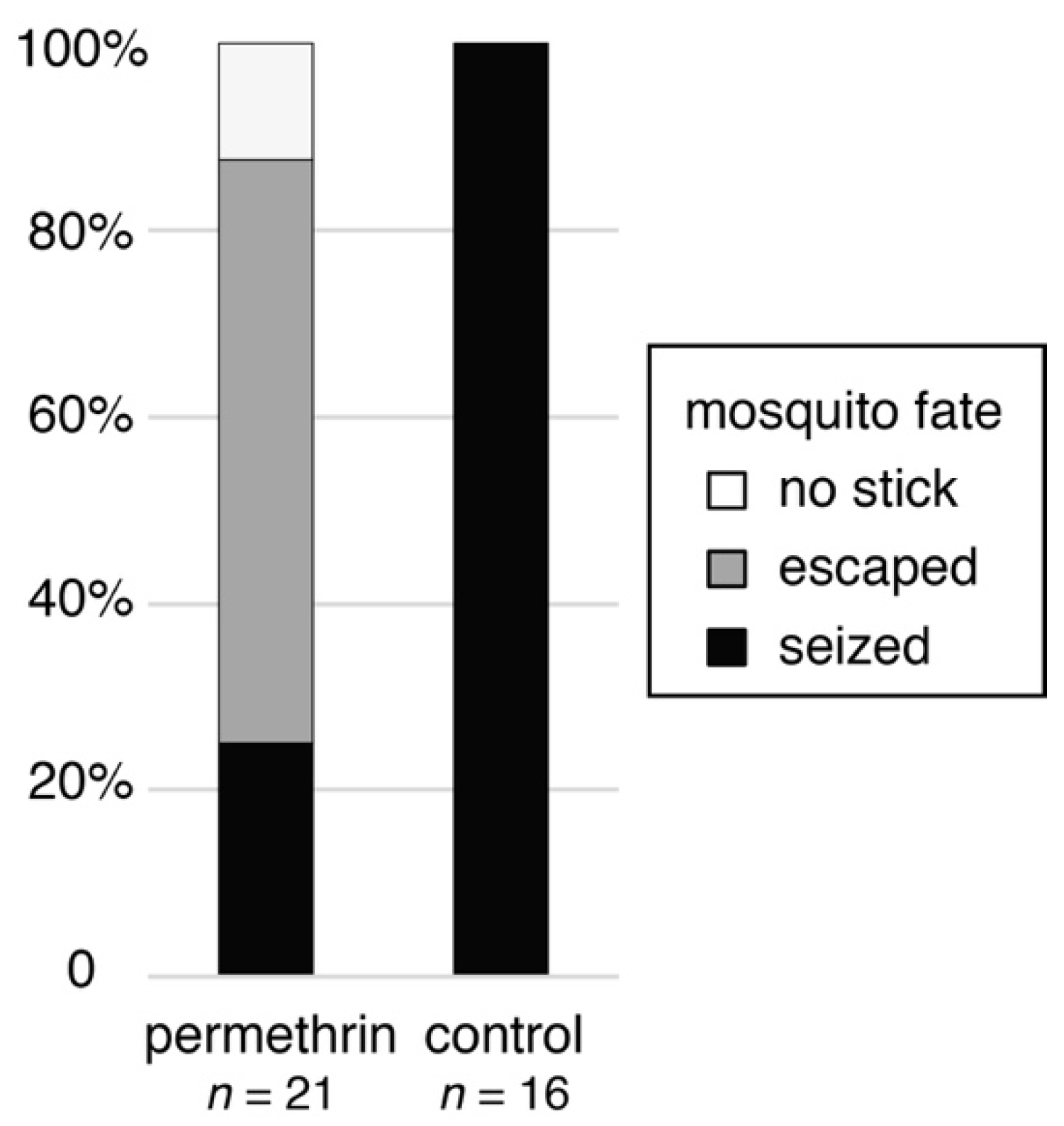
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Miliczky, E.R.; Calkins, C.O.; Horton, D.R. Spider abundance and diversity in apple orchards under three insect pest management programmes in Washington State, U.S.A. Agric. For. Entomol. 2000, 2, 203–215. [Google Scholar] [CrossRef]
- Boyce, W.M.; Lawler, S.P.; Schultz, J.M.; McCauley, S.J.; Kimsey, L.S.; Niemela, M.K.; Nielsen, C.F.; Reisen, W.K. Nontarget effects of the mosquito adulticide pyrethrin applied aerially during a West Nile virus outbreak in an urban California environment. J. Am. Mosq. Control Assoc. 2007, 23, 335–339. [Google Scholar] [CrossRef]
- Pekár, S.; Beneš, J. Aged pesticide residues are detrimental to agrobiont spiders (Araneae). J. Appl. Entomol. 2008, 132, 614–622. [Google Scholar] [CrossRef]
- Pasquet, A.; Tupinier, N.; Mazzia, C.; Capowiez, Y. Exposure to spinosad affects orb-web spider (Agalenatea redii) survival, web construction and prey capture under laboratory conditions. J. Pest. Sci. 2016, 89, 507–515. [Google Scholar] [CrossRef]
- Bogya, S.; Marko, V.; Szinetar, C. Effect of pest management systems on foliage- and grass-dwelling spider communities in an apple orchard in Hungary. Int. J. Pest. Manag. 2000, 46, 241–250. [Google Scholar] [CrossRef]
- Samu, F.; Matthews, G.A.; Lake, D.; Vollrath, F. Spider webs are efficient collectors of agrochemical spray. Pestic. Sci. 1992, 36, 47–51. [Google Scholar] [CrossRef]
- Townley, M.A.; Tillinghast, E.K. Orb web recycling in Araneus cavaticus (Araneae, Araneidae) with an emphasis on the adhesive spiral component, gabamide. J. Arachnol. 1988, 16, 303–319. [Google Scholar]
- Carico, J.E. Web removal patterns in orb-weaving spiders. In Spiders: Webs, Behavior, and Evolution; Shear, W.A., Ed.; Stanford University Press: Stanford, CA, USA, 1986; pp. 306–318. [Google Scholar]
- Breed, A.L.; Levine, V.D.; Peakall, D.B.; Witt, P.N. The fate of the intact orb web of the spider Araneus diadematus Cl. Behaviour 1964, 23, 43–60. [Google Scholar]
- Peakall, D.B. Conservation of web proteins in the spider, Araneus diadematus. J. Exp. Zool. 1971, 176, 257–264. [Google Scholar] [CrossRef]
- Opell, B.D. Economics of spider orb-webs: The benefits of producing adhesive capture thread and of recycling silk. Funct. Ecol. 1998, 12, 613–624. [Google Scholar] [CrossRef]
- Focks, D.A.; Kloter, K.O.; Carmichael, G.T. The impact of sequential ultra-low volume ground aerosol applications of malathion on the population-dynamics of Aedes aegypti (L). Am. J. Trop. Med. Hyg. 1987, 36, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Alto, B.W.; Lampman, R.L.; Kesavaraju, B.; Muturi, E.J. Pesticide-induced release from competition among competing Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Costanzo, K.; Kesavaraju, B.; Lampman, R.; Alto, B.W. Interaction of a pesticide and larval competition on life history traits of Culex pipiens. Acta Trop. 2010, 116, 141–146. [Google Scholar] [CrossRef]
- Kesavaraju, B.; Brey, C.W.; Farajollahi, A.; Evans, H.L.; Gaugler, R. Effect of malathion on larval competition between Aedes albopictus and Aedes atropalpus (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 479–484. [Google Scholar] [CrossRef]
- Stoddard, P.K. Managing Aedes aegypti populations in the first Zika transmission zones in the continental United States. Acta Trop. 2018, 187, 108–118. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Hormiga, G. Species delimitation of the North American orchard-spider Leucauge venusta (Walckenaer, 1841) (Araneae, Tetragnathidae). Mol. Phylogenet. Evol. 2018, 121, 183–197. [Google Scholar] [CrossRef]
- Opell, B.D.; Bond, J.E.; Warner, D.A. The effects of capture spiral composition and orb-web orientation on prey interception. Zoology 2006, 109, 339–345. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Efficacy Testing of Insecticides for Indoor and Outdoor Ground Applied Space Spray Applications. 2019. Available online: http://apps.who.int/iris/bitstream/handle/10665/70070/WHO_HTM_NTD_WHOPES_2009.2_eng.pdf?sequence=1 (accessed on 1 February 2021).
- Hoskins, W.M.; Gordon, H.T. Arthropod resistance to chemicals. Annu. Rev. Entomol. 1956, 1956, 89–122. [Google Scholar] [CrossRef]
- Samu, F.; Vollrath, F. Spider orb web as bioassay for pesticide side-effects. Entomol. Exp. Appl. 1992, 62, 117–124. [Google Scholar] [CrossRef]
- Shaw, E.M.; Wheater, C.P.; Langan, A.M. The effects of cypermethrin on Tenuiphantes tenuis (Blackwall, 1852): Development of a technique for assessing the impact of pesticides on web building in spiders (Araneae: Linyphiidae). Acta Zool. Bulg. 2006, 1, 173–179. [Google Scholar]
- Mulder, T.; Mortimer, B.; Vollrath, F. Functional flexibility in a spider’s orb web. J. Exp. Biol. 2020, 223, jeb234070. [Google Scholar] [CrossRef] [PubMed]
- Everts, J.W.; Willemsen, I.; Stulp, M.; Simons, L.; Aukema, B.; Kammenga, J. The toxic effect of deltamethrin on linyphiid and erigonid spiders in connection with ambient-temperature, humidity, and predation. Arch. Environ. Contam. Toxicol. 1991, 20, 20–24. [Google Scholar] [CrossRef]
- Pekár, S. Side effect of synthetic pesticides on spiders. In Spider Ecophysiology; Nentwig, W., Ed.; Springer: Berlin, Germany, 2012; pp. 415–427. [Google Scholar]
- Marc, P.; Canard, A.; Ysnel, F. Spiders (Araneae) useful for pest limitation and bioindication. Ag. Ecosyst. Environ. 1999, 74, 229–273. [Google Scholar] [CrossRef]
- Juliano, S.A. Species interactions among larval mosquitoes: Context dependence across habitat gradients. Annu. Rev. Entomol. 2009, 54, 37–56. [Google Scholar] [CrossRef]
- Gilpin, M.E.; Mcclelland, G.A.H. Systems-analysis of the yellow-fever mosquito Aedes aegypti. Forts. Zool. 1979, 25, 355–388. [Google Scholar]
- Dye, C. Intraspecific competition amongst larval Aedes aegypti—Food exploitation or chemical interference. Ecol. Entomol. 1982, 7, 39–46. [Google Scholar] [CrossRef]
- Dye, C. Competition amongst larval Aedes aegypti—The role of interference. Ecol. Entomol. 1984, 9, 355–357. [Google Scholar] [CrossRef]
- Wray, A.K.; Jusino, M.A.; Banik, M.T.; Palmer, J.M.; Kaarakka, H.; White, J.P.; Lindner, D.L.; Gratton, C.; Peery, M.Z. Incidence and taxonomic richness of mosquitoes in the diets of little brown and big brown bats. J. Mammal. 2018, 99, 668–674. [Google Scholar] [CrossRef]
- Wetzler, G.C.; Boyles, J.G. The energetics of mosquito feeding by insectivorous bats. Can. J. Zool. 2017, 96, 373–377. [Google Scholar] [CrossRef]
- Nyffeler, M.; Benz, G. Spiders in natural pest-control—A review. J. Appl. Entomol. 1987, 103, 321–339. [Google Scholar] [CrossRef]
- Michalko, R.; Pekar, S.; Entling, M.H. An updated perspective on spiders as generalist predators in biological control. Oecologia 2019, 189, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Nyffeler, M.; Benz, G. Foraging ecology and predatory importance of a guild of orb-weaving spiders in a grassland habitat. J. Appl. Entomol. 1989, 107, 166–184. [Google Scholar] [CrossRef]
- Henaut, Y.; Garcia-Ballinas, J.A.; Alauzet, C. Variations in web construction in Leucauge venusta (Araneae, Tetragnathidae). J. Arachnol. 2006, 34, 234–240. [Google Scholar] [CrossRef]
- Muma, M.H. Spiders in Florida citrus groves. Fla. Entomol. 1975, 58, 83–90. [Google Scholar] [CrossRef]
- Strickman, D.; Sithiprasasna, R.; Southard, D. Bionomics of the spider, Crossopriza lyoni (Araneae, Pholcidae), a predator of dengue vectors in Thailand. J. Arachnol. 1997, 25, 194–201. [Google Scholar]
- Ndava, K.; Diaz Llera, S.; Manyanga, P. The future of mosquito control: The role of spiders as biological control agents: A review. Int. J. Mosq. Res. 2018, 5, 6–11. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhoades, S.N.; Stoddard, P.K. Nonlethal Effects of Pesticides on Web-Building Spiders Might Account for Rapid Mosquito Population Rebound after Spray Application. Appl. Sci. 2021, 11, 1360. https://doi.org/10.3390/app11041360
Rhoades SN, Stoddard PK. Nonlethal Effects of Pesticides on Web-Building Spiders Might Account for Rapid Mosquito Population Rebound after Spray Application. Applied Sciences. 2021; 11(4):1360. https://doi.org/10.3390/app11041360
Chicago/Turabian StyleRhoades, Stefan N., and Philip K. Stoddard. 2021. "Nonlethal Effects of Pesticides on Web-Building Spiders Might Account for Rapid Mosquito Population Rebound after Spray Application" Applied Sciences 11, no. 4: 1360. https://doi.org/10.3390/app11041360
APA StyleRhoades, S. N., & Stoddard, P. K. (2021). Nonlethal Effects of Pesticides on Web-Building Spiders Might Account for Rapid Mosquito Population Rebound after Spray Application. Applied Sciences, 11(4), 1360. https://doi.org/10.3390/app11041360







