Oil-In-Water Microemulsion Encapsulation of Antagonist Drugs Prevents Renal Ischemia-Reperfusion Injury in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
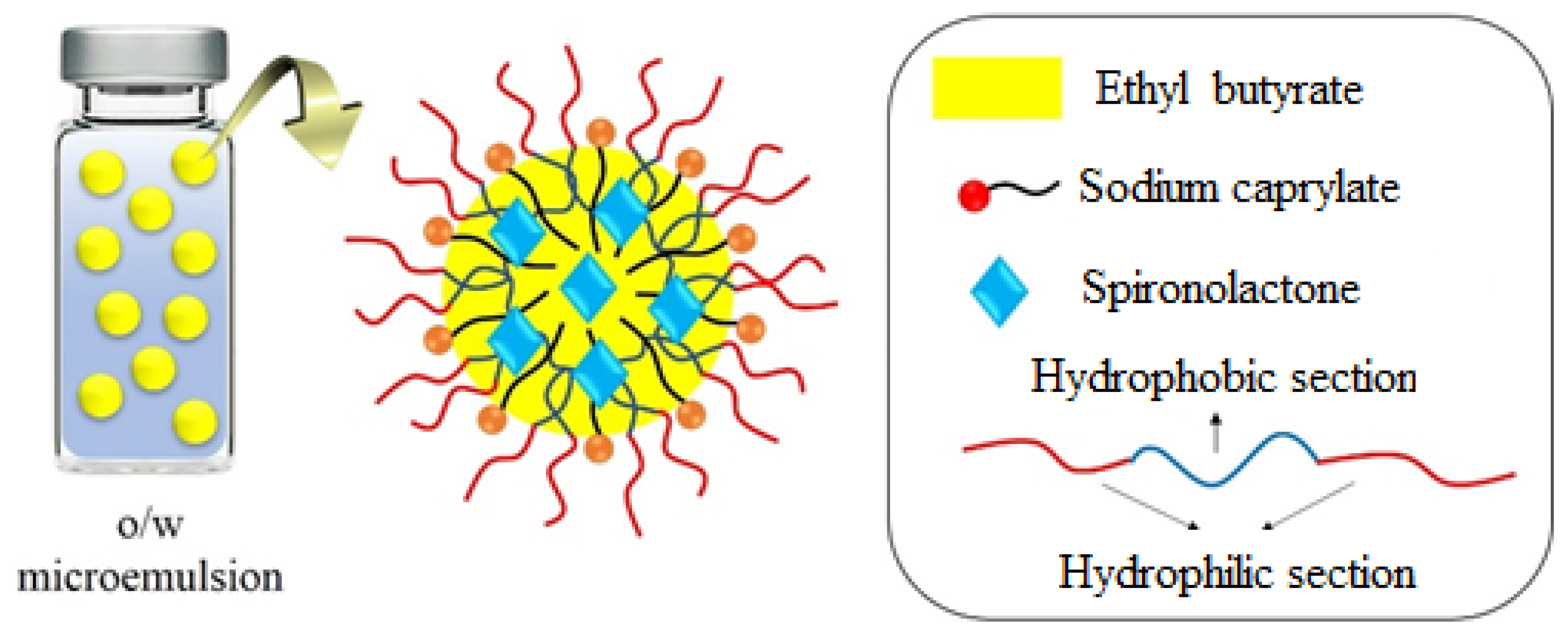
2.2. Synthesis of SP-Incorporated Microemulsions
2.3. The Size Characterization of SP-Loaded Microemulsions by Digital Light Scattering (DLS)
2.4. In Vivo Studies
2.4.1. Animals and Experimental Design
2.4.2. Renal I/R Model
2.4.3. Assessment of Oxidant/Antioxidant Markers
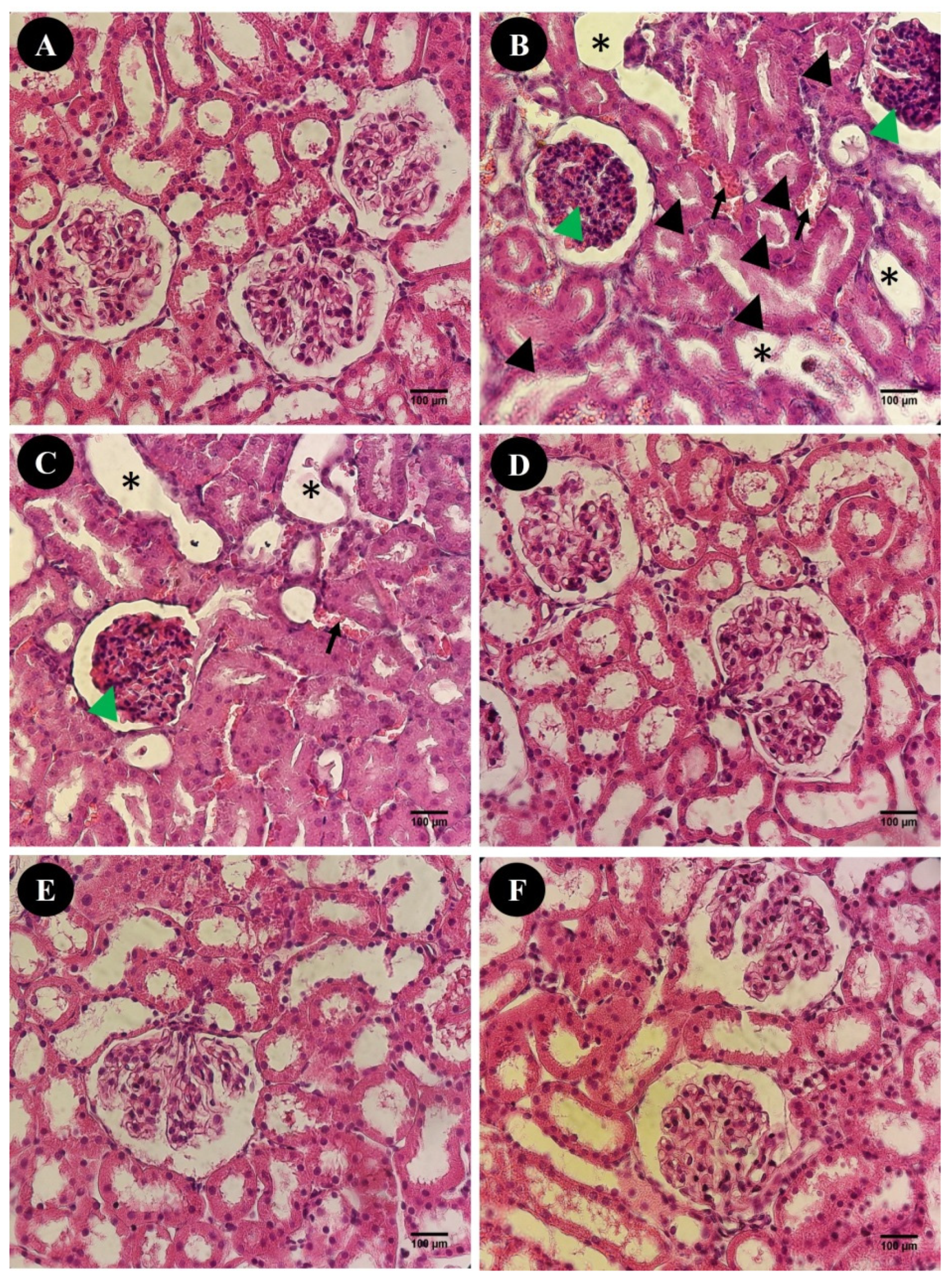
2.4.4. Histological Evaluation
2.4.5. Measurement of Renal Biomarkers
2.4.6. Statistical Analysis
3. Results
3.1. Physical Characterization of Oil-In-Water Microemulsions Containing SP
3.2. In Vivo Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weight, S.C.; Bell, P.R.F.; Nicholson, M.L. Renal ischae-mia-reperfusion injury. Br. J. Surg. 1996, 83, 162–170. [Google Scholar] [PubMed]
- Kelly, K.J.; Molitoris, B. Acute renal failure in the new millennium: Time to consider combination therapy. Semin. Nephrol. 2000, 20, 4–19. [Google Scholar] [PubMed]
- Matejovic, M.; Ince, C.; Chawla, L.S.; Blantz, R.; Molitoris, B.A.; Rosner, M.H.; Okusa, M.D.; Kellum, J.A.; Ronco, C. Renal Hemodynamics in AKI: In Search of New Treatment Targets. J. Am. Soc. Nephrol. 2016, 27, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K. Acute Renal Failure: Much More Than a Kidney Disease. Semin. Nephrol. 2006, 26, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Blasi, E.R.; Rocha, R.; Rudolph, A.E.; Blomme, E.A.; Polly, M.L.; McMahon, E.G. Aldosterone/salt induces renal inflammation and fi-brosis in hypertensive rats. Kidney Int. 2003, 63, 1791–1800. [Google Scholar] [CrossRef]
- Greene, E.L.; Kren, S.; Hostetter, T.H. Role of aldosterone in the remnant kidney model in the rat. J. Clin. Investig. 1996, 98, 1063–1068. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, C.H.; Kim, H.S.; Jee, Y.H.; Song, H.K.; Lee, M.H.; Han, K.H.; Kim, H.K.; Kang, Y.S.; Han, J.Y.; et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J. Am. Soc. Nephrol. 2006, 17, 1362–1372. [Google Scholar] [CrossRef]
- Trachtman, H.; Weiser, A.; Valderrama, E.; Morgado, M.; Palmer, L.S. Prevention of renal fibrosis by spironolactone in mice with complete unilateral ureteral obstruction. J. Urol. 2004, 172, 1590–1594. [Google Scholar] [CrossRef]
- Hostetter, T.H.; Ibrahim, H.N. Aldosterone in Chronic Kidney and Cardiac Disease. J. Am. Soc. Nephrol. 2003, 14, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rojas, J.; Blanco, J.A.; Cruz, C.; Trujillo, J.; Vaidya, V.S.; Uribe, N.; Bonventre, J.V.; Gamba, G.; Bobadilla, N.A. Mineralocorticoid receptor blockade confers renoprotection in preexisting chronic cyclosporine ne-phrotoxicity. Am. J. Physiol.-Renal Physiol. 2007, 292, F131–F139. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pozos, K.; Barrera-Chimal, J.; Garzón-Muvdi, J.; Pérez-Villalva, R.; Rodríguez-Romo, R.; Cruz, C.; Gamba, G.; Bobadilla, N.A. Recovery from ischemic acute kidney injury by spironolactone administration. Nephrol. Dial. Transplant. 2012, 27, 3160–3169. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Vilet, J.M.; Ramírez, V.; Cruz, C.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Renal ischemia-reperfusion injury is prevented by the mineralocor-ticoid receptor blocker spironolactone. Am. J. Phys.-Renal Physiol. 2007, 293, F78–F86. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fan, T.-T.; Ji, Y.-S.; Yu, J.-Y.; Wu, S.; Zhang, L. Spironolactone alleviates diabetic nephropathy through promoting autophagy in podocytes. Int. Urol. Nephrol. 2019, 51, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.L.; Zhang, J.M.; An, N.; Tao, Y.; Yu, W.F.; Wu, F.X. Spirono-lactone rescues renal dysfunction in obstructive jaundice rats by upregulating ACE2 expression. J. Cell Commun. Signal. 2019, 13, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Abolghasmi, R.; Taziki, O. Efficacy of low dose spironolactone in chronic kidney disease with resistant hypertension. Saudi J. Kidney Dis. Transplant. 2011, 22, 75–78. [Google Scholar]
- Blouza, I.L.; Charcosset, C.; Sfar, S.; Fessi, H. Preparation and characterization of spironolactone-loaded nanocapsules for paediatric use. Int. J. Pharm. 2006, 325, 124–131. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Kostarelos, K. Liposome–nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomedicine 2007, 2, 85–98. [Google Scholar] [CrossRef]
- Elbayoumi, T.A.; Torchilin, V.P. Current trends in liposome re-search. Methods Mol. Biol. 2010, 605, 1–27. [Google Scholar]
- Giddam, A.K.; Zaman, M.; Skwarczynski, M.; Toth, I. Liposome based delivery system for vaccine candidates: Constructing an effective formu-lation. Nanomedicine 2012, 7, 1877–1893. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.-J.; Ma, Y.-Q.; Zhang, X.; Zhang, L.; Zhang, Y.-D.; Su, C.-C.; Xiang, G.-A.; Zhang, M.-P.; Lin, Z.-C.; Wei, L.-Q.; et al. Inflammatory monocyte/macrophage modulation by lipo-some-entrapped spironolactone ameliorates acute lung injury in mice. Nanomedicine 2016, 11, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Badran, M.; Elmowafy, M.; Soliman, G.M. Spirono-lactone-loaded leciplexes as potential topical delivery systems for female acne: In vitro appraisal and ex vivo skin permeability studies. Pharmaceutics 2020, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Murthy, K.N.C.; Patil, B.S. Enhanced colon cancer chemoprevention of curcumin by nanoencapsulation with whey protein. Eur. J. Pharmacol. 2016, 789, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Minaei, A.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cyto-toxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Onsori, H.; Rahmati, M. Investigation of the anti-cancer effects of free and PLGA-PAA encapsulated Hydroxytyrosol on the MCF-7 breast cancer cell line. Curr. Mol. Med. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Varshney, M.; Morey, T.E.; Shah, D.O.; Flint, J.A.; Moudgil, B.M.; Seubert, C.N.; Dennis, D.M. Pluronic microemulsions as nanoreservoirs for ex-traction of bupivacaine from normal saline. J. Am. Chem. Soc. 2004, 126, 5108–5112. [Google Scholar] [CrossRef]
- Finsy, R. Particle sizing by quasi-elastic light scattering. Adv. Colloid Interface Sci. 1994, 52, 79–143. [Google Scholar] [CrossRef]
- Rahdar, A.; Almasi-Kashi, M.; Khan, A.M.; Aliahmad, M.; Salimi, A.; Guettari, M.; Kohne, H.E.G. Effect of ion exchange in naaot surfactant on droplet size and location of dye within rhodamine b (rhb)-containing micro-emulsion at low dye concentration. J. Mol. Liquids 2018, 252, 506–513. [Google Scholar] [CrossRef]
- Brown, W. Dynamic Light Scattering: The Method and Some Application; Clarendon Press: Oxford, England, 1993. [Google Scholar]
- Feria, I.; Pichardo, I.; Juárez, P.; Ramírez, V.; González, M.A.; Uribe, N.; García-Torres, R.; López-Casillas, F.; Gamba, G.; Bobadilla, N.A. Thera-peutic benefit of spironolactone in experimental chronic cyclosporine A ne-phrotoxicity. Kidney Int. 2003, 63, 43–52. [Google Scholar] [CrossRef]
- Mcauley, F.T.; Whiting, P.H.; Thomson, A.W.; Simpson, J.G. The influence of enalapril or spironolactone on experimental cyclosporin ne-phrotoxicity. Biochem. Pharm. 1987, 36, 699–703. [Google Scholar] [CrossRef]
- Saraç, F.; Kilincaslan, H.; Kılıc, E.; Koldaş, M.; Terzi, E.H.; Aydogdu, I.; Kilic, E.; Kılıncaslan, H. Methylene blue attenuates renal ischemia–reperfusion injury in rats. J. Pediatr. Surg. 2015, 50, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Roberts, L.J. Measurement of Lipid Peroxidation. Free. Radic. Res. 1998, 28, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase Activity. In CRC Hand-Book of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Caraway, W.T. Uric acid. In Standard Methods of Clinical Chemistry; Academic Press: New York, NY, USA, 1963; pp. 239–247. [Google Scholar]
- Molitoris, B.A.; Sutton, T.A. Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int. 2004, 66, 496–499. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Nasri, S.; Beyzaei, H.; Barani, M.; Trant, J. The synthesis of methotrexate-loaded F127 microemulsions and their in vivo toxicity in a rat model. J. Mol. Liq. 2020, 313, 113449. [Google Scholar] [CrossRef]
- Rahdar, A.; Taboada, P.; Hajinezhad, M.R.; Barani, M.; Beyzaei, H. Effect of tocopherol on the properties of Pluronic F127 mi-croemulsions: Physico-chemical characterization and in vivo toxicity. J. Mol. Liq. 2019, 277, 624–630. [Google Scholar] [CrossRef]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Adeli-Sardou, M. Evaluation of Carum-loaded Niosomes on Breast Cancer Cells: Physicochemical Properties, In Vitro Cytotoxicity, Flow Cytometric, DNA Fragmenta-tion and Cell Migration Assay. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Barani, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Lohrasbi-Nejad, A.; Nematollahi, M.H. A new formulation of hydrophobin-coated niosome as a drug carrier to cancer cells. Mater. Sci. Eng. C 2020, 113, 110975. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.J. Aldosterone and end-organ damage. Curr. Opin. Nephrol. Hypertens. 2005, 14, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Abe, Y. Molecular Mechanisms and Therapeutic Strategies of Chronic Renal Injury: Renoprotective Effects of Aldosterone Blockade. J. Pharmacol. Sci. 2006, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rojas, J.M.; Derive, S.; Blanco, J.A.; Cruz, C.; De La Maza, L.M.; Gamba, G.; Bobadilla, N.A. Renocortical mRNA expression of vaso-active factors during spironolactone protective effect in chronic cyclosporine nephrotoxicity. Am. J. Physiol. Renal Physiol. 2005, 289, F1020–F1030. [Google Scholar]
- Barrera-Chimal, J.; Pérez-Villalva, R.; Rodríguez-Romo, R.; Reyna, J.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Spironolactone prevents chronic kid-ney disease caused by ischemic acute kidney injury. Kidney Int. 2013, 83, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Rahman, M.; Shokoji, T.; Nagai, Y.; Zhang, G.-X.; Sun, G.-P.; Kimura, S.; Yukimura, T.; Kiyomoto, H.; Kohno, M.; et al. Aldosterone Stimulates Reactive Oxygen Species Production through Activation of NADPH Oxidase in Rat Mesangial Cells. J. Am. Soc. Nephrol. 2005, 16, 2906–2912. [Google Scholar] [CrossRef] [PubMed]
- McInnes, G.T.; Asbury, M.J.; Ramsay, L.E.; Shelton, J.R.; Harrison, I.R. Effect of micronization on the bioavailability and pharmacologic activ-ity of spironolactone. J. Clin. Pharmacol. 1982, 22, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Yusuff, N.T.; York, P.; Chrystyn, H.; Bramley, P.N.; Swallow, R.D.; Tuladhar, B.R.; Losowsky, M.S. Improved bioavailability from a spironolactone beta-cyclodextrin complex. Eur. J. Clin. Pharmacol. 1991, 40, 507–511. [Google Scholar] [CrossRef]
- Abosehmah-Albidy, A.Z.M.; York, P.; Wong, V.; Losowsky, M.S.; Chrystyn, H. Improved bioavailability and clinical response in patients with chronic liver disease following the administration of a spironolactone: β-cyclodextrin complex. Br. J. Clin. Pharmacol. 1997, 44, 35–39. [Google Scholar] [CrossRef]


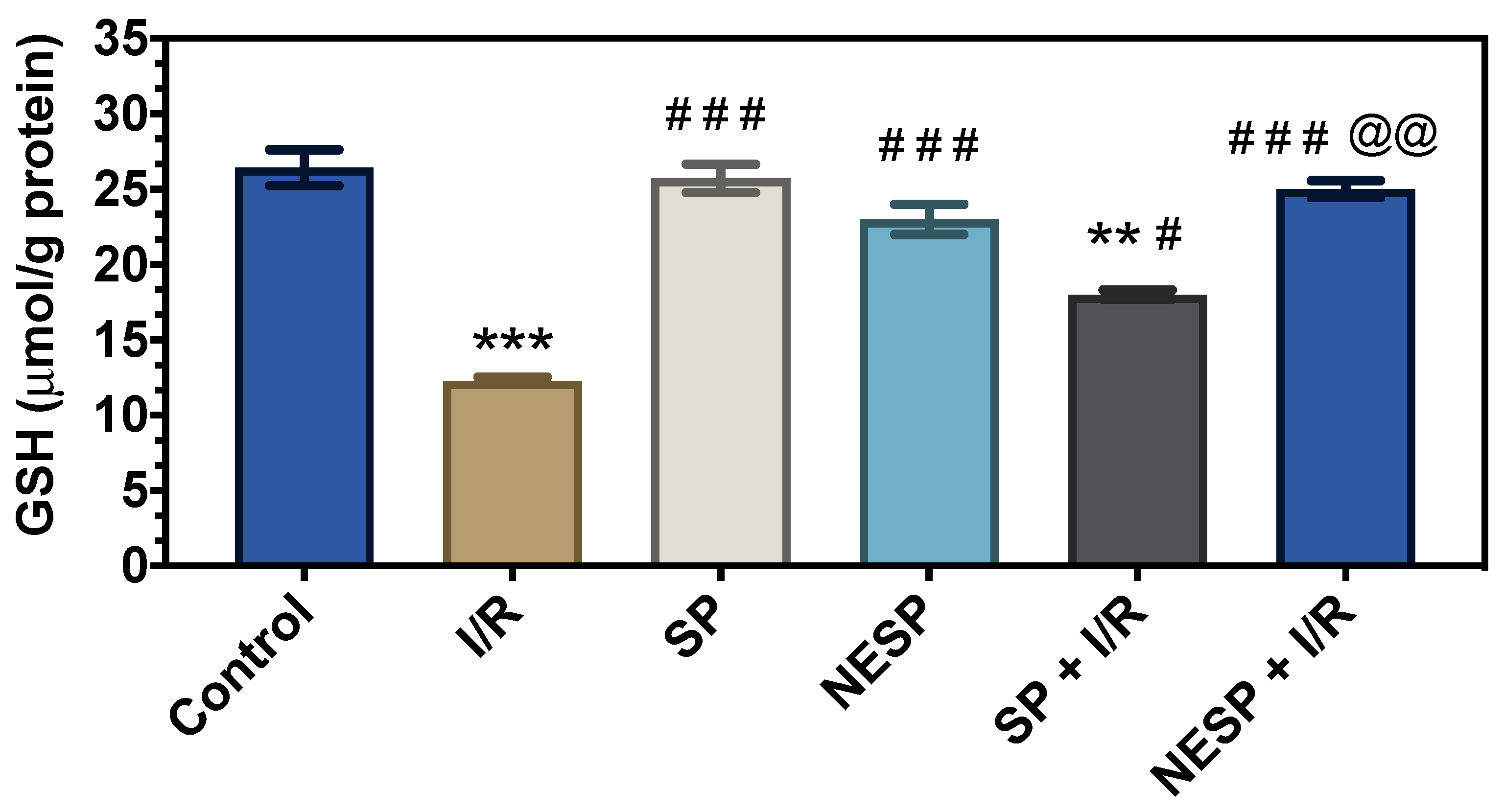

| Markers | Control | I/R | SP | NESP | SP + I/R | NESP + I/R |
|---|---|---|---|---|---|---|
| Urea (mg/dL) | 5.97 ± 0.99 | 49.11 ± 3.11 *** | 6.21 ± 0.42 ### | 7.31 ± 0.97 ### | 32.34 ± 3.08 ***## | 12.14 ± 1.00 ###@@ |
| Creatinine (mg/dL) | 0.38 ± 0.04 | 1.98 ± 0.23 *** | 0.19 ± 0.02 ### | 0.29 ± 0.01 ### | 1.12 ± 0.09 **# | 0.41 ± 0.01 ###@ |
| TP (g/dL) | 6.84 ± 0.21 | 2.98 ± 0.09 *** | 5.78 ± 0.43 ### | 7.58 ± 0.51 ### | 4.54 ± 0.09 **# | 6.11 ± 0.07 ###@ |
| Uric acid (mg/dL) | 1.35 ± 0.30 | 5.98 ± 0.39 *** | 0.97 ± 0.02 ### | 1.31 ± 0.11 ### | 4.20 ± 0.07 ***## | 1.98 ± 0.25 ###@@ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanein, P.; Rahdar, A.; Barani, M.; Baino, F.; Yari, S. Oil-In-Water Microemulsion Encapsulation of Antagonist Drugs Prevents Renal Ischemia-Reperfusion Injury in Rats. Appl. Sci. 2021, 11, 1264. https://doi.org/10.3390/app11031264
Hasanein P, Rahdar A, Barani M, Baino F, Yari S. Oil-In-Water Microemulsion Encapsulation of Antagonist Drugs Prevents Renal Ischemia-Reperfusion Injury in Rats. Applied Sciences. 2021; 11(3):1264. https://doi.org/10.3390/app11031264
Chicago/Turabian StyleHasanein, Parisa, Abbas Rahdar, Mahmood Barani, Francesco Baino, and Siamak Yari. 2021. "Oil-In-Water Microemulsion Encapsulation of Antagonist Drugs Prevents Renal Ischemia-Reperfusion Injury in Rats" Applied Sciences 11, no. 3: 1264. https://doi.org/10.3390/app11031264
APA StyleHasanein, P., Rahdar, A., Barani, M., Baino, F., & Yari, S. (2021). Oil-In-Water Microemulsion Encapsulation of Antagonist Drugs Prevents Renal Ischemia-Reperfusion Injury in Rats. Applied Sciences, 11(3), 1264. https://doi.org/10.3390/app11031264








