Biosynthesis of Polyhydroxyalkanoates from Defatted Chlorella Biomass as an Inexpensive Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Microalgae Biomass
2.2. Microbial Strains
2.3. Media Preparation
2.4. Algal Biomass Pretreatment and Reducing Sugar Quantification
2.5. PHA Production via Batch Cultivation in Shake Flasks
2.6. Analytical Techniques
2.6.1. Determination of Cell Growth and Dry Cell Weight (DCW)
2.6.2. Reducing Sugar Quantification during the Fermentation Process
2.6.3. Quantification of Intracellular PHA and Carotenoid Content
2.7. PHA Characterization
2.8. Upscaling of PHA and Carotenoid Co-Production by H. mediterranei in a Fermenter
3. Results and Discussion
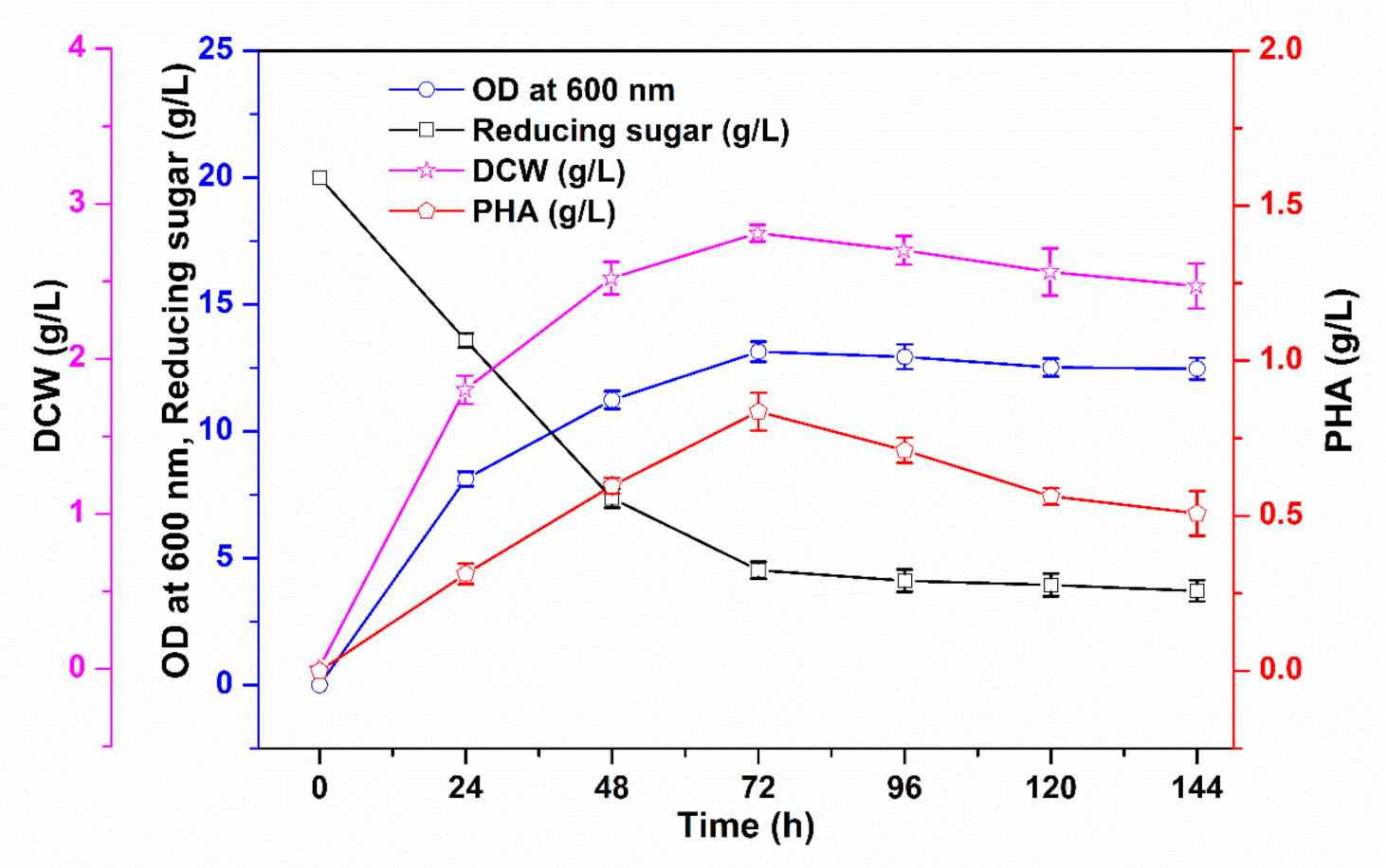
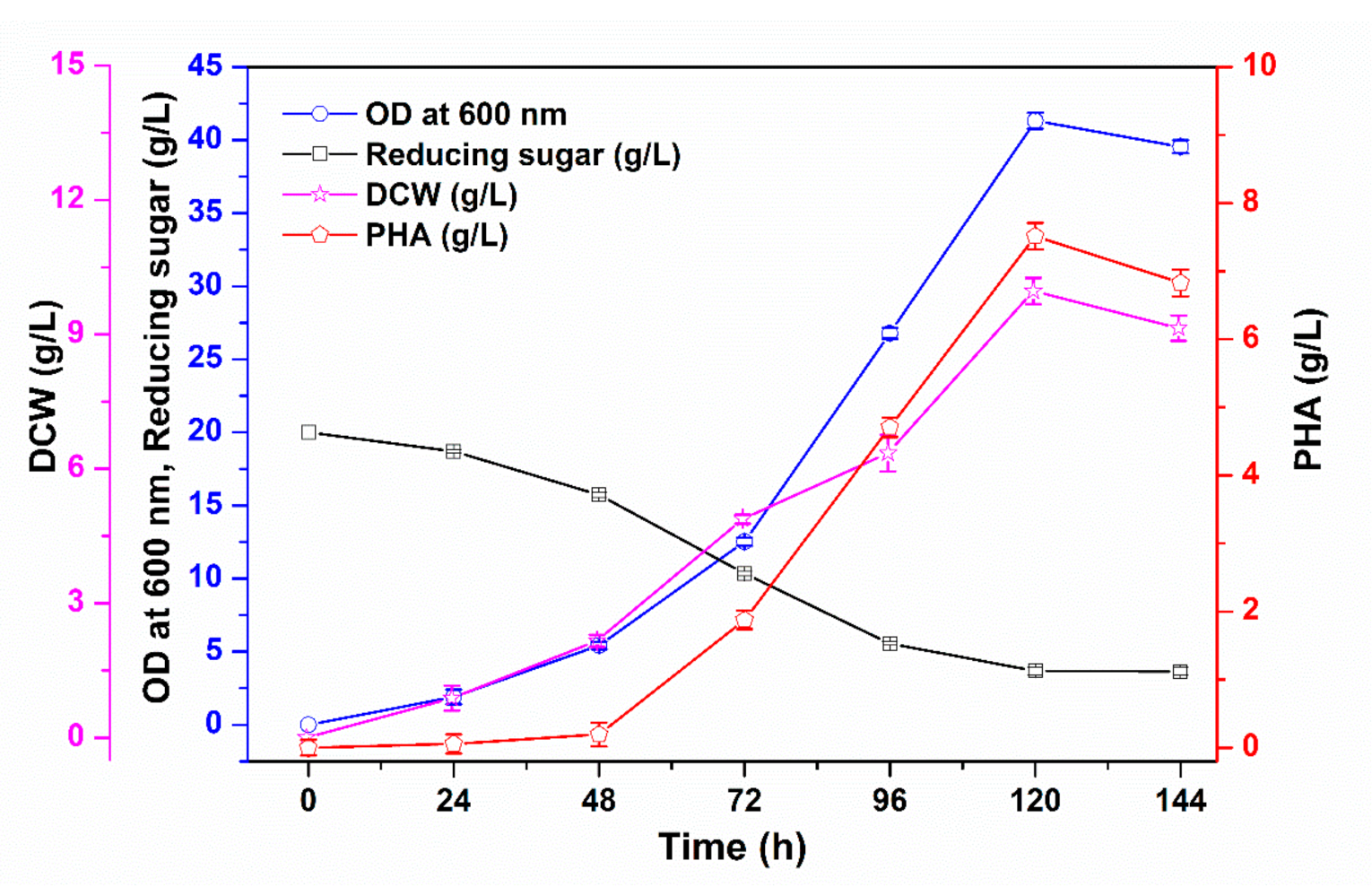
3.1. PHA Production via Batch Cultivation in Shake Flasks
3.2. Characterization of PHA Polymers
3.2.1. FTIR Analysis
3.2.2. 1H NMR
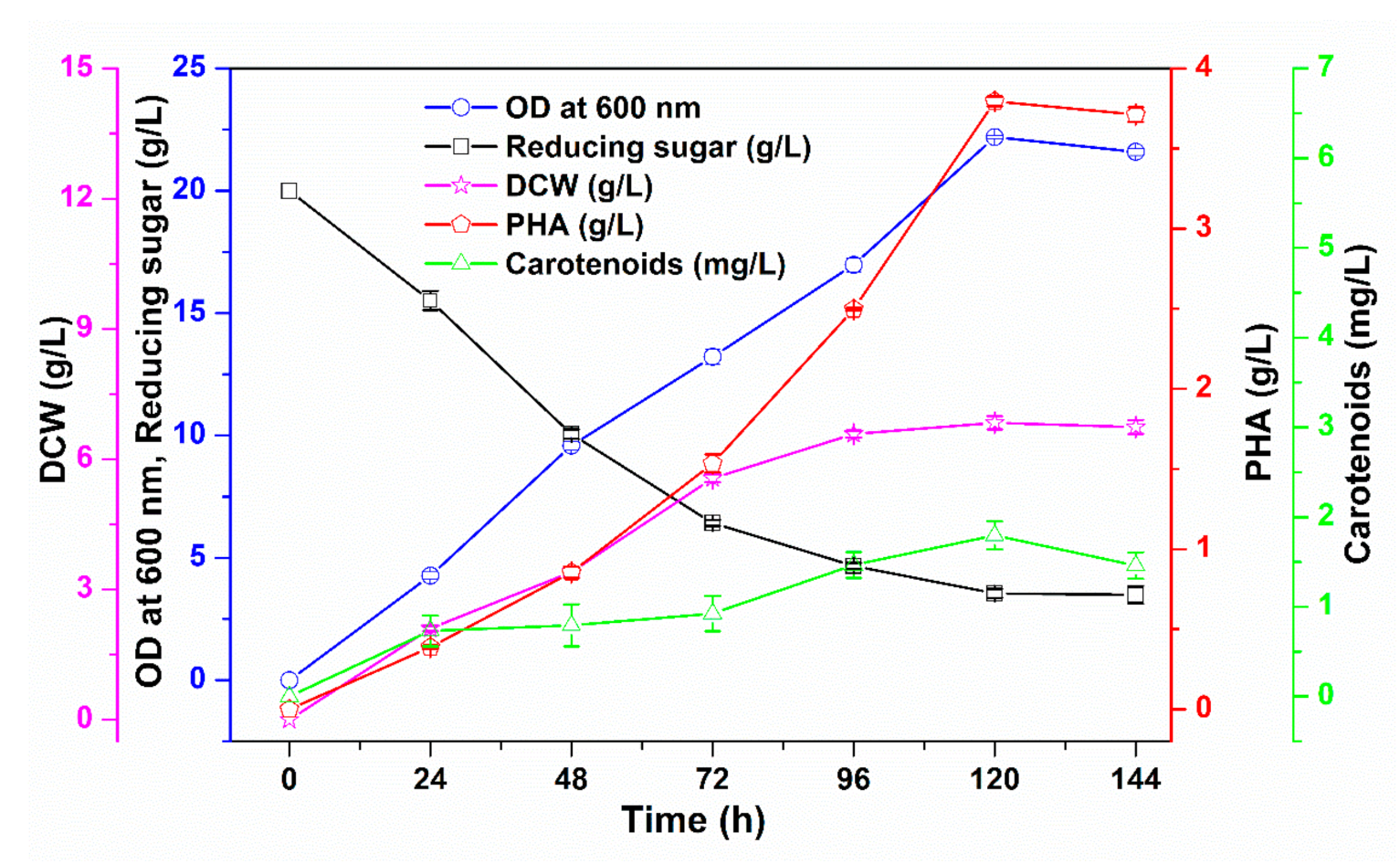
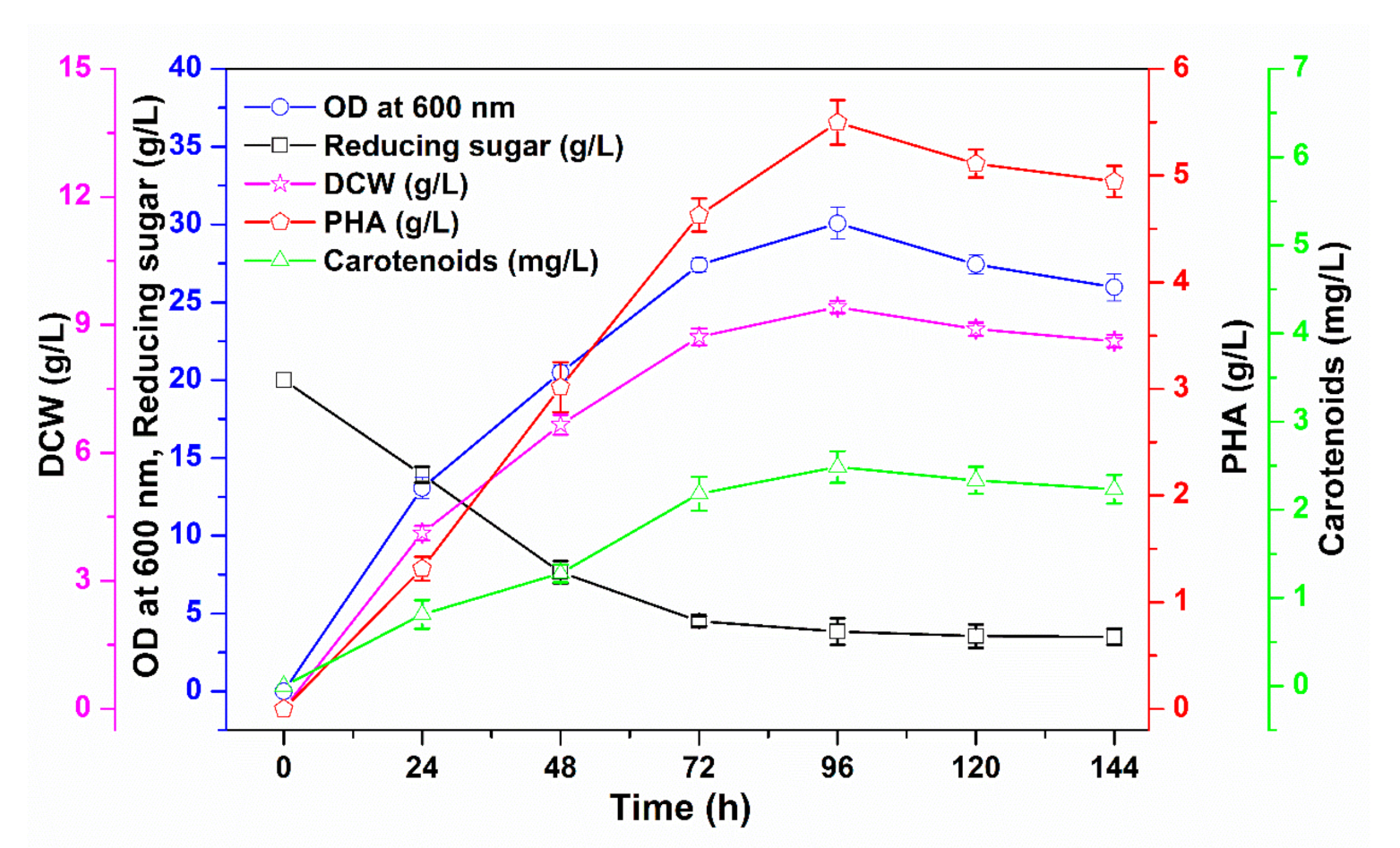
3.3. Upscaling of PHA and Carotenoid Co-Production in a Fermenter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCB | defatted Chlorella biomass |
| HCl | hydrochloric acid |
| PHA | polyhydroxyalkanoate |
| PHB | poly(3-hydroxybutyrate) |
| 3HB | 3-hydroxybutyrate |
| 3HV | 3-hydroxyvalerate |
| P (3HB-co-3HV) | poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| OD | optical density |
| DCW | dry cell weight |
References
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A roadmap towards green packaging: The current status and future outlook for polyesters in the packaging industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Cho, S.K.; Kim, D.S.; Ghodake, G.S.; Kadam, A.; Kumar, G.; Bharagava, R.N.; Banu, R.; Shin, H.S. Pretreatment of kenaf (Hibiscus cannabinus L.) biomass feedstock for polyhydroxybutyrate (PHB) production and characterization. Bioresour. Technol. 2019, 282, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Duan, K.J.; Huang, S.Y.; Chen, C.W. Production of polyhydroxyalkanoates from inexpensive extruded rice bran and starch by Haloferax mediterranei. J. Ind. Microbiol. Biotechnol. 2006, 33, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Song, H.S.; Jeon, J.M.; Choi, K.Y.; Yang, Y.H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kim, B.S. Paracoccus sp. strain LL1 as a single cell factory for the conversion of waste cooking oil to polyhydroxyalkanoates and carotenoids. Appl. Food Biotechnol. 2019, 6, 53–60. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Kim, J.H.; Kim, M.S.; Kim, J.; Hong, J.W.; Hong, Y.G.; Kim, H.J.; Jeon, J.M.; Kim, S.H.; Ahn, J.; et al. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess. Biosyst. Eng. 2018, 41, 229–235. [Google Scholar] [CrossRef]
- Ghosh, S.; Gnaim, R.; Greiserman, S.; Fadeev, L.; Gozin, M.; Golberg, A. Macroalgal biomass subcritical hydrolysates for the production of polyhydroxyalkanoate (PHA) by Haloferax mediterranei. Bioresour. Technol. 2019, 271, 166–173. [Google Scholar] [CrossRef]
- Azizi, N.; Najafpour, G.; Younesi, H. Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (PHB) production using Cupriavidus necator. Int. J. Biol. Macromol. 2017, 101, 1029–1040. [Google Scholar] [CrossRef]
- Alkotaini, B.; Koo, H.; Kim, B.S. Production of polyhydroxyalkanoates by batch and fed-batch cultivations of Bacillus megaterium from acid-treated red algae. Korean J. Chem. Eng. 2016, 33, 1669–1673. [Google Scholar] [CrossRef]
- Khomlaem, C.; Aloui, H.; Deshmukh, A.R.; Yun, J.-H.; Kim, H.-S.; Napathorn, S.C.; Kim, B.S. Defatted Chlorella biomass as a renewable carbon source for polyhydroxyalkanoates and carotenoids co-production. Algal Res. 2020, 51, 102068. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Prommuak, C.; Pavasant, P.; Quitain, A.T.; Goto, M.; Shotipruk, A. Simultaneous Production of Biodiesel and Free Lutein from Chlorella vulgaris. Chem. Eng. Technol. 2013, 36, 733–739. [Google Scholar] [CrossRef]
- Agwa, O.K.; Nwosu, I.G.; Abu, G.O. Bioethanol Production from Chlorella vulgaris Biomass Cultivated with Plantain (Musa paradisiaca) Peels Extract. Adv. Biosci. Biotechnol. 2017, 08, 478–490. [Google Scholar] [CrossRef]
- Al-lwayzy, S.H.; Yusaf, T.; Al-Juboori, R.A. Biofuels from the fresh water microalgae Chlorella vulgaris (FWM-CV) for diesel engines. Energies 2014, 7, 1829–1851. [Google Scholar] [CrossRef]
- Muhammad, M.; Aloui, H.; Khomlaem, C.; Hou, C.T.; Kim, B.S. Production of polyhydroxyalkanoates and carotenoids through cultivation of different bacterial strains using brown algae hydrolysate as a carbon source. Biocatal. Agric. Biotechnol. 2020, 30, 101852. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, H.R.; Hou, C.T. Effect of surfactant on the production of oxygenated unsaturated fatty acids by Bacillus megaterium ALA2. New Biotechnol. 2010, 27, 33–37. [Google Scholar] [CrossRef]
- Melanie, S.; Winterburn, J.B.; Devianto, H. Production of Biopolymer Polyhydroxyalkanoates (PHA) by Extreme Halophilic Marine Archaea Haloferax mediterranei in Medium with Varying Phosphorus Concentration. J. Eng. Technol. Sci. 2018, 50, 255–271. [Google Scholar] [CrossRef]
- Gouda, M.K.; Swellam, A.E.; Omar, S.H. Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol. Res. 2001, 156, 201–207. [Google Scholar] [CrossRef]
- Kim, N.J.; Li, H.; Jung, K.; Chang, H.N.; Lee, P.C. Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour. Technol. 2011, 102, 7466–7469. [Google Scholar] [CrossRef]
- Nygaard, D.; Yashchuk, O.; Hermida, É.B. Evaluation of culture medium on poly(3-hydroxybutyrate) production by Cupriavidus necator ATCC 17697: Application of the response surface methodology. Heliyon 2019, 5, 1–18. [Google Scholar] [CrossRef]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Piotrowska-Seget, Z.; Radecka, I.K. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.Y.; Hussin, M.H.; Baidurah, S. Biosynthesis of poly(3-hydroxybutyrate) (PHB) by Cupriavidus necator from various pretreated molasses as carbon source. Biocatal. Agric. Biotechnol. 2019, 17, 51–59. [Google Scholar] [CrossRef]
- Ferre-Guell, A.; Winterburn, J. Increased production of polyhydroxyalkanoates with controllable composition and consistent material properties by fed-batch fermentation. Biochem. Eng. J. 2019, 141, 35–42. [Google Scholar] [CrossRef]
- Zuo, Z.Q.; Xue, Q.; Zhou, J.; Zhao, D.H.; Han, J.; Xiang, H. Engineering Haloferax mediterranei as an efficient platform for high level production of lycopene. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pais, J.; Serafim, L.S.; Freitas, F.; Reis, M.A.M. Conversion of cheese whey into poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Haloferax mediterranei. New Biotechnol. 2016, 33, 224–230. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Saha, J.; Haldar, S.; Bhowmic, A.; Mukhopadhyay, U.K.; Mukherjee, J. Production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei using rice-based ethanol stillage with simultaneous recovery and re-use of medium salts. Extremophiles 2014, 18, 463–470. [Google Scholar] [CrossRef]
- Ansari, S.; Fatma, T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, Optimization and Characterization. PLoS ONE 2016, 11, e0158168. [Google Scholar] [CrossRef]
- Alsafadi, D.; Al-Mashaqbeh, O. A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. New Biotechnol. 2017, 34, 47–53. [Google Scholar] [CrossRef]
- Raho, S.; Carofiglio, V.E.; Montemurro, M.; Miceli, V.; Centrone, D.; Stufano, P.; Schioppa, M.; Pontonio, E.; Rizzello, C.G. Production of the polyhydroxyalkanoate PHBV from ricotta cheese exhausted whey by Haloferax mediterranei fermentation. Foods 2020, 9, 1459. [Google Scholar] [CrossRef]
- Gahlawat, G.; Soni, S.K. Valorization of waste glycerol for the production of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus necator and extraction in a sustainable manner. Bioresour. Technol. 2017, 243, 492–501. [Google Scholar] [CrossRef]
- Alsafadi, D.; Ibrahim, M.I.; Alamry, K.A.; Hussein, M.A.; Mansour, A. Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci. Total Environ. 2020, 737, 139716. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. Recycling of waste streams of the biotechnological poly(hydroxyalkanoate) production by Haloferax mediterranei on whey. Int. J. Polym. Sci. 2015, 2015. [Google Scholar] [CrossRef]
- Koller, M.; Puppi, D.; Braunegg, F. Comparing Chemical and Enzymatic Hydrolysis of Whey Lactose to Generate Feedstocks for Haloarchaeal Poly(3-hydroxybutyrate-co-3- hydroxyvalerate) Biosynthesis. Int. J. Pharm. Sci. Res. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- López-Cuellar, M.R.; Alba-Flores, J.; Rodríguez, J.N.G.; Pérez-Guevara, F. Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int. J. Biol. Macromol. 2011, 48, 74–80. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khomlaem, C.; Aloui, H.; Kim, B.S. Biosynthesis of Polyhydroxyalkanoates from Defatted Chlorella Biomass as an Inexpensive Substrate. Appl. Sci. 2021, 11, 1094. https://doi.org/10.3390/app11031094
Khomlaem C, Aloui H, Kim BS. Biosynthesis of Polyhydroxyalkanoates from Defatted Chlorella Biomass as an Inexpensive Substrate. Applied Sciences. 2021; 11(3):1094. https://doi.org/10.3390/app11031094
Chicago/Turabian StyleKhomlaem, Chanin, Hajer Aloui, and Beom Soo Kim. 2021. "Biosynthesis of Polyhydroxyalkanoates from Defatted Chlorella Biomass as an Inexpensive Substrate" Applied Sciences 11, no. 3: 1094. https://doi.org/10.3390/app11031094
APA StyleKhomlaem, C., Aloui, H., & Kim, B. S. (2021). Biosynthesis of Polyhydroxyalkanoates from Defatted Chlorella Biomass as an Inexpensive Substrate. Applied Sciences, 11(3), 1094. https://doi.org/10.3390/app11031094






