1. Introduction
Polypoidal choroidal vasculopathy (PCV) is a distinct phenotype of exudative age-related macular degeneration (AMD) and is characterized by polyp-like nodules and branching vascular networks, which are hyperfluorescent on indocyanine-green angiography (ICGA) [
1,
2]. A previous study reported better visual prognosis in patients with PCV than in patients with other forms of exudative AMD [
3]. However, other studies demonstrated that the best-corrected visual acuity (BCVA) deteriorated significantly in the natural course and half of the patients who experienced repeated bleeding and leakage, resulting in chorio-retinal atrophy and vision loss [
4,
5].
There are two main treatment modalities for PCV, including photodynamic therapy (PDT) and intravitreal injection (IVI) of anti-vascular endothelial growth factor (VEGF) agents. VEGF plays an important role in exudative AMD and PCV, and VEGF levels in aqueous solution elevated in patients with PCV [
6]. Anti-VEGF agents, such as ranibizumab and aflibercept, have a favorable visual outcome for treating PCV, but a large number of injections are required to maintain the vision. In three initial monthly injections followed by an as-needed reinjection regimen, the mean number of injections ranged from 4.2 to 5.0 during a one-year follow-up [
7,
8,
9,
10]. PDT is effective in occlusion of the polypoidal vascular lesions, and the complete polyp regression rate of PDT alone is greater than that of anti-VEGF monotherapy [
11]. However, PDT may induce choroidal ischemia and retinal pigment epithelium damage, which may contribute to VEGF production [
12]. PDT combined with anti-VEGF is thought to have synergistic effects on polyp regression and a good visual outcome.
In the EVEREST II study, patients receiving combination therapy of PDT with intravitreal ranibizumab (IVR) had a favorable visual outcome and complete polyp regression, and few injections were required [
13]. Other studies also reported preferable visual outcomes of combination therapy of PDT with either IVR or intravitreal aflibercept (IVA) [
14,
15,
16,
17,
18]. However, the different efficacy of combination therapy with different anti-VEGF agents was not well established.
The purpose of this study is to compare the two-year visual and anatomical outcomes of combination therapy of PDT with IVA or IVR for patients with PCV in order to investigate the clinical factors with a final visual outcome and retreatment.
2. Methods
A retrospective medical chart review was performed for 55 eyes from 55 patients with PCV treated by a combination therapy of either IVA or IVR with prompt PDT at Far Eastern Memorial Hospital in Taiwan from August 2015 to November 2018. There were 30 patients in the IVA/PDT group treated from August 2015 to December 2017, and 25 patients in the IVR/PDT group from January to November 2018. This study was approved by the Institutional Review Board of Far Eastern Memorial Hospital (ethical approval office reference number: FEMH-107160-E), and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants before treatment.
Prior to the initial treatments, all patients received comprehensive ophthalmic examinations, including measurements of (BCVA) using Snellen E chart, intraocular pressure, slit-lamp biomicroscopy, color fundus photography, spectral-domain optical coherence tomography (SD-OCT) (RTVue, Optovue Inc., Fremont, CA, USA), fundus fluorescein, and indocyanine green angiography (FA/ICGA) (HRA, Heidelberg Engineering, Heidelberg, Germany). Central retinal thickness (CRT) was measured by SD-OCT.
The diagnosis of PCV was made by the presence of polypoidal choroidal vascular lesions with or without abnormal vascular networks on ICGA. Exclusion criteria included patients with diabetic retinopathy, retinal vessel occlusion, rhegmatogenous retinal detachment, uveitis, glaucoma, or pregnancy. If the patient had PCV of both eyes, the eye treated first was included. If a patient received treatment for both eyes simultaneously, the eye with worse BCVA was included for analysis.
All subjects received combination therapy with IVI of aflibercept (2.0 mg in 0.05 mL) or ranibizumab (0.5 mg in 0.05 mL) followed by standard PDT within a week of the IVI. The PDT was administered according to the standard protocol [
19]. After the initial treatment, follow-up examinations including BCVA, slit-lamp biomicroscopy, fundus examination, and OCT were performed every 3 months, and all patients were followed for at least 24 months. Angiographic regression of polypoidal vascular lesions in ICGA was evaluated three months after the initial treatment.
Retreatment included IVI monotherapy or combination therapy. In the IVA/PDT group, repeated IVA monotherapy was administered in cases with recurrent or persistent submacular or intramacular fluid but complete regression of choroidal vascular polyps. Repeated combination therapy with IVA and PDT was performed in cases having partial or no polyp regression without dry macula. Repeated IVR alone or combined with PDT was performed using the same protocol in recurrent or refractory cases in the IVR/PDT group. Numbers of additional IVI monotherapy or additional combination therapy were recorded and the units were times. The retreatment rate was defined as patients receiving retreatment of all patients in each group.
Baseline data and treatment outcomes were compared between the two groups. Primary outcomes were the changes in BCVA and the rate of complete polyp regression. Secondary outcome measures were the changes in CRT, and the rate of dry macula. The BCVA was converted from a Snellen E chart to a logarithm of the minimum angle of resolution (LogMAR) for statistical analysis. Patients with BCVA gain or loss ≥ 0.3 LogMAR units were recorded. Dry macula means no subretinal and intraretinal fluid at macula on OCT.
Statistical analysis was performed using SPSS (V. 20.0, IBM, Armonk, New York, NY, USA). A paired t-test was used to compare LogMAR BCVA and CRT before and after treatment. An independent t-test was used to evaluate differences of continuous variables between the two groups. A chi-square test was used to evaluate differences of categorical variables between the two groups. Pearson’s correlation coefficient was performed to investigate the correlations of clinical factors and visual outcome or retreatment. A p-value less than 0.05 was considered a statistical significance.
3. Results
Baseline characteristics comparison between the two treatment groups was presented in
Table 1. There were no significant differences in baseline characteristics between the two groups except for gender since there were more male patients in the IVA/PDT group than those in the IVR/PDT group.
Figure 1 showed the changes of BCVA of the two groups. The mean logMAR BCVA in the IVA/PDT group significantly improved from 0.73 ± 0.65 prior to the initial treatment to 0.60 ± 0.69 at 6 months (
p = 0.045), 0.51 ± 0.59 at 12 months (
p = 0.010), 0.58 ± 0.61 at 18 months (
p = 0.021), and 0.55 ± 0.52 at 24 months (
p = 0.022) after the treatment. In the IVR/PDT group, the mean logMAR BCVA significantly improved from 0.76 ± 0.64 at baseline to 0.53 ± 0.64 at 6 months (
p = 0.008) and 0.48 ± 0.55 at 12 months (
p = 0.015), but the BCVA changed to 0.59 ± 0.65 at 18 months (
p = 0.113) and 0.61 ± 0.69 at 24 months (
p = 0.256) insignificantly when compared to baseline BCVA. There were no significant differences in change of BCVA between the two groups at every 6-month visit.
At the 24-month follow-up, BCVA gain ≥ 0.3 LogMAR was achieved in 23.3% and 40.0% patients of IVA/PDT and IVR/PDT, respectively. BCVA loss ≥ 0.3 LogMAR was recorded in 6.7% and 24.0% of patients in IVA/PDT and IVR/PDT groups, respectively. There were no significant differences in BCVA gain or BCVA loss ≥ 0.3 LogMAR between the two groups (
Table 2).
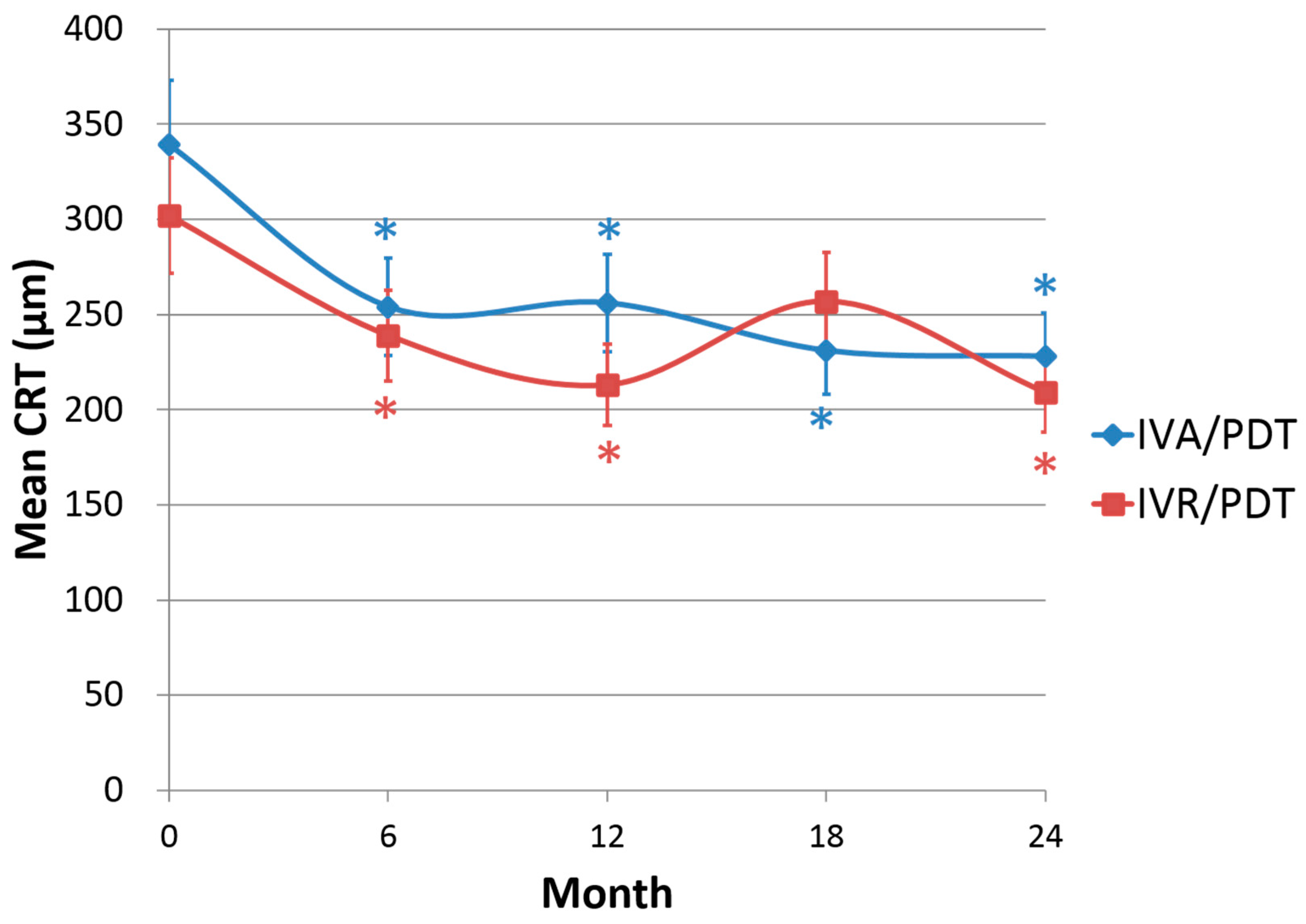
The CRT decreased significantly from 339 ± 96 μm at baseline to 228 ± 44 μm at 24-month in IVA/PDT, and from 302 ± 59 μm at baseline to 209 ± 38 μm at 24-month in IVR/PDT.
Figure 2 shows the changes of CRT of the two groups, which were significantly reduced at every 6-month visit compared to baseline in both groups (
p < 0.001) except for the 18-month follow-up in the IVR/PDT group (
p = 0.092). The change in CRT did not differ between the two groups at every 6-month visit.
Complete polyp regression rate at 3-month was 53.3% in the IVA/PDT group, and 52.0% in the IVR/PDT group. Between the two groups, the complete polyp regression rate did not differ (
p = 0.921). In the IVA/PDT group, the dry macula rate was 93.3% at 6 months, and then maintained at 96.7% at the 12-month, 18-month, and 24-month period. In the IVR/PDT group, the dry macula rate was 72.0% at the 6-month, 96.0% at the 12-month, 80.0% at the 18-month, and 92.0% at the 24-month periods. There were significant differences in the 6-month dry macula rate (
p = 0.033) and the 18-month dry macula rate (
p = 0.048) (
Table 2).
During the 24-month follow-up, retreatment was performed in 26.7% of patients in the IVA/PDT group, and in 60.0% of patients in the IVR/PDT group. The retreatment rate was significantly different between the two groups (p = 0.013). The mean additional IVI monotherapy was 0.13 ± 0.35 times in the IVA/PDT group and 0.68 ± 0.85 times in the IVR/PDT group. The mean additional combination therapy was 0.23 ± 0.43 times in the IVA/PDT group and 0.52 ± 0.59 times in the IVR/PDT group. The differences in additional IVI monotherapy and additional combination therapy were statistically significant (p = 0.005 and p = 0.048, respectively).
There was a significant correlation of baseline BCVA and final BCVA (r = 0.663,
p < 0.001). Younger patients had a better visual outcome (r = 0.328,
p = 0.014). Visual outcome was not related to gender, lens status, baseline CRT, retreatment, or complete polyp regression. Retreatment was significantly associated with complete polyp regression at the 3-month period (
p = 0.024), but was not associated with baseline characteristics.
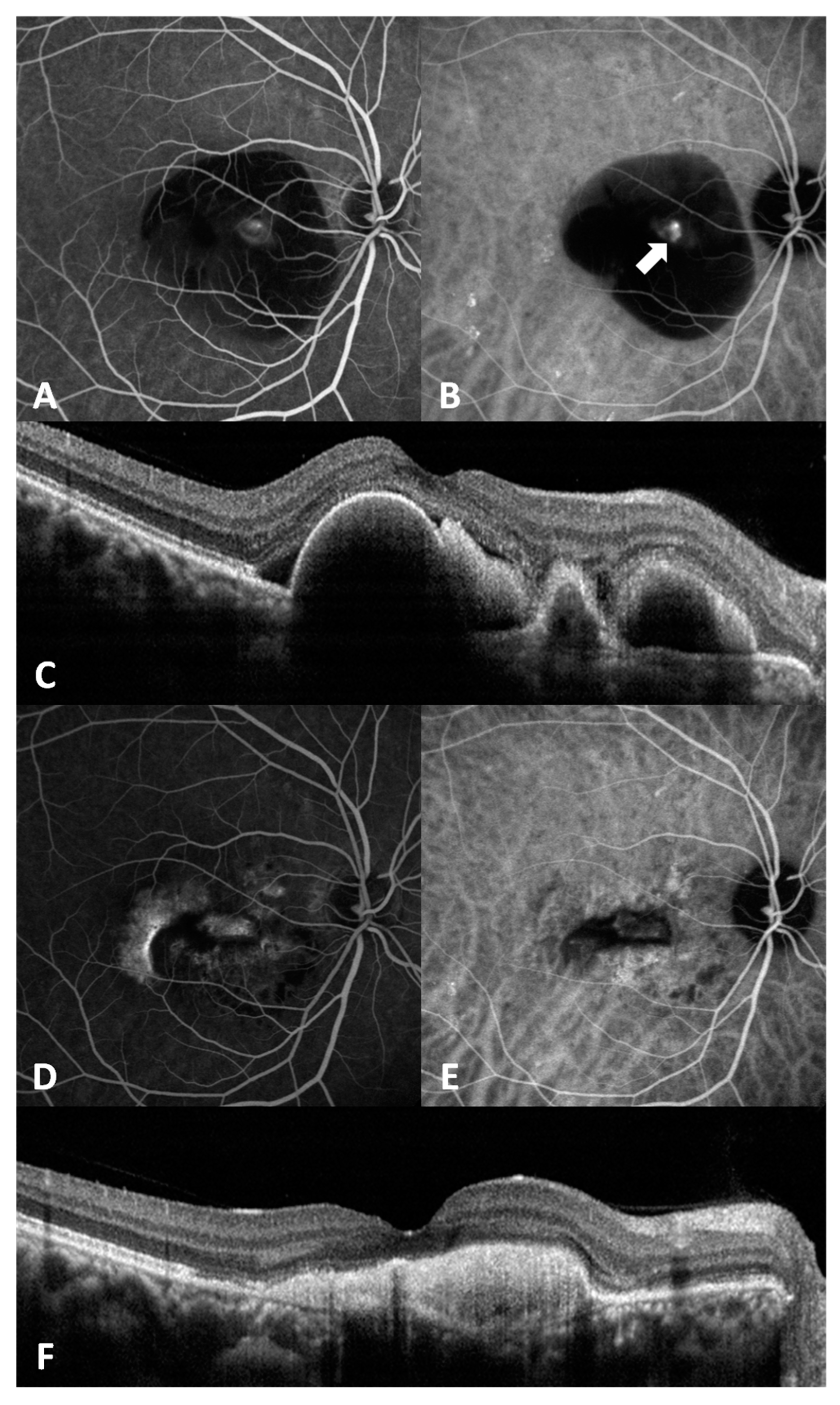
Figure 3 shows representative images of a case.
4. Discussion
Previous studies also compared combination therapy of PDT with IVA or IVR for treating PCV. Sakurada Y. et al. and Ito A. et al. reported no significant differences in changes in BCVA or CRT between the two groups 12 months after treatment [
20,
21], which were similar to our results at the 12-month follow-up. However, Kikushima W. et al. demonstrated significantly better BCVA in the IVA/PDT group at 18 and 24 months [
22]. In our study, the BCVA improvement was significant in the IVA/PDT group, but not significant in the IVR/PDT group at the 18-month and 24-month follow-ups compared to baseline. However, there was no significant difference in the change of BCVA between the two groups.
As for the change of CRT, previous studies reported significant reduction of CRT and subfoveal choroidal thickness in either the IVA/PDT group or the IVR/PDT group during a one-year follow-up period [
20,
21]. Additionally, Ito A. et al. demonstrated the change in subfoveal choroidal thickness was significantly larger in the IVA/PDT group than that in the IVR/PDT group [
21]. In our study, the CRT reduction was not significant in the IVR/PDT group at the 18-month follow-up when compared to baseline.
There was a significant difference in the dry macula rate at 18 months between the two groups, and the result was consistent with poor BCVA and CRT in the IVR/PDT group at the 18-month period. At the 24-month follow-up, the CRT reduced and the dry macula rate was achieved in 92.0% of patients in the IVR/PDT group, but the BCVA did not improve. This implies that, after retreatment for the recurrent disease, chorioretinal atrophy might develop, and the visual outcome was not as favorable as the anatomical outcome.
As for the rate of complete polyp regression, Ito A. et al. reported 76.9% in the IVA/PDT group and 78.2% in the IVR/PDT group at 12 months after treatment, without a significant difference between the two groups (
p = 0.57). Other studies reported 70.0% to 87.5% of the 3-month period for the complete polyp regression rate in patients receiving IVA/PDT [
17,
18], and 77.8% of the 6-month period for the complete polyp regression rate in patients receiving IVR/PDT. In our study, the 3-month polyp regression rate in both groups was inferior to that of previous studies (53.3% in the IVA/PDT group and 52.0% in the IVR/PDT group).
In this present study, there was significantly less retreatment, additional IVI monotherapy, and additional combination therapy in the IVA/PDT group than those in the IVR/PDT group. Sakurada Y. et al. also reported less additional combination therapy in the IVA/PDT group than that in the IVR/PDT group but without a statistically significant difference (
p = 0.08) [
20]. Kikushima W. et al. reported no differences in the retreatment rate, additional PDT, or IVI number between the two groups [
22].
We demonstrated that younger patients and patients with better baseline BCVA had better visual outcome. Sakurada Y. et al. reported better visual outcome was correlated with better initial BCVA, a smaller greatest linear dimension, and greater subfoveal choroidal thickness [
20]. Kikushima W. et al. reported better final BCVA was associated with the female sex, better initial BCVA, IVA/PDT treatment, and a smaller greatest linear dimension [
22].
This is the first study of comparison of the two-year outcome of combination therapy of PDT with IVA or IVR for PCV, which only enrolled participants in Taiwan. However, there were some limitations of the study. First, this study was retrospective and we could not control for certain confounding factors, including patients’ physical condition and lifestyle. Second, our case number was relatively small. Third, there was a significant difference of gender between the two groups in our study, and this may be attributed to the small sample size. Finally, only patients with persistent or recurrent disease received repeated ICGA examination during 6 months to 24 months, so we could not obtain the data of a complete polyp regression rate through the whole period of the study.
In summary, both the IVA/PDT and IVR/PDT were effective in treating PCV. Although there were no significant differences of final BCVA or CRT between the two groups, patients in the PDT/IVA group experienced less disease recurrence and needed fewer PDT numbers and injection numbers than patients in the IVR/PDT group. Combination therapy with PDR and IVA is a good treatment choice for eyes with PCV.
Author Contributions
Conceptualization, J.-K.W.; methodology, J.-K.W.; software, H.-Y.W.; validation, J.-K.W., T.-L.H., P.-Y.C., W.-T.H., Y.-R.H., F.-T.C., and Y.-J.C.; formal analysis, H.-Y.W.; investigation, H.-Y.W.; resources, J.-K.W., Y.-R.H., F.-T.C., and Y.-J.C.; data curation, H.-Y.W.; writing—original draft preparation, H.-Y.W.; writing—review and editing, J.-K.W.; visualization, T.-L.H., P.-Y.C., W.-T.H., Y.-R.H., F.-T.C., and Y.-J.C.; supervision, T.-L.H., P.-Y.C., W.-T.H., Y.-R.H., F.-T.C., and Y.-J.C.; project administration, J.-K.W.; funding acquisition, J.-K.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Far Eastern Memorial Hospital (ethical approval office reference number: FEMH-107160-E), and was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Research data will be available after personal request for the responding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yannuzzi, L.A.; Sorenson, J.; Spaide, R.F.; Lipson, B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990, 10, 1–8. [Google Scholar] [CrossRef]
- Spaide, R.F.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.; Orlach, D.A. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995, 15, 100–110. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Ciardella, A.; Spaide, R.F.; Rabb, M.; Freund, K.B.; Orlock, D.A. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch. Ophthalmol. 1997, 115, 478–485. [Google Scholar] [CrossRef]
- Bessho, H.; Honda, S.; Imai, H.; Negi, A. Natural course and funduscopic findings of polypoidal choroidal vasculopathy in a Japanese population over 1 year of follow-up. Retina 2011, 31, 1598–1602. [Google Scholar] [CrossRef]
- Uyama, M.; Wada, M.; Nagai, Y.; Matsubara, T.; Matsunaga, H.; Fukushima, I.; Takahashi, K.; Matsumura, M. Polypoidal choroidal vasculopathy: Natural history. Am. J. Ophthalmol. 2002, 133, 639–648. [Google Scholar] [CrossRef]
- Matsuoka, M.; Ogata, N.; Otsuji, T.; Nishimura, T.; Takahashi, K.; Matsumura, M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br. J. Ophthalmol. 2004, 88, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, T.; Higuchi, M.; Matsushita, T.; Kosaka, S.; Matsushita, R.; Takami, K.; Ohtsuka, H.; Ariga, H. One-year results of three monthly ranibizumab injections and as-needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am. J. Ophthalmol. 2012, 154, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Oishi, A.; Kojima, H.; Mandai, M.; Honda, S.; Matsuoka, T.; Oh, H.; Kita, M.; Nagai, T.; Fujihara, M.; Bessho, N.; et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am. J. Ophthalmol. 2013, 156, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Maruyama-Inoue, M.; Sato, S.; Yamane, S.; Kadonosono, K. Intravitreal injection of aflibercept in patients with polypoidal choroidal vasculopathy: A 3-year follow-up. Retina 2018, 38, 2001–2009. [Google Scholar] [CrossRef]
- Inoue, M.; Yamane, S.; Taoka, R.; Arakawa, A.; Kadonosono, K. Aflibercept for polypoidal choroidal vasculopathy: As needed versus fixed interval dosing. Retina 2016, 36, 1527–1534. [Google Scholar] [CrossRef]
- Koh, A.; Lee, W.K.; Chen, L.J.; Chen, S.J.; Hashad, Y.; Kim, H.; Lai, T.Y.; Pilz, S.; Ruamviboonsuk, P.; Tokaji, E.; et al. EVEREST study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012, 32, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Tatar, O.; Adam, A.; Shinoda, K.; Stalmans, P.; Eckardt, C.; Lüke, M.; Bartz-Schmidt, K.U.; Grisanti, S. Expression of VEGF and PEDF in choroidal neovascular membranes following verteporfin photodynamic therapy. Am. J. Ophthalmol. 2006, 142, 95–104. [Google Scholar] [CrossRef]
- Koh, A.; Lai, T.Y.Y.; Takahashi, K.; Wong, T.Y.; Chen, L.J.; Ruamviboonsuk, P.; Tan, C.S.; Feller, C.; Margaron, P.; Lim, T.H.; et al. Efficacy and Safety of Ranibizumab With or Without Verteporfin Photodynamic Therapy for Polypoidal Choroidal Vasculopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2017, 135, 1206–1213. [Google Scholar] [CrossRef]
- Saito, M.; Iida, T.; Kano, M.; Itagaki, K. Two-year results of combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2099–2110. [Google Scholar] [CrossRef]
- Sakurada, Y.; Iijima, H. Two-year results of photodynamic therapy with or without intravitreal ranibizumab for polypoidal choroidal vasculopathy. J. Ocul. Pharmacol. Ther. 2013, 29, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kaneko, H.; Kataoka, K.; Hattori, K.; Ra, E.; Tsunekawa, T.; Fukukita, H.; Haga, F.; Ito, Y.; Terasaki, H. Comparison between 1-year outcomes of aflibercept with and without photodynamic therapy for polypoidal choroidal vasculopathy: Retrospective observation study. PLoS ONE 2017, 12, e0176100. [Google Scholar] [CrossRef]
- Matsumiya, W.; Honda, S.; Otsuka, K.; Miki, A.; Nagai, T.; Imai, H.; Kusuhara, S.; Nakamura, M. One-year outcome of combination therapy with intravitreal aflibercept and verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Kikushima, W.; Sakurada, Y.; Sugiyama, A.; Tanabe, N.; Kume, A.; Iijima, H. Comparison of initial treatment between 3-monthly intravitreal aflibercept monotherapy and combined photodynamic therapy with single intravitreal aflibercept for polypoidal choroidal vasculopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 311–316. [Google Scholar] [CrossRef]
- Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials--TAP report. Arch. Ophthalmol. 1999, 117, 1329–1345. [Google Scholar] [CrossRef]
- Sakurada, Y.; Sugiyama, A.; Tanabe, N.; Kikushima, W.; Kume, A.; Iijima, H. Choroidal thickness as a prognostic factor of photodynamic therapy with aflibercept or ranibizumab for polypoidal choroidal vasculopathy. Retina 2017, 37, 1866–1872. [Google Scholar] [CrossRef]
- Ito, A.; Maruyama-Inoue, M.; Kitajima, Y.; Sato, S.; Inoue, T.; Yamane, S.; Kadonosono, K. Comparison of one-year results of photodynamic therapy combined with ranibizumab or aflibercept for treating polypoidal choroidal vasculopathy. PLoS ONE 2020, 15, e0235213. [Google Scholar] [CrossRef] [PubMed]
- Kikushima, W.; Sakurada, Y.; Sugiyama, A.; Yoneyama, S.; Tanabe, N.; Matsubara, M.; Mabuchi, F.; Iijima, H. Comparison of two-year outcomes after photodynamic therapy with ranibizumab or aflibercept for polypoidal choroidal vasculopathy. Sci. Rep. 2017, 7, 16461. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).