Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Academies of Sciences, Engineering, and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [PubMed]
- Unluer, C.; Al-Tabbaa, A. Characterization of Light and Heavy Hydrated Magnesium Carbonates Using Thermal Analysis. J. Therm. Anal. Calorim. 2014, 115, 595–607. [Google Scholar]
- Farhang, F.; Oliver, T.K.; Rayson, M.; Brent, G.; Stockenhuber, M.; Kennedy, E. Experimental study on the precipitation of magnesite from thermally activated serpentine for CO2 sequestration. Chem. Eng. J. 2016, 303, 439–449. [Google Scholar]
- Glasser, F.P.; Jauffret, G.; Morrison, J.; Galvez-Martos, J.-L.; Patterson, N.; Imbabi, M.S.-E. Sequestering CO2 by Mineralization into Useful Nesquehonite-Based Products. Front. Energy Res. 2013, 4, 3. [Google Scholar]
- Frykstrand, S.; Forsgren, J.; Mihranyan, A.; Strømme, M. On the pore forming mechanism of Upsalite, a micro- and mesoporous magnesium carbonate. Microporous Mesoporous Mater. 2014, 190, 99–104. [Google Scholar]
- Yang, J.; Alvebratt, C.; Lu, X.; Bergström, C.A.S.; Strömme, M.; Welch, K. Amorphous magnesium carbonate nanoparticles with strong stabilizing capability for amorphous ibuprofen. Int. J. Pharm. 2018, 548, 515–521. [Google Scholar]
- Cheung, O.; Zhang, P.; Frykstrand, S.; Zheng, H.; Yang, T.; Sommariva, M.; Zou, X.; Strömme, M. Nanostructure and pore size control of template-free synthesised mesoporous magnesium carbonate. RSC Adv. 2016, 6, 74241–74249. [Google Scholar]
- Frykstrand, S.; Forsgren, J.; Cheung, O.; Zhang, P.; Hong, J.; Strømme, M.; Ferraz, N. Study of mesoporous magnesium carbonate in contact with whole human blood. RSC Adv. 2016, 6, 52810–52816. [Google Scholar]
- Vall, M.; Hultberg, J.; Strömme, M.; Cheung, O. Carbon dioxide adsorption on mesoporous magnesium carbonate. Energy Procedia 2019, 158, 4671–4676. [Google Scholar]
- Shahwan, T.; Zünbül, B.; Eroğlu, A.E.; Yılmaz, S. Effect of magnesium carbonate on the uptake of aqueous zinc and lead ions by natural kaolinite and clinoptilolite. Appl. Clay Sci. 2005, 30, 209–218. [Google Scholar]
- Wang, P.; Shen, T.; Li, X.; Tang, Y.; Li, Y. Magnetic Mesoporous Calcium Carbonate-Based Nanocomposites for the Removal of Toxic Pb(II) and Cd(II) Ions from Water. ACS Appl. Nano Mater. 2020, 3, 1272–1281. [Google Scholar]
- Shan, Q.; Zhang, Y.; Xue, X. Removal of copper from wastewater by using the synthetic nesquehonite. Environ. Prog. Sustain. Energy 2013, 3, 543–546. [Google Scholar]
- Castilleja-Escobedo, O.; Sánchez-García, R.E.; Nigama, K.D.P.; López-Salinas, J.L. Directional displacement of non-aqueous fluids through spontaneous aqueous imbibition in porous structures. Chem. Eng. Sci. 2020, 228, 115959. [Google Scholar]
- Kim, K.D.; Kim, Y.D.; Kim, S.W. Synthesis of Porous Magnesium Oxide Cubes with Nano-Grain Structure in Supercritical CO2/Ethanol Solution. J. Nanosci. Nanotechnol. 2002, 11, 5723–5728. [Google Scholar]
- de Vito, C.; Ferrini, V.; Mignardi, S.; Cagnetti, M.; Leccese, F. Progress in carbon dioxide sequestration via carbonation of aqueous saline wastes. Period. Mineral. 2012, 81, 333–344. [Google Scholar]
- Nakashima, Y.; Takai, C.; Razavi-Khosroshahi, H.; Suthabanditpong, W.; Fuji, M. Synthesis of ultra-small hollow silica nanoparticles using the prepared amorphous calcium carbonate in one-pot process. Adv. Powder Technol. 2018, 29, 904–908. [Google Scholar]
- DeSimone, J.M. Practical Approaches to Green Solvents. Science 2002, 297, 799–803. [Google Scholar]
- Yoo, Y.; Kang, D.; Choi, E.; Park, J.; Hugh, I. Morphology control of magnesium carbonate for CO2 utilization using Mg2+ ions in industrial wastewater depending on length of alkyl chain of primary alkanolamine, reaction temperature, CO2 concentration, and Mg2+/Na+ ratio. Chem. Eng. J. 2019, 370, 237–250. [Google Scholar]
- Sim, S.; Cole, I.S.; Choi, Y.S.; Birbilis, N. A review of the protection strategies against internal corrosion for the safe transport of supercritical CO2 via steel pipelines for CCS purposes. Int. J. Greenh. Gas Control 2014, 29, 185–199. [Google Scholar]
- Langmuir, D. Stability of Carbonates in the system MgO-CO2-H2O. J. Geol. 2015, 73, 730–754. [Google Scholar]
- Perry, C.T.; Salter, M.A.; Harborne, A.R.; Crowley, S.F.; Jelks, H.L.; Wilson, R.W. Fish as major carbonate mud producers and missing components of the tropical carbonate factory. Proc. Natl. Acad. Sci. USA 2011, 108, 3865–3869. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Ouyang, J.; Yang, H. Synthesis and characterization of nesquehonite (MgCO3·3H2O) powders from natural talc. Powder Technol. 2016, 292, 169–175. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mg-carbonate system—effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Botha, A. DTA and FT-IR analysis of the rehydration of basic magnesium carbonate. J. Therm. Anal. Calorim. 2003, 71, 987–996. [Google Scholar] [CrossRef]
- Forsgren, J.; Frykstrand, S.; Grandfield, K.; Mihranyan, A.; Strömme, M. A Template-Free, Ultra-Adsorbing, High Surface Area Carbonate Nanostructure. PLoS ONE 2013, 8, e68486. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Freeman, J.J.; Jolliff, B.L.; Chou, I. Sulfates on Mars: A systematic Raman spectroscopic study of hydration states of magnesium sulfates. Geochim. Cosmochim. Acta 2006, 70, 6118–6135. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials (Basel) 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Sing, K.S.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ferrini, V.; de Vito, C.; Mignardi, S. Synthesis of nesquehonite by reaction of gaseous CO2 with Mg chloride solution: Its potential role in the sequestration of carbon dioxide. J. Hazard. Mater. 2009, 168, 832–837. [Google Scholar] [CrossRef]

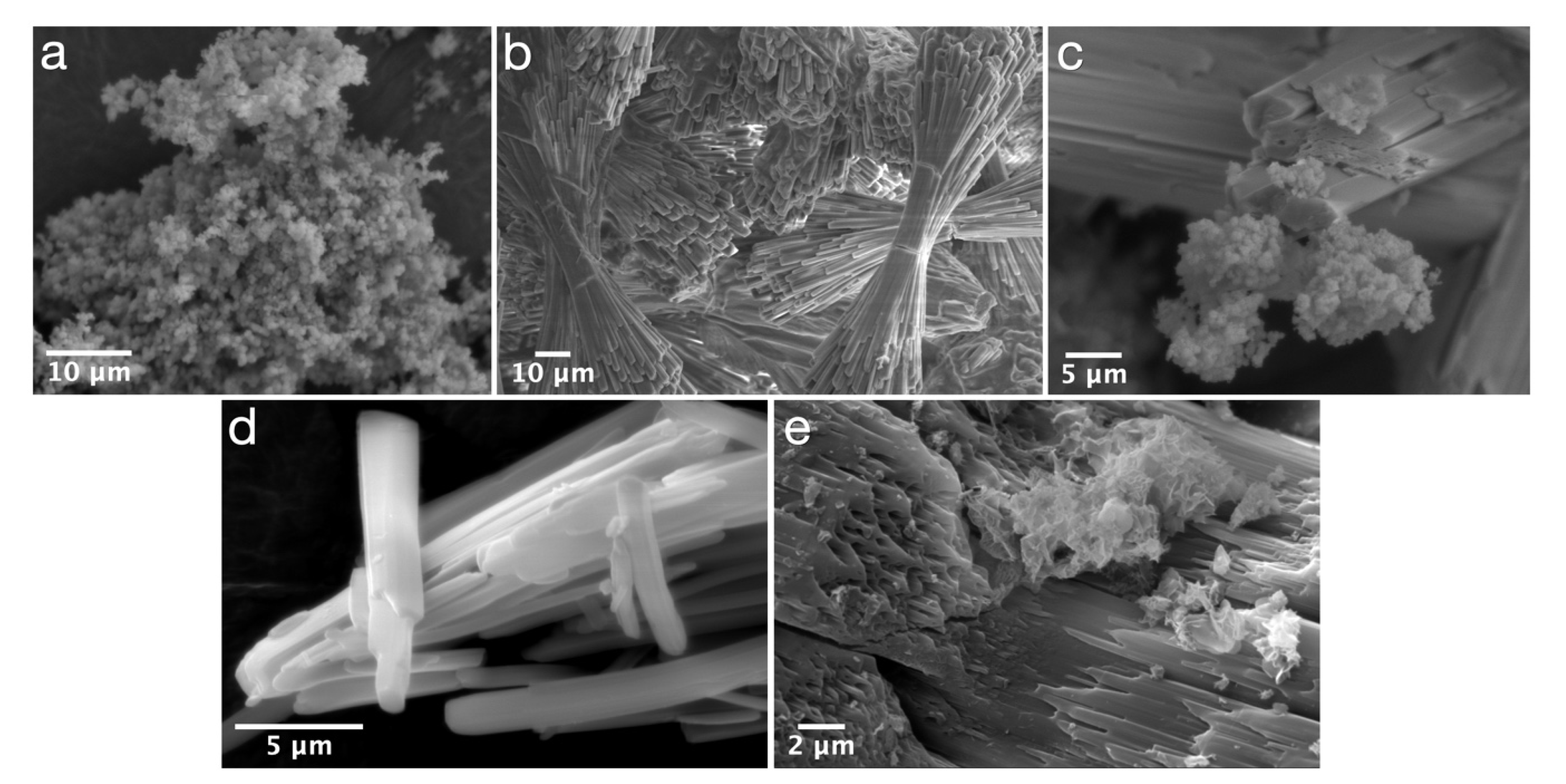
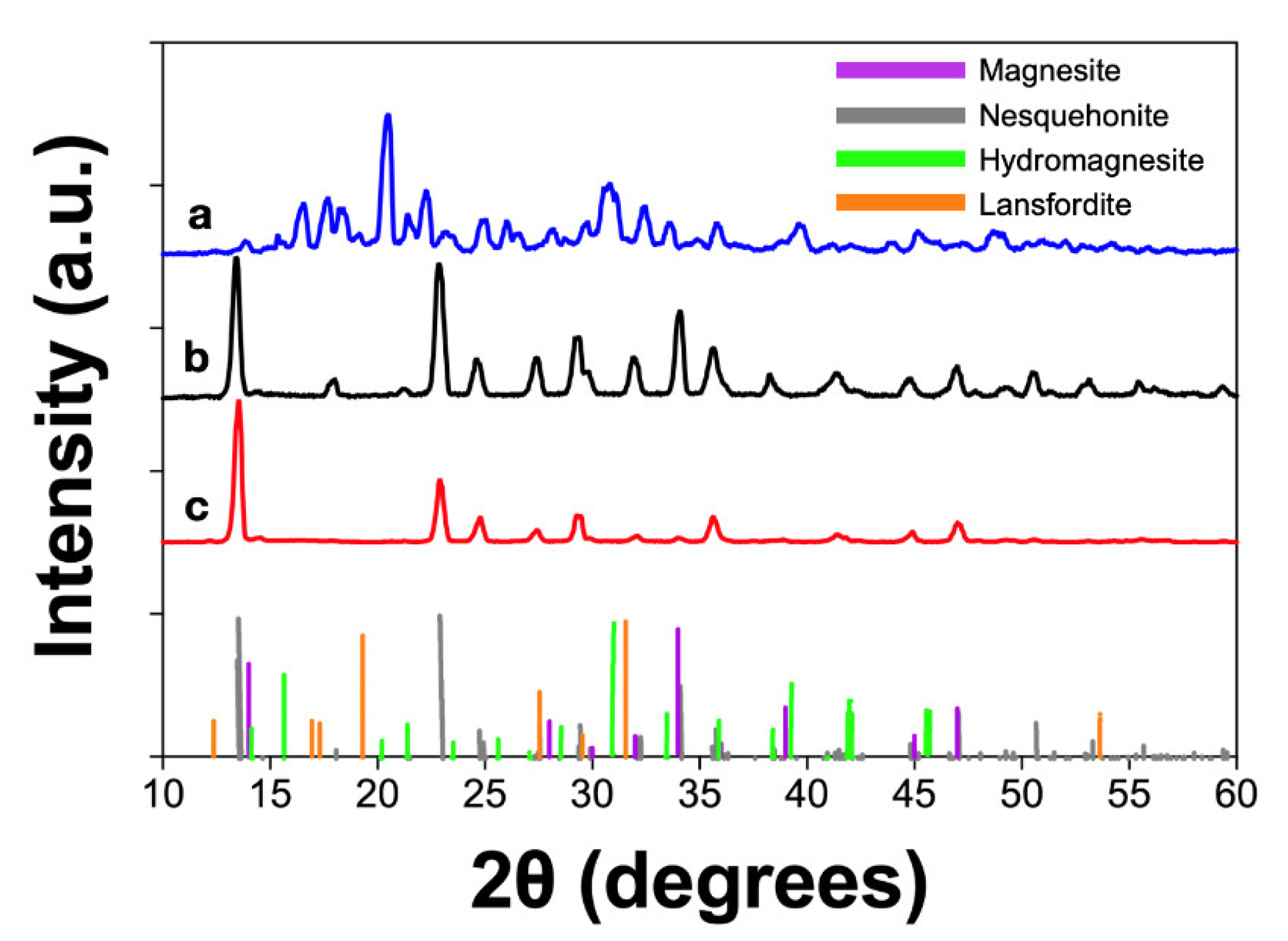
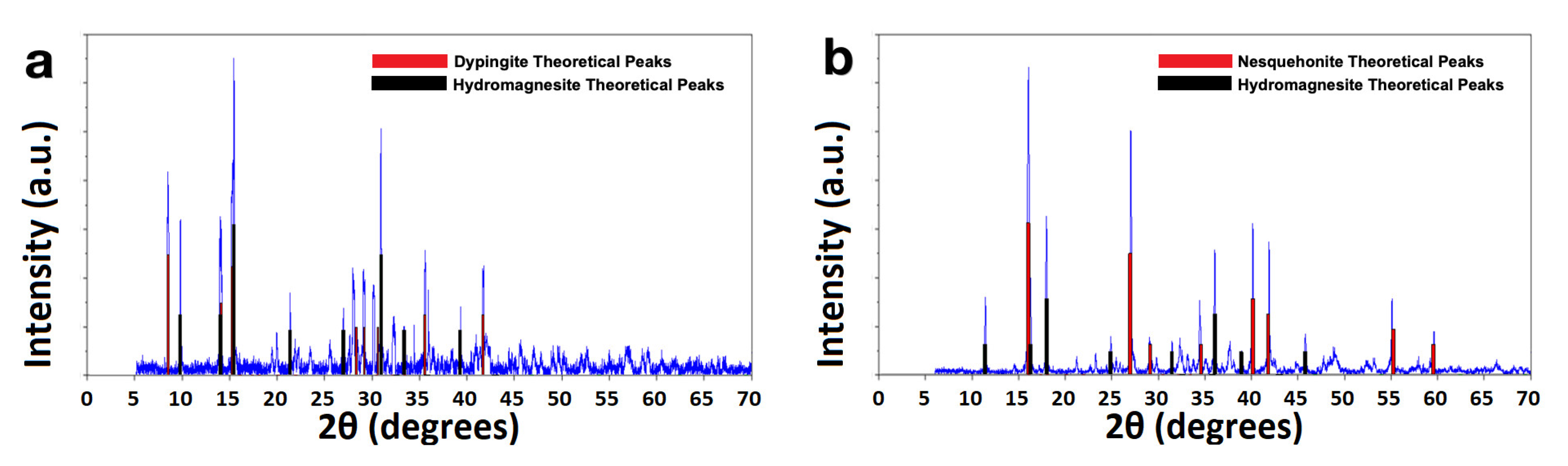
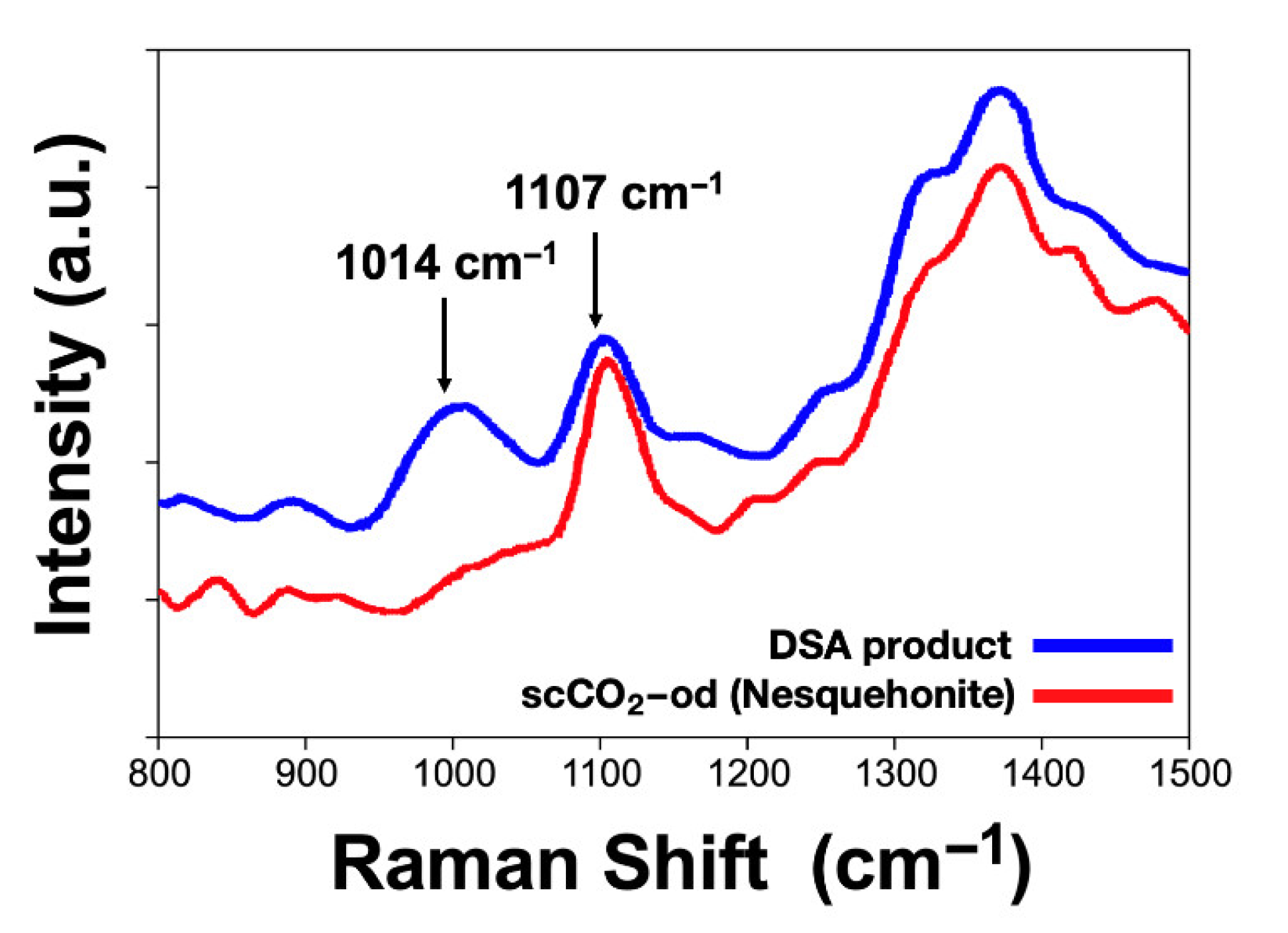
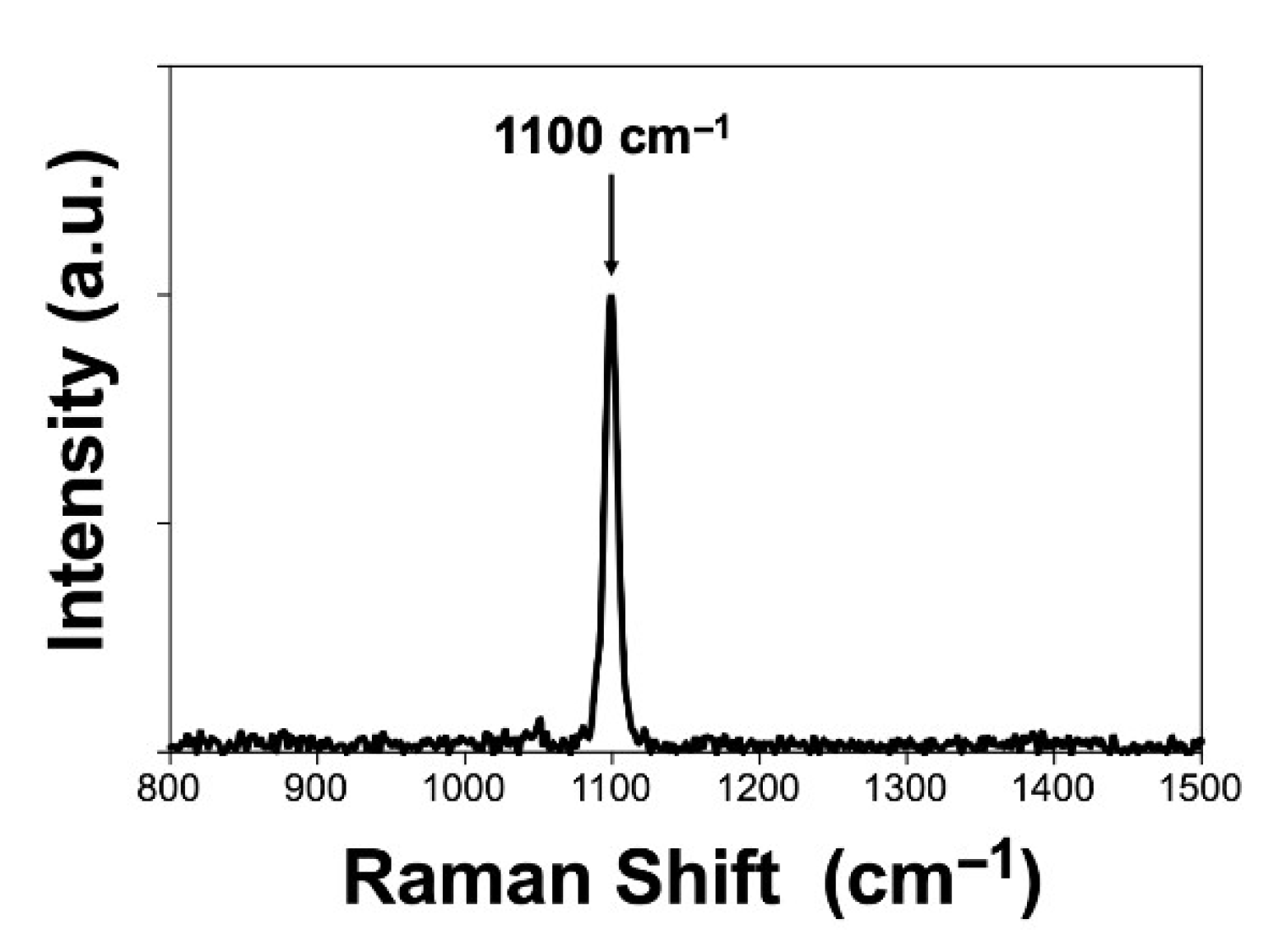
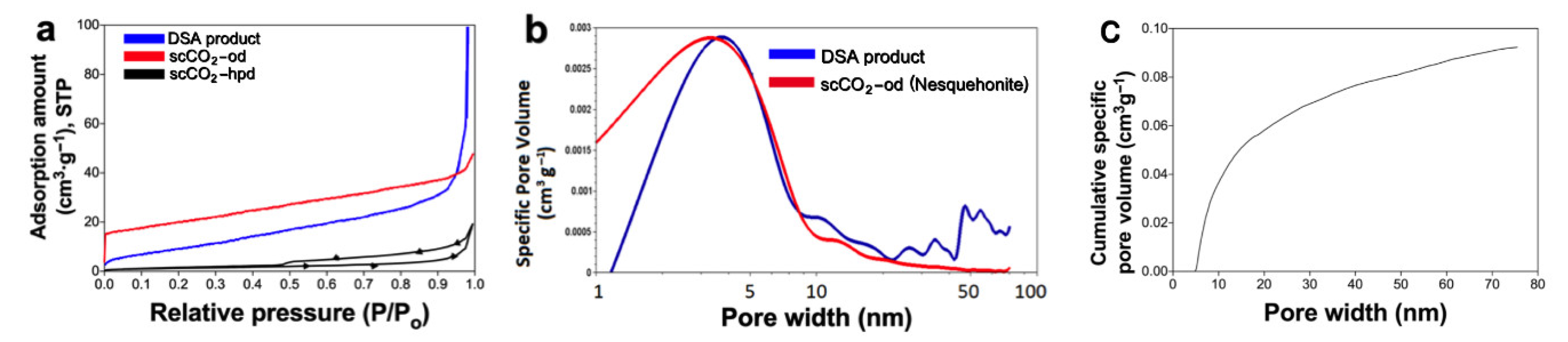
| Experimental Method | Precursors Salts | Reaction Time | Reaction Temperature | Drying Temperature |
|---|---|---|---|---|
| Aqueous Synthesis | MgCl2·6H2O | 25 and 60 min | 5, 21, and 70 °C | 40 °C |
| Mg(NO3)2·6H2O | ||||
| MgSO4·7H2O | ||||
| scCO2 Synthesis | Mg(NO3)2·6H2O | 12 h | 35–40 °C | 40 °C |
| 100 °C |
| Phase | scCO2-od | scCO2-hpd | DSA |
|---|---|---|---|
| Magnesite | 0% | 20% | 0% |
| Nesquehonite | 65% | 0% | 54% |
| Lansfordite | 27% | 60% | 40% |
| Hydromagnesite | 8% | 20% | 6% |
| Sample | Specific Surface Area (m2g−1) | Average Pore Size (nm) |
|---|---|---|
| scCO2-od nesquehonite | 70.4 | 1.02 |
| DSA | 39.1 | 3.78 |
| MgCl2 + NaHCO3 + KOH | 11.8 | 50 |
| Commercial carbonate | 18.1 | 4.89 |
| scCO2 -hpd | 19.8 | 5.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Macías, F.J.; Ortiz-Castillo, J.E.; López-Lara, E.; García-Cuéllar, A.J.; López-Salinas, J.L.; García-Pérez, C.A.; Castilleja-Escobedo, O.; Vega-Cantú, Y.I. Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide. Appl. Sci. 2021, 11, 1141. https://doi.org/10.3390/app11031141
Rodríguez-Macías FJ, Ortiz-Castillo JE, López-Lara E, García-Cuéllar AJ, López-Salinas JL, García-Pérez CA, Castilleja-Escobedo O, Vega-Cantú YI. Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide. Applied Sciences. 2021; 11(3):1141. https://doi.org/10.3390/app11031141
Chicago/Turabian StyleRodríguez-Macías, Fernando J., José E. Ortiz-Castillo, Erika López-Lara, Alejandro J. García-Cuéllar, José L. López-Salinas, César A. García-Pérez, Orlando Castilleja-Escobedo, and Yadira I. Vega-Cantú. 2021. "Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide" Applied Sciences 11, no. 3: 1141. https://doi.org/10.3390/app11031141
APA StyleRodríguez-Macías, F. J., Ortiz-Castillo, J. E., López-Lara, E., García-Cuéllar, A. J., López-Salinas, J. L., García-Pérez, C. A., Castilleja-Escobedo, O., & Vega-Cantú, Y. I. (2021). Syntheses of Nanostructured Magnesium Carbonate Powders with Mesoporous Structures from Carbon Dioxide. Applied Sciences, 11(3), 1141. https://doi.org/10.3390/app11031141







