The Link between Periodontal Disease and Oral Cancer—A Certainty or a Never-Ending Dilemma?
Abstract
1. Introduction
2. Periodontal Disease Etiopathogenesis
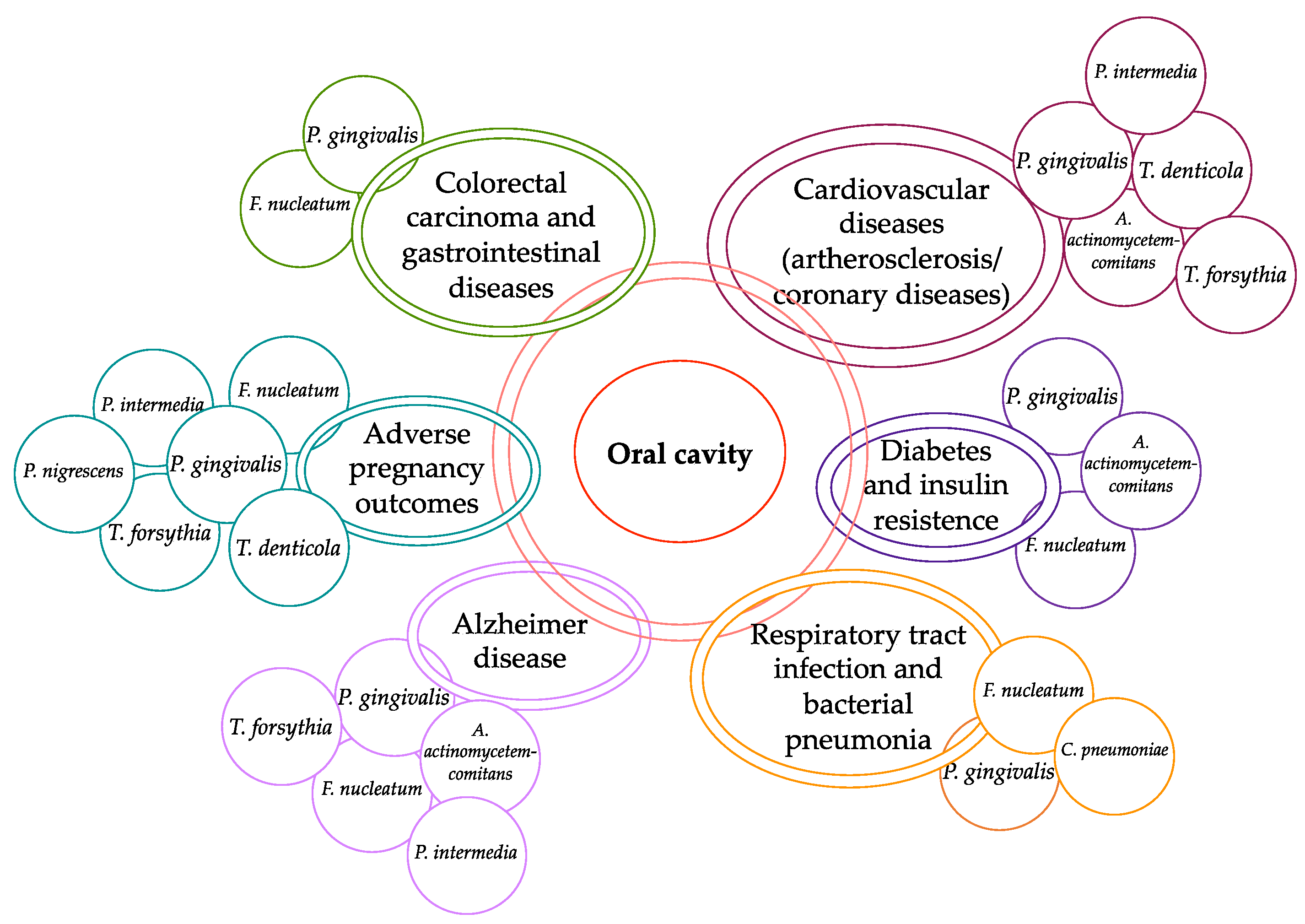
3. Periodontal Disease and Systemic Disorders
4. Periodontal Disease and Oral Cancer
4.1. Oral Cancer
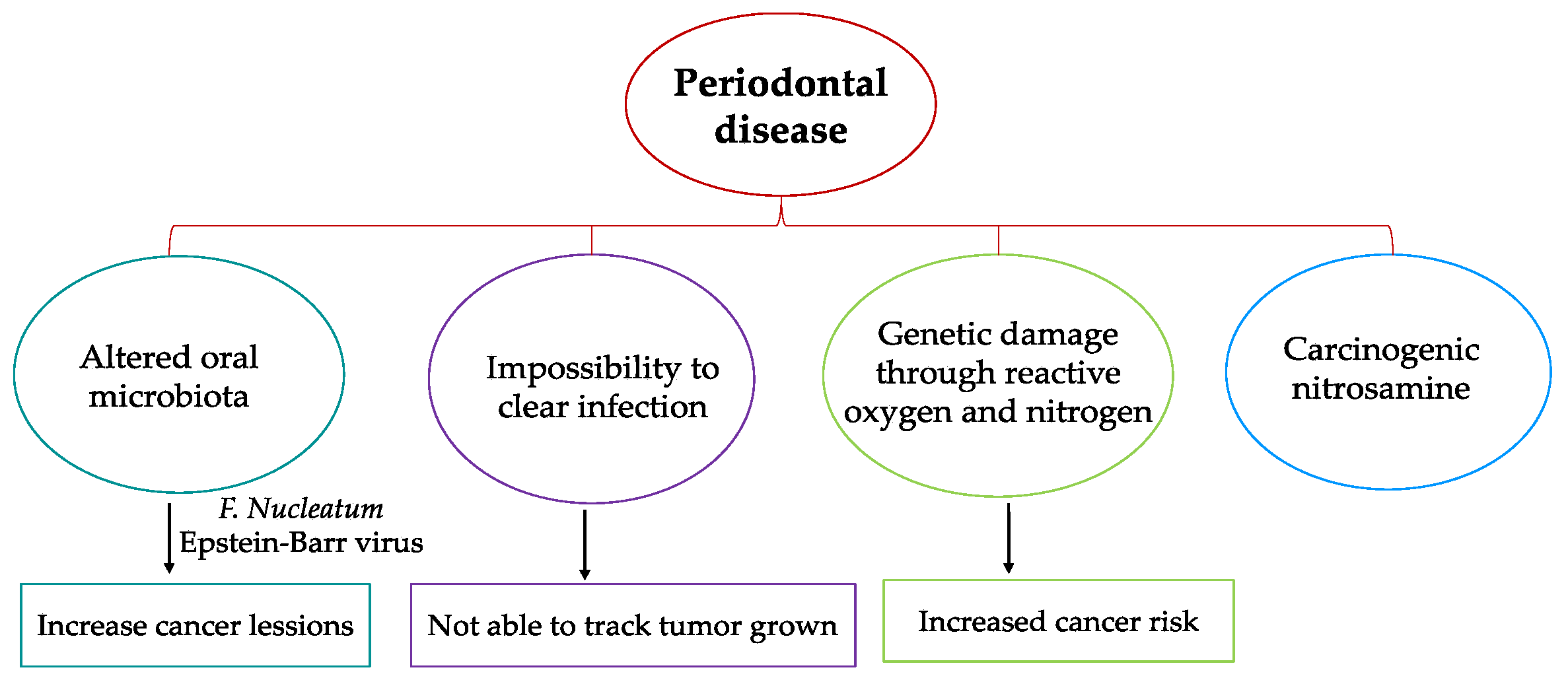
4.2. Hypotheses for Etiology of a Link between Periodontal Disease and Oral Cancer
4.3. The Link between Periodontal Disease and Oral Cancer-Current Vision
4.4. Potential Confounding Factors of the Periodontal Disease and Oral Cancer Link
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usui, M.; Onizuka, S.; Sato, T.; Kokabu, S.; Ariyoshi, W.; Nakashima, K. Mechanism of alveolar bone destruction in periodontitis—Periodontal bacteria and inflammation. Jpn. Dent. Sci. Rev. 2021, 57, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A multifaceted disease of tooth-supporting tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Gao, L.; Kang, M.; Zhang, M.J.; Sailani, M.R.; Kuraji, R.; Martinez, A.; Ye, C.; Kamarajan, P.; Le, C.; Zhan, L.; et al. Polymicrobial periodontal disease triggers a wide radius of effect and unique virome. NPJ Biofilms Microbiomes 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Loesche, W.J.; Grossman, N.S. Periodontal disease as a specific, albeit chronic, infection: Diagnosis and treatment. Clin. Microbiol. Rev. 2001, 14, 727–752. [Google Scholar] [CrossRef]
- Amar, S.; Han, X. The impact of periodontal infection on systemic diseases. Med. Sci. Monit. 2003, 9, 291–299. [Google Scholar]
- Oindrila, P.; Payal, A.; Michael, M.; Shampa, C. Inflammation in periodontal disease: Possible link to vascular disease. Front. Physiol. 2021, 11, 609614. [Google Scholar]
- Salhi, L.; Rompen, E.; Sakalihasan, N.; Teughels, W.; Michel, J.B.; Lambert, F. Can Periodontitis Influence the Progression of Abdominal Aortic Aneurysm? A Systematic Review. Angiology 2019, 70, 479–491. [Google Scholar] [CrossRef]
- Sinisalo, J.; Paronen, J.; Mattila, K.J.; Syrjala, M.; Alfthan, G.; Palosuo, T.; Nieminen, M.S.; Vaarala, O. Relation of inflammation to vascular function in patients with coronary heart disease. Atherosclerosis 2000, 149, 403–411. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Wand, H.C.; Merchant, A.T.; Rimm, E.B. Periodontal disease and biomarkers related to cardiovascular disease. J. Dent. Res. 2004, 83, 151–155. [Google Scholar] [CrossRef]
- Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible association of periodontal disease and macular degeneration: A case-control study. Dent. J. 2020, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Toti, P.; Pilone, V.; Carinci, F.; Lauritano, D.; Sbordone, L. The association between periodontitis and human colorectal cancer: Genetic and pathogenic linkage. Life 2020, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Liu, Y.; Meyer, M.; Giovannucci, E.; Joshipura, K. Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. Lancet Oncol. 2008, 9, 550–558. [Google Scholar] [CrossRef]
- Abnet, C.C.; Kamangar, F.; Dawsey, S.M.; Stolzenberg-Solomon, R.Z.; Albanes, D.; Pietinen, P.; Virtamo, J.; Taylor, P.R. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand. J. Gastroenterol. 2005, 40, 681–687. [Google Scholar] [CrossRef]
- Tezal, M.; Sullivan, M.A.; Reid, M.E.; Marshall, J.R.; Hyland, A.; Loree, T.; Lillis, C.; Hauck, L.; Wactawski-Wende, J.; Scannapieco, F.A. Chronic periodontitis and the risk for tongue cancer. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 450–454. [Google Scholar] [CrossRef]
- Brkic, Z.; Pavlić, V. Periodontology: The historical outline from ancient times until the 20th Century. Vojnosanit. Pregl. 2017, 74, 193–199. [Google Scholar] [CrossRef]
- Billings, M.; Holtfreter, B.; Papapanou, P.N.; Mitnik, G.L.; Kocher, T.; Dye, B.A. Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J. Periodontol. 2018, 89, 140–158. [Google Scholar] [CrossRef]
- Raitapuro-Murray, T.; Molleson, T.I.; Hughes, F.J. The prevalence of periodontal disease in a Romano-British population c. 200–400 AD. Br. Dent. J. 2014, 217, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Richards, D. Oral diseases affect some 3. 9 billion people. Evid. Based Dent. 2013, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- CDC researchers find close to half of American adults have periodontitis. J. Can. Dent. Assoc. 2012, 78, c136.
- Gjermo, P.; Rosing, C.K.; Susin, C.; Oppermann, R. Periodontal diseases in Central and South America. Periodontol. 2000 2002, 29, 70–78. [Google Scholar]
- Van Winkelhoff, A.J.; Winkel, E.G. Antibiotics in periodontics: Right or wrong? J. Periodontol. 2009, 80, 1555–1558. [Google Scholar] [CrossRef]
- Negrato, C.A.; Tarzia, O.; Jovanovič, L.; Chinellato, L.E.M. Periodontal disease and diabetes mellitus. J. Appl. Oral Sci. 2013, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M. Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health 2015, 15, S5. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, 159–172. [Google Scholar] [CrossRef]
- Saglie, F.R.; Caranza, F.A.; Newman, M.G.; Cheng, L.; Lewin, K.J. Identification of tissue invading bacteria in human periodontal diseases. J. Periodontal Res. 1982, 17, 452–455. [Google Scholar] [CrossRef]
- Peković, D.D.; Fillery, E.D. Identification of bacteria in immunopathological mechanisms of human periodontal diseases. J. Periodontal Res. 1984, 19, 329–351. [Google Scholar] [CrossRef]
- Page, R.C.; Schroeder, H.E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab. Investig. 1976, 34, 235–249. [Google Scholar] [PubMed]
- Page, R.C.; Kornman, K.S. The pathogenesis of human periodontitis: An introduction. Periodontol. 2000 1997, 14, 9–11. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol. 2000 2005, 38, 13–87. [Google Scholar] [CrossRef] [PubMed]
- Listgarten, M.A. Pathogenesis of periodontitis. J. Clin. Periodontol. 1986, 13, 418–430. [Google Scholar] [CrossRef]
- Offenbacher, S.; Heasman, P.A.; Collins, J.G. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J. Periodontol. 1993, 64, 432–444. [Google Scholar]
- Grau, A.J.; Becher, H.; Ziegler, C.M.; Lichy, C.; Buggle, F.; Kaiser, C.; Lutz, R.; Bültmann, S.; Preusch, M.; Dörfer, C.E. Periodontal disease as a risk factor for ischemic stroke. Stroke. Cereb. Circ. 2004, 35, 496–501. [Google Scholar] [CrossRef]
- Nakamura, I.; Takahashi, N.; Jimi, E.; Udagawa, N.; Suda, T. Regulation of osteoclast function. Mod. Rheumatol. 2012, 22, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, V.; Schoenmaker, T.; de Vries, T.J.; Everts, V. Direct cell-cell contact between periodontal ligament fibroblasts and osteoclast precursors synergistically increases the expression of genes related to osteoclastogenesis. J. Cell. Physiol. 2010, 222, 565–573. [Google Scholar] [CrossRef]
- Bloemen, V.; Schoenmaker, T.; de Vries, T.J.; Everts, V. IL-111β favors osteoclastogenesis via supporting human periodontal ligament fibroblasts. J. Cell. Biochem. 2011, 112, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, I.E.; Pflanz, S. The emerging role of Interleukin 27 in inflammatory arthritis and bone destruction. Cytokine Growth Factor Rev. 2013, 24, 115–121. [Google Scholar] [CrossRef]
- Fujihara, R.; Usui, M.; Yamamoto, G.; Nishii, K.; Tsukamoto, Y.; Okamatsu, Y.; Sato, T.; Asou, Y.; Nakashima, K.; Yamamoto, M. Tumor necrosis factor- enhances RANKL expression in gingival epithelial cells via protein kinase A signaling. J. Periodontal Res. 2014, 49, 508–517. [Google Scholar] [CrossRef]
- Okui, T.; Aoki, Y.; Ito, H.; Honda, T.; Yamazaki, K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J. Dent. Res. 2012, 91, 574–579. [Google Scholar] [CrossRef]
- Sun, Y.; Shu, R.; Li, C.L.; Zhang, M.Z. Gram-negative periodontal bacteria induce the activation of Toll-like receptors 2 and 4, and cytokine production in human periodontal ligament cells. J. Periodontol. 2010, 81, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Parlak, H.M.; Karaarslan, D.; Ide, S.; Çelik, H.H.; Karabulut, E.; Ertan, A.A.; Alev, F. Analysis of the nano and microstructures of the cervical cementum and saliva in periodontitis: A pilot study. J. Oral Biosci. 2021, 63, 370–377. [Google Scholar] [CrossRef]
- Saranya, K.K.N.; Maradi, A.; Chithresan, K.; Janakiram, S.H.; Krishna, P. Molecular signatures in infrared spectra of saliva in healthy, chronic and aggressive periodontitis. Vib. Spectrosc. 2020, 111, 103179. [Google Scholar] [CrossRef]
- Kawamoto, D.; Pontes Lucas Amado, P.; Albuquerque-Souza, E.; Bueno, M.R.; Campos Vale, G.; Saraiva, L.; Pinto Alves Mayer, M. Chemokines and cytokines profile in whole saliva of patients with periodontitis. Cytokine 2020, 135, 155197. [Google Scholar] [CrossRef] [PubMed]
- Altin, K.T.; Topcuoglu, N.; Duman, G.; Umsal, M.; Celik, A.; Kuvvetli, S.S.; Kasikci, E.; Sahin, F.; Kulekci, G. Antibacterial effects of saliva substitutes containing lysozyme or lactoferrin against Streptococcus mutans. Arch. Oral Biol. 2021, 129, 105183. [Google Scholar] [CrossRef]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Interleukin-1β induces human cementoblasts to support osteoclastogenesis. Int. J. Oral Sci. 2017, 9, e5. [Google Scholar] [CrossRef] [PubMed]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontol. 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Nakanishi-Matsui, M.; Yano, S.; Matsumoto, N.; Futai, M. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem. Biophys. Res. Commun. 2012, 425, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Desta, T.; Raptis, M.; Darveau, R.P.; Graves, D.T. P. gingivalis and E. coli lipopolysaccharides exhibit different systemic but similar local induction of inflammatory markers. J. Periodontol. 2008, 79, 1241–1247. [Google Scholar]
- Preshaw, P.M.; Hefti, A.F.; Jepsen, S. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. A review. J. Clin. Periodontol. 2004, 31, 697–707. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Pietruska, M.; Pietruski, J.; Skurska, A.; Dolinska, E.; Heumann, C.; Auschill, T.M.; Sculen, A. Six-month results following treatment of aggressive periodontitis with antimicrobial photodynamic therapy or amoxicillin and metronidazole. Clin. Oral Investig. 2014, 18, 2129–2135. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, T. Triggering role of focal infection in the induction of extra-palmoplantar lesions and pustulotic arthro-osteitis associated with palmoplantar pustulosis. In Recent Advances in Tonsils and Mucosal Barriers of the Upper Airways; Karger: Basel, Switzerland, 2011; Volume 72, pp. 89–92. [Google Scholar]
- Mattila, K. Does periodontitis cause heart disease? Eur. Heart J. 2003, 24, 2079–2080. [Google Scholar] [CrossRef]
- Jin, L.S.; Chiu, G.K.C.; Corbet, E.F. Are periodontal diseases risk factors for certain systemic disorders-what matters to medical practitioners? Hong Kong Med. J. 2003, 9, 31–37. [Google Scholar] [PubMed]
- Konkel, J.E.; O’Boyle, C.; Krishnan, S. Distal consequences of oral inflammation are still discussed. Front. Immunol. 2019, 10, 1403. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Fodor, A. Utilizing ‘omics’ tools to study the complex gut ecosystem. Adv. Exp. Med. Biol. 2014, 817, 25–38. [Google Scholar]
- Saggioro, A. Leaky gut, microbiota, and cancer: An incoming hypothesis. J. Clin. Gastroenterol. 2014, 48, 62–66. [Google Scholar] [CrossRef] [PubMed]
- McAllister, F.; Housseau, F.; Sears, C.L. Microbiota and immune responses in colon cancer: More to learn. Cancer J. 2014, 20, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Monsarrat, P.; Blaizot, A.; Kémoun, P.; Ravaud, P.; Nabet, C.; Sixou, M.; Vergnes, J.-N. Clinical research activity in periodontal medicine: A systematic mapping of trial registers. J. Clin. Periodontol. 2016, 43, 390–400. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Barnett, A.H.; Claffey, N.; Davis, M.; Gadsby, R.; Kellett, M.; Lip, G.Y.; Thackray, S. The potential impact of periodontal disease on general health: A consensus view. Curr. Med. Res. Opin. 2008, 24, 1635–1643. [Google Scholar] [CrossRef]
- Grossi, S.G.; Genco, R.J. Periodontal disease and diabetes mellitus: A two-way relationship. Ann. Periodontol. 1998, 3, 52–61. [Google Scholar] [CrossRef]
- Saremi, A.; Nelson, R.G.; Tulloch-Reid, M.; Hanson, R.L.; Sievers, M.L.; Taylor, G.W.; Shlossman, M.; Bennett, P.H.; Genco, R.; Knowler, W.C. Periodontal disease and mortality in type 2 diabetes. Diabetes Care 2005, 28, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, A.; Leake, J. Systematic review of the association between respiratory diseases and oral health. J. Periodontol. 2006, 77, 1465–1482. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Calanan, R.M.; Genco, R.J.; Wilding, G.E.; Hovey, K.M.; Trevisan, M.; Wactawski-Wende, J. Osteoporosis and oral infection: Independent risk factors for oral bone loss. J. Dent. Res. 2008, 87, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Ruiz, V.E.; Carroll, J.D.; Moss, S.F. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011, 305, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Dizdar, O.; Hayran, M.; Guven, D.C.; Yılmaz, T.B.; Taheri, S.; Akman, A.C.; Bilgin, E.; Hüseyin, B.; Berker, E. Increased cancer risk in patients with periodontitis. Curr. Med. Res. Opin. 2017, 33, 2195–2200. [Google Scholar] [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal disease, tooth loss, and cancer risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Chung, M.; York, B.R.; Michaud, D.S. Oral health and cancer. Curr. Oral Health Rep. 2019, 6, 130–137. [Google Scholar] [CrossRef]
- Güven, D.C.; Dizdar, Ö.; Akman, A.C.; Berker, E.; Yekedüz, E.; Ceylan, F.; Başpınar, B.; Akbıyık, İ.; Aktaş, B.Y.; Yüce, D.; et al. Evaluation of cancer risk in patients with periodontal diseases. Turk. J. Med. Sci. 2019, 49, 826–831. [Google Scholar] [CrossRef]
- Printz, C. Study adds evidence to link between gum disease and cancer risk: Researchers observe connection with gastric, esophageal cancer. Cancer 2021, 127, 495–496. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; Weiss, N.S. An exploration of the periodontitis–cancer association. Ann. Epidemiol. 2003, 13, 312–316. [Google Scholar] [CrossRef]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Kademani, D. Oral cancer. Mayo Clin. Proc. 2007, 82, 878–887. [Google Scholar] [CrossRef]
- Szturz, P.; Vermorken, J.B. Treatment of elderly patients with squamous cell carcinoma of the head and neck. Front. Oncol. 2016, 6, 199. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Pitiyage, G.; Kumarasiri, P.V.; Liyanage, R.L.; Dias, K.D.; Tilakaratne, W.M. Clinical and histopathologic parameters in survival of oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Koontongkaew, S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 2013, 4, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M. Rudolf Virchow. Emerg. Infect. Dis. 2008, 14, 1480–1481. [Google Scholar] [CrossRef]
- Feller, L.L.; Khammissa, R.R.; Kramer, B.B.; Lemmer, J.J. Oral squamous cell carcinoma inrelation to field precancerisation: Pathobiology. Cancer Cell Int. 2013, 13, 31. [Google Scholar] [CrossRef]
- Cavallo, F.; De Giovanni, C.; Nanni, P.; Forni, G.; Lollini, P.L. 2011: The immune hallmarks of cancer. Cancer Immunol. Immunother. 2011, 60, 319–326. [Google Scholar] [CrossRef]
- Bagan, J.; Sarrion, G.; Jimenez, Y. Oral cancer: Clinical features. Oral Oncol. 2010, 46, 414–417. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar]
- Yang, C.Y.; Yeh, Y.M.; Yu, H.Y.; Chin, C.Y.; Hsu, C.W.; Liu, H.; Huang, P.J.; Hu, S.N.; Liao, C.T.; Chang, K.P.; et al. Oral microbiota community dynamics associated with oral squamous cell carcinoma staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Kantarci, A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol. 2000. 2015, 69, 255–273. [Google Scholar] [CrossRef]
- Meyer, M.S.; Joshipura, K.; Giovannucci, E.; Michaud, D.S. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control 2008, 19, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Tillonen, J.; Homann, N.; Rautio, M.; Jousimies-Somer, H.; Salaspuro, M. Role of yeasts in the salivary acetaldehyde production from ethanol among risk groups for ethanol-associated oral cavity cancer. Alcohol. Clin. Exp. Res. 1999, 23, 1409–1415. [Google Scholar] [CrossRef]
- Loos, B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005, 76, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.; Ohshima, H.; Nair, U.J.; Bartsch, H. Endogenous formation of nitrosamines and oxidative DNA-damaging agents in tobacco users. Crit. Rev. Toxicol. 1996, 26, 149–161. [Google Scholar] [CrossRef]
- Franco, E.L.; Kowalski, L.P.; Oliveira, B.V.; Curado, M.P.; Pereira, R.N.; Silva, M.E.; Fava, A.S.; Torloni, H. Risk factors for oral cancer in Brazil: A case-control study. Int. J. Cancer 1989, 43, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Boyle, P.; Hu, H.; Duan, J.; Jiang, P.; Ma, D.; Shui, L.; Niu, S.; Scully, C.; MacMahon, B. Dentition, oral hygiene, and risk of oral cancer: A case-control study in Beijing, People’s Republic of China. Cancer Causes Control 1990, 1, 235–241. [Google Scholar] [CrossRef]
- Marshall, J.R.; Graham, S.; Haughey, B.P.; Shedd, D.; O’Shea, R.; Brasure, J.; Wilkinson, G.S.; West, D. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Oral Oncol. Eur. J. Cancer 1992, 28, 9–15. [Google Scholar] [CrossRef]
- Wynder, E.L.; Bross, I.J.; Feldman, R.M. A study of the etiological factors in cancer of the mouth. Cancer 1957, 10, 1300–1323. [Google Scholar] [CrossRef]
- Graham, S.; Dayal, H.; Rohrer, T.; Swanson, M.; Sultz, H.; Shedd, D.; Fischman, S. Dentition, diet, tobacco, and alcohol in the epidemiology of oral cancer. J. Natl. Cancer Inst. 1977, 59, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, T.; Wildt, J.; Frydenberg, M.; Elbrond, O.; Nielsen, J.E. Case-control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control 1995, 6, 57–67. [Google Scholar] [CrossRef]
- de Rezende, C.P.; Ramos, M.B.; Daguila, C.H.; Dedivitis, R.A.; Rapoport, A. Oral health changes in patients with oral and oropharyngeal cancer. Braz. J. Otorhinolaryngol. 2008, 74, 596–600. [Google Scholar] [CrossRef]
- Guha, N.; Boffetta, P.; Filho, V.W.; Neto, J.E.; Shangina, O.; Zaridze, D.; Curado, M.P.; Koifman, S.; Matos, E.; Menezes, A.; et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: Results of two multicentric case-control studies. Am. J. Epidemiol. 2007, 166, 1159–1173. [Google Scholar] [CrossRef]
- Hiraki, A.; Matsuo, K.; Suzuki, T.; Kawase, T.; Tajima, K. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1222–1227. [Google Scholar] [CrossRef]
- Mathur, R.; Singhavi, H.R.; Malik, A.; Nair, S.; Chaturvedi, P. Role of Poor Oral Hygiene in Causation of Oral Cancer—A Review of Literature. Indian J. Surg. Oncol. 2019, 10, 184–195. [Google Scholar] [CrossRef]
- Inaba, H.; Sugita, H.; Kuboniwa, M.; Iwai, S.; Hamada, M.; Noda, T.; Morisaki, I.; Lamont, R.J.; Amano, A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell. Microbiol. 2014, 16, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Galvão-Moreira, L.V.; da Cruz, M.C.F.N. Oral microbiome, periodontitis and risk of head and neck cancer. Oral Oncol. 2016, 53, 17–19. [Google Scholar] [CrossRef]
- Groeger, S.; Domann, E.; Gonzales, J.R.; Chakraborty, T.; Meyle, J. B7-H1 and B7-DC receptors of oral squamous carcinoma cells are upregulated by Porphyromonas gingivalis. Immunobiology 2011, 216, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Sepehr, A.; Kamangar, F.; Fahimi, S.; Saidi, F.; Abnet, C.C.; Dawsey, S.M. Poor oral health as a risk factor for esophageal squamous dysplasia in northeastern Iran. Anticancer Res. 2005, 25, 543–546. [Google Scholar]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Al-Hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum-symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.; et al. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major periodontopathic pathogen overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef]
- Peters, B.A.; Wu, J.; Pei, Z.; Yang, L.; Purdue, M.P.; Freedman, N.D.; Jacobs, E.J.; Gapstur, S.M.; Hayes, R.B.; Ahn, J. Oral microbiome composition reflects prospective risk for esophageal cancers. Cancer Res. 2017, 77, 6777–6787. [Google Scholar] [CrossRef]
- Gao, S.G.; Yang, J.Q.; Ma, Z.K.; Yuan, X.; Zhao, C.; Wang, G.C.; Wei, H.; Feng, X.S.; Qi, Y.J. Preoperative serum immunoglobulin G and A antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer 2018, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Alshaikh, A.; Kim, S.; Chun, C.; Mehrazarin, S.; Lee, J.; Lux, R.; Kim, R.H.; Shin, K.H.; Park, N.H.; et al. Porphyromonas gingivalis impairs oral epithelial barrier through targeting GRHL2. J. Dent. Res. 2019, 98, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Wang, Q.; Li, C.; Liu, J.; Zhang, D.; Zhang, S.; Pan, Y. Identification of potential candidate genes of oral cancer in response to chronic infection with Porphyromonas gingivalis using bioinformatical analyses. Front. Oncol. 2019, 9, 91. [Google Scholar] [CrossRef]
- Dye, B.A.; Choudhary, K.; Shea, S.; Papapanou, P.N. Serum antibodies to periodontal pathogens and markers of systemic inflammation. J. Clin. Periodontol. 2005, 32, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Gani, D.K.; Lakshmi, D.; Krishnan, R.; Emmadi, P. Evaluation of C-reactive protein and interleukin-6 in the peripheral blood of patients with chronic periodontitis. J. Indian Soc. Periodontol. 2009, 13, 69–74. [Google Scholar] [CrossRef]
- Nagy, K.N.; Sonkodi, I.; Szoke, I.; Nagy, E.; Newman, H.N. The microflora associated with human oral carcinomas. Oral Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Bergström, J. Tobacco smoking and chronic destructive periodontal disease. Odontology 2004, 92, 1–8. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Drangsholt, M.; Spiekerman, C.; DeRouen, T.A. Periodontitis-systemic disease associations in the presence of smoking-causal or coincidental? Periodontol. 2000 2002, 30, 51–60. [Google Scholar] [CrossRef]
- Hung, H.C.; Colditz, G.; Joshipura, K.J. The association between tooth loss and the self-reported intake of selected CVD-related nutrients and foods among US women. Community Dent. Oral Epidemiol. 2005, 33, 167–173. [Google Scholar] [CrossRef]
| P. gingivalis promoting oral cancer | Mechanisms |
| Alveolar bone loss is accelerated via IL-17A | |
| Activation of immunologic and inflammatory reactions via IL-1, IL-6, IL-8, TNF-α | |
| Creating favorable microenvironment via PDCD1LG2 | |
| Degaradation of tumor microenvironment via MMP9 | |
| Opsonization of bacteria for complement-binding and activation of complement (CRP) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surlari, Z.; Virvescu, D.I.; Baciu, E.-R.; Vasluianu, R.-I.; Budală, D.G. The Link between Periodontal Disease and Oral Cancer—A Certainty or a Never-Ending Dilemma? Appl. Sci. 2021, 11, 12100. https://doi.org/10.3390/app112412100
Surlari Z, Virvescu DI, Baciu E-R, Vasluianu R-I, Budală DG. The Link between Periodontal Disease and Oral Cancer—A Certainty or a Never-Ending Dilemma? Applied Sciences. 2021; 11(24):12100. https://doi.org/10.3390/app112412100
Chicago/Turabian StyleSurlari, Zinovia, Dragoș Ioan Virvescu, Elena-Raluca Baciu, Roxana-Ionela Vasluianu, and Dana Gabriela Budală. 2021. "The Link between Periodontal Disease and Oral Cancer—A Certainty or a Never-Ending Dilemma?" Applied Sciences 11, no. 24: 12100. https://doi.org/10.3390/app112412100
APA StyleSurlari, Z., Virvescu, D. I., Baciu, E.-R., Vasluianu, R.-I., & Budală, D. G. (2021). The Link between Periodontal Disease and Oral Cancer—A Certainty or a Never-Ending Dilemma? Applied Sciences, 11(24), 12100. https://doi.org/10.3390/app112412100








