Abstract
(1) Background: Stimulating oropharyngeal transient receptor potential (TRP) channels inhibits muscle cramping by triggering a supraspinal reflex to reduce α-motor neuron hyperexcitability. This study investigated whether the longer stimulation of the TRP channels via mouth rinsing with PJ is more effective than drinking PJ at inhibiting an electrically induced muscle cramp (EIMC). Both conditions were compared to the control (water). (2) Methods: The tibial nerves in 11 cramp-prone adults were percutaneously stimulated to elicit an EIMC of the flexor hallucis brevis in three trials that took place one week apart from each other. At cramp onset, the participants received mouth rinsing and expelling PJ (25 mL), ingesting PJ (1 mL∙kg−1 body-mass (BM)), or ingesting water (1 mL∙kg−1 BM). Cramp onset and offset were induced by electromyography, and the severity of discomfort was recorded using a visual analogue scale (VAS). (3) Results: The median time to cramp cessation as a percentage of water was 82.8 ± 14.63% and 68.6 ± 47.78% for PJ ingestion and PJ mouth rinsing, respectively. These results had large variability, and no statistically significant differences were observed. There were also no differences in perceived cramp discomfort between conditions, despite the hazard ratios for the time taken to reach VAS = 0, which was higher than water (control) for PJ ingestion (22%) and mouth rinsing (35%) (p = 0.66 and 0.51, respectively). (4) Conclusions: The data suggest no difference in cramp duration and perceived discomfort between PJ and water.
1. Introduction
Exercise-associated muscle cramps (EAMC) are involuntary, spasmodic, often painful contractions of the skeletal muscles that can last several minutes [1,2]. They generally occur in multi-joint muscle groups, such as the hamstrings or gastrocnemius during or following exercise, when the muscles are active and shortened [1,3] (e.g., hip extension with knee flexion may cause a hamstring cramp). These cramps are common amongst recreational and competitive athletes of various sports and ability levels [3,4,5] They are associated with elevated concentrations of muscle damage biomarkers [6] and local muscle fatigue, thereby impairing function [4].
There has been conjecture regarding the cause of EAMC, with the “electrolyte-depletion and dehydration theory” and the “altered neuromuscular control theory” being predominant [2,3,7,8,9,10,11]. The former theory is based on anecdotal reports dating back over a century [10,12], which postulate that EAMC are due to fluid and electrolyte depletion caused by exercise induced sweating [3]. It is believed that sweat loss leads to a reduction in plasma volume during exercise [8], which causes a contracture of the interstitial space [13,14]. Recent research suggests that the pathophysiological mechanism that triggers EAMC is multifactorial; however, the primary cause is believed to be the result of altered neuromuscular control [9,14,15]. The “altered neuromuscular control theory” postulates that neuromuscular fatigue and muscle overload cause an imbalance in inhibitory and excitatory impulses to the muscle, causing EAMC [1,9,10]. Given that EAMC generally occur when the muscles are shorted, reduced tension in the tendon may decrease inhibitory feedback from the Golgi tendon organ afferents [1,9] which, when coupled with the excitatory impulses from the muscle spindles, cause an imbalance between the inhibitory and excitatory drives to the “α”-motor neurons [1,9,10,16]. This leads to greater excitability at the spinal level and enhanced “α”-motor neuron discharge to the muscle fibres [1,16]. Initially, this presents as a muscle fasciculation when measuring electromyographic (EMG) activity; however, a localised muscle cramp may develop if fatiguing exercise persists [7].
Many potential remedies (e.g., stretching, massage therapy, kinesio-taping) have been developed to treat EAMC with varying levels of efficacy [14]. Ingesting pickle juice (PJ), a solution that contains acetic acid and high concentrations of salt, has become popular [10,14]. Miller et al. [17] reported that ingesting 1 mL∙kg−1 body-mass (BM) of PJ during an electrically induced muscle cramp (EIMC) reduced cramp time by up to 45%. Given that EIMCs are strongly correlated with EAMC [16], the authors concluded that the inhibitory effects of PJ on EAMC may be caused by acetic acid [17]. The sour taste of acetic acid is thought to stimulate the oropharyngeal receptors to trigger a supraspinal reflex that increases inhibitory neurotransmitter activity, potentially activating interneurons to postsynaptically inhibit the “α”-motor neuron pool of the cramping muscle [17]. This notion would support the “altered neuromuscular control theory”; however, evidence for this is lacking.
The oropharynx is host to a vast array of receptors, including taste receptors [18] through which the peripheral gustatory system can gather multisensory information and communicates this via neural pathways to the brainstem [18]. Transient receptor potential (TRP) channels are the nociceptor ion channels within the oropharynx [19]. Although little is known about these receptors [20,21], they have been found to be stimulated by weak acids such as acetic acid [22]. The activation of the transient receptor potential channels (TRP) is involved in sensory transduction through chemical stimulation [23]. There is a very large number of TRP’s in this superfamily that are able to produce diverse physiological responses to temperature and chemical stimuli [24]. Transient receptor potential channel vanilloid 1 (TRPV1) for example opens when the temperature is greater than 40 °C and in the presence of capsaicin [24]. Strong excitatory sensory stimuli can result in a generalized reduction in efferent neural output [24]. Based on this neural mechanism, studies have assessed the use of mouse rinsing in order to preferentially stimulate the taste receptors to elicit a physiological response [25,26,27,28]. For example, mouth rinsing with a carbohydrate solution triggers a neural response that results in an ergogenic effect on endurance exercise performance [25,27]. The mouth rinse modality has gained popularity as a means of intervention delivery [26] and may be an appropriate modality for PJ delivery during muscle cramping to induce the inhibition of EAMC via stimulation from acetic acid [17]. Therefore, this study investigated whether mouth rinsing with PJ (1) reduced the duration of an EIMC compared to water, (2) reduced the discomfort associated with an EIMC more than water, and (3) was superior to ingesting PJ at inhibiting an EIMC in active cramp-prone adults.
2. Materials and Methods
2.1. Participants
Nineteen currently active adults aged 18–35 years volunteered to participate in this study. All of the participants were completing three sessions of exercise per week throughout the study. Only volunteers who had experienced EAMC within the past six months were considered for the study. Volunteers were excluded from participating in the study if they reported: (1) any lower limb injury or surgery within the past six months; (2) any past adverse reactions to electrical stimulation; (3) an allergy to pickles; and (4) having been diagnosed with any cardiovascular, neurological, or blood-borne pathologies, as electrical stimulation may trigger adverse physiological processes in these conditions [29]. Participants (n = 8) were further excluded if they failed to experience an EIMC in their flexor hallucis brevis (FHB) when stimulated at 24 Hz during the familiarisation session.
Therefore, the final number of participants was 11 (females n = 3; mean ± SD, age = 27.1 ± 5.0 years, height = 176.8 ± 9.2 cm, BM = 76.3 ± 14.4 kg). All of the procedures were approved by the Curtin University Human Research Ethics Committee (HRE2018-0651), and all participants provided written informed consent.
2.2. Study Design
A randomised, repeated measures crossover design was used to ascertain the effect of three conditions: (1) mouth rinsing with 25 mL of PJ (Pickle Juice Sport, The Pickle Juice Company, Mesquite, TX, USA) for 10 s, then expelling [24]; (2) ingesting 1 mL∙kg−1 BM of PJ (Pickle Juice Sport, The Pickle Juice Company, Mesquite, TX, USA); (3) ingesting 1 mL∙kg−1 BM of room temperature water [17], on cramp duration and on the severity of discomfort experienced from an EIMC in the FHB of active cramp-prone adults. Pickle juice mouth rinse and ingestion will be henceforth referred to as PJMR, and PJI, respectively.
2.3. Experimental Procedures
2.3.1. Familiarisation Session
The participants attended a familiarisation session where the study procedures were explained and to screen whether the electrical stimulation of their FHB was tolerated and productive of a cramp. The FHB was selected because low frequency percutaneous stimulation of this muscle induces cramps that have both intra- and inter-sessional reliability [17,30]. Participants were asked which leg was their dominant kicking leg. The non-dominant leg was screened for cramp induction during familiarisation, whilst the dominant leg was stimulated during testing [17]. This prevented any residual local physiological changes from the familiarisation session from affecting the results during testing, which commonly occurred the following day [17].
During the familiarisation session, the participant sat upright on a plinth with their legs fully extended and with their ankles placed over the edge. On the non-dominant leg, two Ag/AgCl EMG electrodes were positioned 2 cm apart on the FHB mid-belly [30], and a reference electrode was placed on the ipsilateral lateral malleolus using standard EMG preparatory procedures [31] (see Figure 1). This leg was then prepared for cramp induction using electrical stimulation with a constant current stimulator (model DS7A, Digitimer Ltd., Hertfordshire, UK). The stimulation conductor was positioned inferior to the medial malleolus (Figure 1), and the tibial nerve was stimulated sub-maximally 2–4 times with a 1 ms electrical stimulus at 200 mA. The site that stimulated the greatest hallux flexion was marked for replication during cramp induction. The EMG electrodes were then connected to a computer via a Bortec AMT-8 EMG amplifier (Bortec Biomedical Ltd., Calgary, AB, Canada) and an NI USB-6221 analogue to digital converter (National Instruments, Austin, TX, USA), which began recording the time and EMG from the initiation of the electrical stimulus using the LabView software package (National Instruments, Austin, TX, USA).
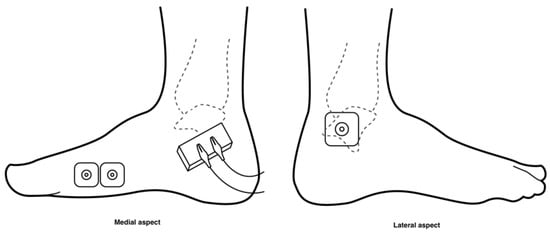
Figure 1.
Diagram of electrode and stimulation conductor placement. The recording electrodes were placed over the belly of the FHB muscle on the medial aspect of the foot. The reference electrode was placed over on the ipsilateral lateral malleolus, and the stimulating conductor was positioned inferior to the medial malleolus.
The participant was shown how to perform an isometric contraction of the non-dominant FHB and was allowed time to practice. Once able, they were instructed to repeat and maintain the contraction until signaled to relax, and were told to indicate when they felt the muscle was about to cramp. When the impending cramp was signaled, a train of electrical stimulation via the tibial nerve was delivered at an intensity of 200 mA for 2 s. The volitional contraction was maintained for 10 s from stimulus initiation, and then the participant was instructed to relax. The initial stimulus frequency was 4 Hz, and a total of eight stimuli were delivered during the first trial [30]. If a cramp was not induced, the participant rested for 1 min, and the process was repeated with the train frequency increased by 2 Hz [30]. This process was repeated until either an EIMC of the FHB was produced or until the frequency reached 24 Hz [30]. The frequency at which a cramp was induced was recorded as the participant’s cramp threshold frequency (CTF), which is the minimum frequency required to stimulate a cramp [32]. This CTF was used to induce all subsequent cramps.
The FHB was considered to be cramping if involuntarily contraction immediately succeeded the cessation of volitional contraction, which was verified by the participant’s self-report of a cramp, sustained hallux flexion, and an average EMG root mean square amplitude >2 SDs greater than the baseline reading [30]. Upon determining the CTF, the protocols for ingesting and mouth rinsing were practiced with water, thus ensuring protocol familiarity prior to experimental sessions.
2.3.2. Experimental Sessions
Assuring inter-sessional standardisation, the participants attended all experimental sessions after having fasted (12 h) and after having abstained from exercise (24 h). Upon arrival, a refractometer (Sper Scientific, Ltd., Scottsdale, AZ, USA) was used to measure urine specific gravity (USG) to ensure each participant was in a dehydrated state, reading >1.050 [33], as dehydration may increase the likelihood of cramp onset [34].
To minimise effects between trials, testing sessions were performed on the dominant leg at least one week apart [17]. The leg was prepared as per the familiarisation session, and electrode positions were marked with permanent marker [30] and were photographed to enable replication in subsequent trials. The participants were instructed to re-mark the sites if fading was observed [30]. During the initial testing session, prior to the first volitional contraction, baseline FHB activation was measured for 15 s while the participant relaxed the muscle. Before cramp induction, the participant was instructed to refrain from stretching the upcoming cramp, allowing maximum duration. At their pre-established CTF, a cramp was induced using a 200 mA stimulation for 2 s. Immediately following cramp detection and verification (10 s after electrical stimulus initiation), the participant was signaled to administer the intervention (PJMR, PJI, or water ingestion). Every 10 s during the cramp, the participant was instructed to rate the severity of discomfort experienced on a 100 mm visual analogue scale (VAS), where 0 = no discomfort and 10 = maximal discomfort. A cramp was considered alleviated when the EMG activity returned to ≤5% of the baseline contraction for 3 s.
2.4. Statistical Analysis
All participants were assessed for their hydration level at the beginning of each experimental session. One-way ANOVA was used to compare the USG mean scores. To determine the effect that mouth rinsing and ingesting PJ had on cramp duration, EMG raw data were rectified and digitised, and then individual values for PJMR and PJI were normalised to the water condition before being analysed. The EMG data was notch filtered at 50 Hz using a 2nd order zero lag Butterworth filter. The data were then smoothed using a moving root mean square window of 1 s. The minimum of these smoothed data was located, and the mean of the surrounding 0.5 s of smoothed data was calculated. The time point at which the smoothed data dropped below 1.5x the minimum smoothed value for 0.1s was then located to determine the offset time of the cramp.
Linear mixed models with random intercept participant effects were used to compare the VAS between conditions over time, and the results were summarised using a graphical representation of the marginal estimates. The time to cramp-end and time to zero discomfort (VAS = 0) between conditions were estimated using Kaplan–Meier survival probabilities. Results were summarised using Kaplan–Meier curves and medians and 95% confidence intervals of times to events. Log Rank tests were used to compare the survival probabilities between conditions. The effect of the condition on the time to zero discomfort was examined using Cox proportional hazards regression, and the effects were summarised using hazard ratios and 95% confidence intervals. The participants were censored if the VAS did not reach zero. Longitudinal and mixed model data were analysed using Stata version 15.1 (StataCorp, College Station, TX, USA); all other data were analysed with IBM SPSS version 24.0 (IBM Corporation, New York, NY, USA). Statistical significance was accepted at p < 0.05.
3. Results
3.1. Urine Specific Gravity
There were no significant differences in USG between the PJMR (mean ± SD; 1.068 ± 0.005), PJI (1.067 ± 0.007), and water (1.066 ± 0.01) conditions (F = 0.069, p = 0.93).
3.2. Cramp Duration
The median time to cramp cessation for the control (water) condition was 50.68 s. As a percentage of the control condition, the median cramp duration of the PJI and PJMR conditions were 82.8% (95% CIs = 54.13–111.50) and 68.6% (95% CIs = 0.00–162.21), respectively (Table 1). However, this time difference was not significantly different between conditions (p = 0.942, Log Rank test). Furthermore, given that the confidence intervals were set at 100 and since the ranges for both PJMR and PJI included the null value of 100, there was no statistical difference between conditions. The low number of participants makes these results inconclusive.

Table 1.
Median cramp duration.
3.3. Severity and Duration of Perceived Cramp Discomfort (VAS)
Linear mixed models with random subject intercepts were used to compare the VAS between conditions over time (Figure 2). The Kaplan–Meier survival probabilities showed no significant difference in time to VAS = 0 between conditions (p = 0.76, Log Rank test). Cox regression was used to compare the PJI and PJMR to water for time to VAS = 0. Although the hazard ratios for both PJI and PJMR were higher (22% and 35%) compared to water these results were not statistically significant (p = 0.67 and 0.51). Note: One participant did not reach zero in any of the conditions and was therefore censored in the survival model.
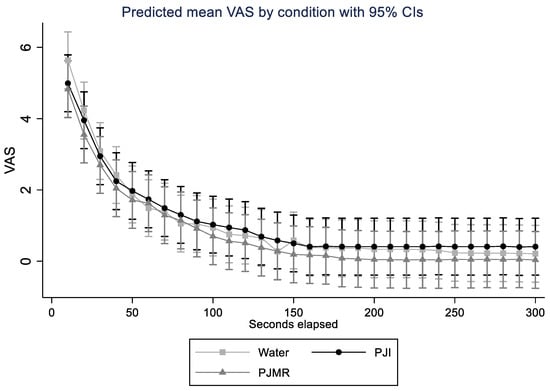
Figure 2.
Linear mixed models with random subject intercepts comparing visual analogue scale (VAS) between conditions over time (seconds).
4. Discussion
This study is the first to report whether mouth rinsing with PJ has any inhibitory effect on EIMCs in active cramp-prone adults. It aimed to determine whether mouth rinsing with PJ (1) reduced the duration of an EIMC when compared to water, (2) reduced the discomfort associated with an EIMC more than water, and (3) was superior to ingesting PJ at inhibiting an EIMC.
There was no significant difference in cramp duration between conditions. Interestingly however, the point estimates for median cramp duration reduced by 17.2% and 31.4% in the PJI and PJMR groups, respectively, compared to water (50.68 s). Similarly, Miller et al. [17] found that cramp duration was reduced by 45% compared to baseline when ingesting PJ (pre: 153.2 ± 23.7 s, post: 84.6 ± 18.5 s). A recent study investigating the acetic acid concentration in various substances found that several pickle brines (acetic acid weight percent (AAwt%) range = 0.406 ± 0.007–0.796 ± 0.007) [33] similar to those used in the study by Miller et al. [17] had a greater AAwt% than the proprietary PJ (0.260 ± 0.006%) [35] used in the current study. The reduced acetic acid concentration in the tested conditions may suggest that a larger sample was needed to account for the concentration difference, potentially contributing to the lack of significance that was observed. Furthermore, unlike the current study, Miller et al. [17] only included cramps that lasted ≥90 s, thus reducing the potential for variability. Although a similar sample and intervention was tested in the current study, the absence of a minimum cramp duration inclusion criterion resulted in wide-ranging variability between participants, making it difficult to assess any changes caused by PJ. Miller et al.’s [17] study reported the reliable electrical induction of long (≥90 s) muscle cramps in the FHB of the participants. However, despite replicating Miller et al.’s [17] cramp induction protocol during pilot testing, the authors were unable to induce muscle cramps reliably. Even upon modifying the protocol, the authors observed marked variability in cramp duration between participants, with few lasting ≥90 s. Consistency was observed in the individuals’ inter-sessional cramp durations, resulting in the inclusion of all of the participants who experienced a FHB EIMC during the familiarisation session, regardless of cramp duration. Considering that few participants experienced long cramps, the current sample is a better representation of the general cramp-prone population. The current study showed no significant changes in the severity or duration of perceived cramp discomfort between conditions during an EIMC. No other studies monitoring the effects of an intervention on perceived discomfort throughout the duration of a cramp have been identified. Other studies testing TRP-activator ingestion have assessed perceived discomfort severity pre- and post-cramp induction, but not during, with mixed findings being reported [32,36]. One study found no changes in the perceived discomfort between the TRP-activator intervention group and placebo control at various time points across the 24 h following the electrical induction of a calf cramp [32]. Whilst another found that during the initial 20 min following volitional calf cramp cessation, the perceived discomfort (on a 1 to 10 rating scale) was significantly lower for the TRP-activator group (3.4 ± 4.6) than it was in the control group (4.0 ± 2.2) [36]. Interestingly, Earp et al. [37] found that the perceived discomfort severity of an EIMC was significantly (p = 0.01) reduced in participants when comparing an electrolyte beverage (2.00 ± 0.54) to a placebo beverage (2.88 ± 0.84). However, unlike the current study, these study protocols had participants ingesting the conditions prior to cramp induction. Therefore, it is not yet known whether TRP-activators or other interventions reduce discomfort during or after muscle cramps when ingested immediately following cramp onset. Muscle cramps are typically offset by the passive stretching of the muscle secondary to pain and loss of function [14]. In this study, the participants were asked to relax and to allow the cramp to subside unaided. The severity of discomfort that was reported by the participants varied greatly, so it is not possible to suggest any relationship between the severity or duration of discomfort and PJ.
Through assessing PJ mouth rinsing and ingestion, this study was able to measure the effects of exclusively stimulating oropharyngeal receptors with PJ. Although the literature suggests that PJ alleviates skeletal muscle cramping by stimulating the oropharyngeal TRP-channels that trigger an inhibitory supraspinal reflex [17,21,32,38], the current study does not support these findings. The authors found no statistical differences in cramp duration or perceived discomfort between PJ ingestion, PJ mouth rinsing, and water in active cramp-prone adults. Despite expanding the inclusion parameters, the authors were unable to induce cramps in eight cramp-prone participants during the familiarisation session, resulting in exclusion of these participants from the study, thereby reducing the total number of datasets collected. Furthermore, the unsecured foot position of the participants during testing may have also contributed to the variability in the data. By not adequately restraining the foot, there is the potential for adjacent muscles to cause reciprocal inhibition that affects FHB activation [39], possibly impacting the results. Improved methods for inducing and maintaining a cramp for long enough to assess the effects of interventions should be a priority for future research.
Given the limitations of the study, recommendations for future research can be suggested. The aetiology of EAMC is still unknown; however, it is likely multifactorial in nature, with altered neuromuscular control [38] and hypohydration contributing to onset [34]. To better understand a mechanism that causes the immediate relief of EAMC, future studies should compare PJ with water by testing mouth rinsing and ingestion for both conditions. Furthermore, the intervention and placebo groups should be considered to enable blinding. However, an appropriate placebo for PJ will be difficult to create and may present challenges with assessment.
5. Conclusions
The present study found no evidence that ingesting or rinsing with PJ is more effective than water for the alleviation of EIMC. However, given the large variability in cramp duration, it is difficult to determine any meaningful effect of PJ on muscle cramps. It is possible even a short duration effect of pickle juice on EIMC would have a practical benefit in the final minutes of a match for a player who is experiencing a cramp. Future studies should seek to determine the most consistent protocol for cramp induction and should continue research into alleviating EIMC quickly with an improved methodological approach to better understand whether mouth rinsing with PJ is, in fact, an efficacious treatment for acute EAMC.
Author Contributions
Conceptualization, J.G., A.P.L., C.J.B., K.J.D., and M.O.; methodology, J.G., A.P.L., C.J.B., and K.J.D.; software, P.D.; validation, J.G., A.P.L., C.J.B., K.J.D., P.D., A.J., and M.O.; formal analysis, A.J.; investigation, J.G. and A.P.L.; resources, J.G., A.P.L., and P.D.; data curation, P.D., J.G., and A.P.L.; writing—original draft preparation, J.G.; writing—review and editing, J.G., A.P.L., C.J.B., K.J.D., P.D., A.J., and M.O.; visualization, J.G., A.J., and A.P.L.; supervision, A.P.L., C.J.B., and K.J.D.; project administration, A.P.L. and J.G.; funding acquisition, A.P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Curtin University (HRE2018-0651).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors would like to thank the participants in this study and Eloise Arnold for her idea, which initiated the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schwellnus, M.P.; Derman, E.W.; Noakes, T.D. Aetiology of skeletal muscle ‘cramps’ during exercise: A novel hypothesis. J. Sports Sci. 1997, 15, 277–285. [Google Scholar] [CrossRef]
- Swash, M.; Czesnik, D.; De Carvalho, M. Muscular cramp: Causes and management. Eur. J. Neurol. 2018, 26, 214–221. [Google Scholar] [CrossRef]
- Miller, K.C.; Stone, M.S.; Huxel, K.C.; Edwards, J.E. Exercise-Associated Muscle Cramps. Sports Health A Multidiscip. Approach 2010, 2, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Minetto, M.A.; Holobar, A.; Botter, A.; Farina, D. Origin and Development of Muscle Cramps. Exerc. Sport Sci. Rev. 2013, 41, 3–10. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Stuempfle, K.J. Muscle Cramping during a 161-km Ultramarathon: Comparison of Characteristics of Those with and without Cramping. Sports Med.-Open 2015, 1, 24. [Google Scholar] [CrossRef]
- Martínez-Navarro, I.; Montoya-Vieco, A.; Collado-Boira, E.; Hernando, B.; Panizo, N.; Hernando, C. Muscle Cramping in the Marathon. J. Strength Cond. Res. 2020, 37, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Schwellnus, M.P. Cause of Exercise Associated Muscle Cramps (EAMC)-altered neuromuscular control, dehydration or electrolyte depletion? Br. J. Sports Med. 2008, 43, 401–408. [Google Scholar] [CrossRef]
- Edouard, P. Exercise associated muscle cramps: Discussion on causes, prevention and treatment. Sci. Sports 2014, 29, 299–305. [Google Scholar] [CrossRef]
- Jahic, D.; Begic, E. Exercise-Associated Muscle Cramp-Doubts About the Cause. Mater. Soc.-Med. 2018, 30, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Shirreffs, S.M. Muscle Cramping During Exercise: Causes, Solutions, and Questions Remaining. Sports Med. 2019, 49, 115–124. [Google Scholar] [CrossRef]
- Swash, M.; De Carvalho, M. Testing electrolyte supplementation for muscle cramp. Muscle Nerve 2019, 60, 499–500. [Google Scholar] [CrossRef]
- Schwellnus, P.M.P. Muscle Cramping in the Marathon: Aetiology and risk factors. Sports Med. 2007, 37, 364–367. [Google Scholar] [CrossRef]
- Schwellnus, M.P.; Drew, N.; Collins, M. Increased running speed and previous cramps rather than dehydration or serum sodium changes predict exercise-associated muscle cramping: A prospective cohort study in 210 Ironman triathletes. Br. J. Sports Med. 2011, 45, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.L.; Churilla, J.R. A narrative review of exercise-associated muscle cramps: Factors that contribute to neuromuscular fatigue and management implications. Muscle Nerve 2016, 54, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Giuriato, G.; Pedrinolla, A.; Schena, F.; Venturelli, M. Muscle cramps: A comparison of the two-leading hypothesis. J. Electromyogr. Kinesiol. 2018, 41, 89–95. [Google Scholar] [CrossRef]
- Panza, G.; Stadler, J.; Murray, D.; Lerma, N.; Barrett, T.; Pettit-Mee, R.; Edwards, J.E. Acute Passive Static Stretching and Cramp Threshold Frequency. J. Athl. Train. 2017, 52, 918–924. [Google Scholar] [CrossRef]
- Miller, K.C.; Mack, G.W.; Knight, K.L.; Hopkins, J.T.; Draper, D.O.; Fields, P.J.; Hunter, I. Reflex Inhibition of Electrically Induced Muscle Cramps in Hypohydrated Humans. Med. Sci. Sports Exerc. 2010, 42, 953–961. [Google Scholar] [CrossRef]
- Simon, S.A.; De Araujo, I.E.; Gutierrez, R.; Nicolelis, M.A.L. The neural mechanisms of gustation: A distributed processing code. Nat. Rev. Neurosci. 2006, 7, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Berdugo, D.; Rofes, L.; Farre, R.; Casamitjana, J.F.; Enrique, A.; Chamizo, J.M.G.; Padrón, A.; Navarro, X.; Clave, P. Localization and expression of TRPV1 and TRPA1 in the human oropharynx and larynx. Neurogastroenterol. Motil. 2015, 28, 91–100. [Google Scholar] [CrossRef]
- Chang, R.B.; Waters, H.; Liman, E.R. A proton current drives action potentials in genetically identified sour taste cells. Proc. Natl. Acad. Sci. USA 2010, 107, 22320–22325. [Google Scholar] [CrossRef]
- Huang, Y.A.; Maruyama, Y.; Stimac, R.; Roper, S.D. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J. Physiol. 2008, 586, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chang, R.B.; Allgood, S.; Silver, W.L.; Liman, E.R. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J. Gen. Physiol. 2011, 137, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Craighead, D.H.; Shank, S.W.; Gottschall, J.S.; Passe, D.H.; Murray, B.; Alexander, L.M.; Kenney, W.L. Ingestion of transient receptor potential channel agonists attenuates exercise-induced muscle cramps. Muscle Nerve 2017, 56, 379–385. [Google Scholar] [CrossRef]
- Mandadi, S.; Nakanishi, S.; Takashima, Y.; Dhaka, A.; Patapoutian, A.; McKemy, D.; Whelan, P. Locomotor networks are targets of modulation by sensory transient receptor potential vanilloid 1 and transient receptor potential melastatin 8 channels. Neuroscience 2009, 162, 1377–1397. [Google Scholar] [CrossRef]
- Ataide-Silva, T.; Souza, M.E.D.C.A.D.; De Amorim, J.F.; Stathis, C.G.; Leandro, C.G.; Lima-Silva, A.E. Can Carbohydrate Mouth Rinse Improve Performance during Exercise? A Systematic Review. Nutrients 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Wickham, K.A.; Spriet, L.L. Administration of Caffeine in Alternate Forms. Sports Med. 2018, 48, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Fares, E.-J.M.; Kayser, B. Carbohydrate Mouth Rinse Effects on Exercise Capacity in Pre- and Postprandial States. J. Nutr. Metab. 2011, 2011, 385962. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.; Bottoms, L.; Flynn, C.; Bradley, E.; Alexander, G.; Mccullagh, S.; Finn, T.; Hurst, H.T. The effect of different durations of carbohydrate mouth rinse on cycling performance. Eur. J. Sport Sci. 2014, 14, 259–264. [Google Scholar] [CrossRef]
- Rennie, S. Electrophysical agents contraindications and precautions: An evidence-based approach to clinical decision making in physical therapy foreword. Physiother. Can. 2010, 62, 1–80. [Google Scholar] [CrossRef]
- Miller, K.C.; Knight, K.L.; Wilding, S.R.; Stone, M.B. Duration of electrically induced muscle cramp increased by increasing stimulation frequency. J. Sport Rehabil. 2012, 21, 182–185. [Google Scholar] [CrossRef]
- Ms, M.B.S.; Edwards, J.E.; Babington, J.P.; Ingersoll, C.D.; Ms, R.M.P. Reliability of an electrical method to induce muscle cramp. Muscle Nerve 2003, 27, 122–123. [Google Scholar] [CrossRef]
- Behringer, M.; Nowak, S.; Leyendecker, J.; Mester, J. Effects of TRPV1 and TRPA1 activators on the cramp threshold frequency: A randomized, double-blind placebo-controlled trial. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 117, 1641–1647. [Google Scholar] [CrossRef]
- Tanner, R.K.; Gore, C.J. Physiological Tests for Elite Athletes, 2nd ed.; Australian Institute of Sport: Champaign, IL, USA, 2013. [Google Scholar]
- Ohno, M.; Lavender, A.P.; Sawai, A. Heat-induced Body Fluid Loss Causes Muscle Cramp during Maximal Voluntary Contraction for the Knee Flexors. Int. J. Sport Health Sci. 2018, 16, 191–199. [Google Scholar] [CrossRef][Green Version]
- Marosek, S.E.H.; Antharam, V.; Dowlatshahi, K. Quantitative Analysis of the Acetic Acid Content in Substances Used by Athletes for the Possible Prevention and Alleviation of Exercise-Associated Muscle Cramps. J. Strength Cond. Res. 2020, 34, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Craighead, D.H.; Shank, S.; Alexander, L.M.; Kenney, W.L. Orally ingested transient receptor potential (TRP) channel activators attenuate the intensity-duration of voluntarily induced muscle cramps in humans. Faseb. J. 2016, 30, lb706. [Google Scholar] [CrossRef]
- Earp, J.E.; Stearns, R.L.; Stranieri, A.; Agostinucci, J.; Lepley, A.S.; Matson, T.; Ward-Ritacco, C.L. Electrolyte beverage consumption alters electrically induced cramping threshold. Muscle Nerve 2019, 60, 598–603. [Google Scholar] [CrossRef]
- Miller, K.C. Myths and Misconceptions About Exercise-Associated Muscle Cramping. ACSM’s Health Fit. J. 2016, 20, 37–39. [Google Scholar] [CrossRef]
- Manning, C.D.; McDonald, P.L.L.; Murnaghan, C.D.; Bawa, P. Reciprocal inhibition versus unloading response during stretch reflex in humans. Exp. Brain Res. 2013, 226, 33–43. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).