Efficient Fuzzy Image Stretching for Automatic Ganglion Cyst Extraction Using Fuzzy C-Means Quantization
Abstract
1. Introduction
2. Fuzzy Stretching with Trapezoid Membership Function in Image Enhancement
3. Cyst Extraction with FCM Algorithm
- Step 1: Initialize the number of cluster c (2 ≤ c < n), exponential weight m (1 ≤ m < ∞), the membership degree u(0), and the error threshold (ε).
- Step 2: Compute the central vector Vij as Equation (8) for {vi | i=1, 2, …, c}.where X is the input pattern, i is the cluster index, and j is the pattern node index. k is the pattern index, n is the number of patterns, and U is the membership function.
- Step 3: Define the FCM cost function J as Equation (9) where dik is the distance between the k-th pattern xk and the central vector of the i-th cluster, and uik is the membership degree of xk among patterns in the i-th cluster.
- Step 4: Compute the difference between the new and previous membership degrees (Uik (r + 1) − Uik(r)). If the difference is larger than the error threshold (ε), then go to Step 2; otherwise the algorithm stops.
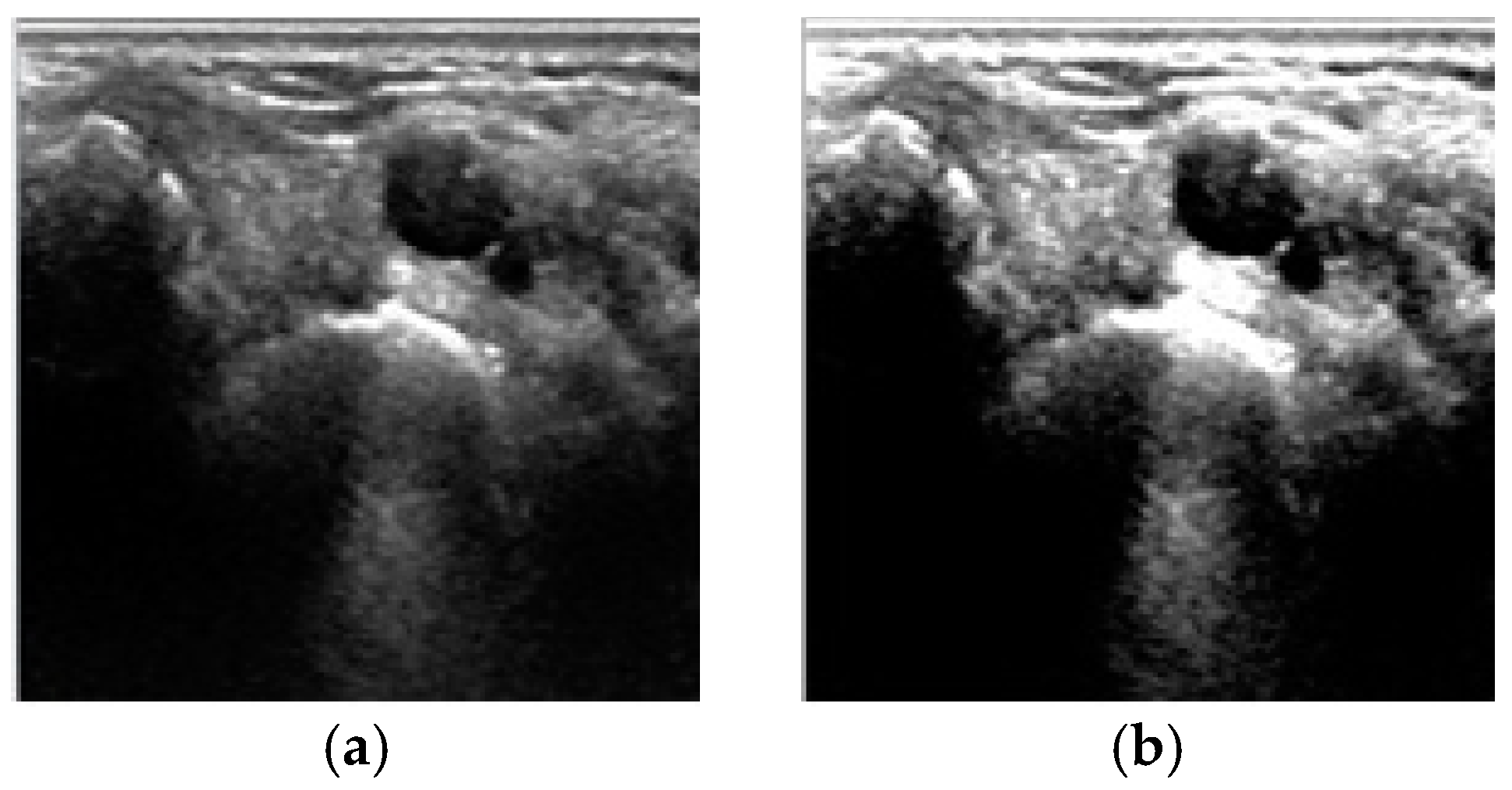
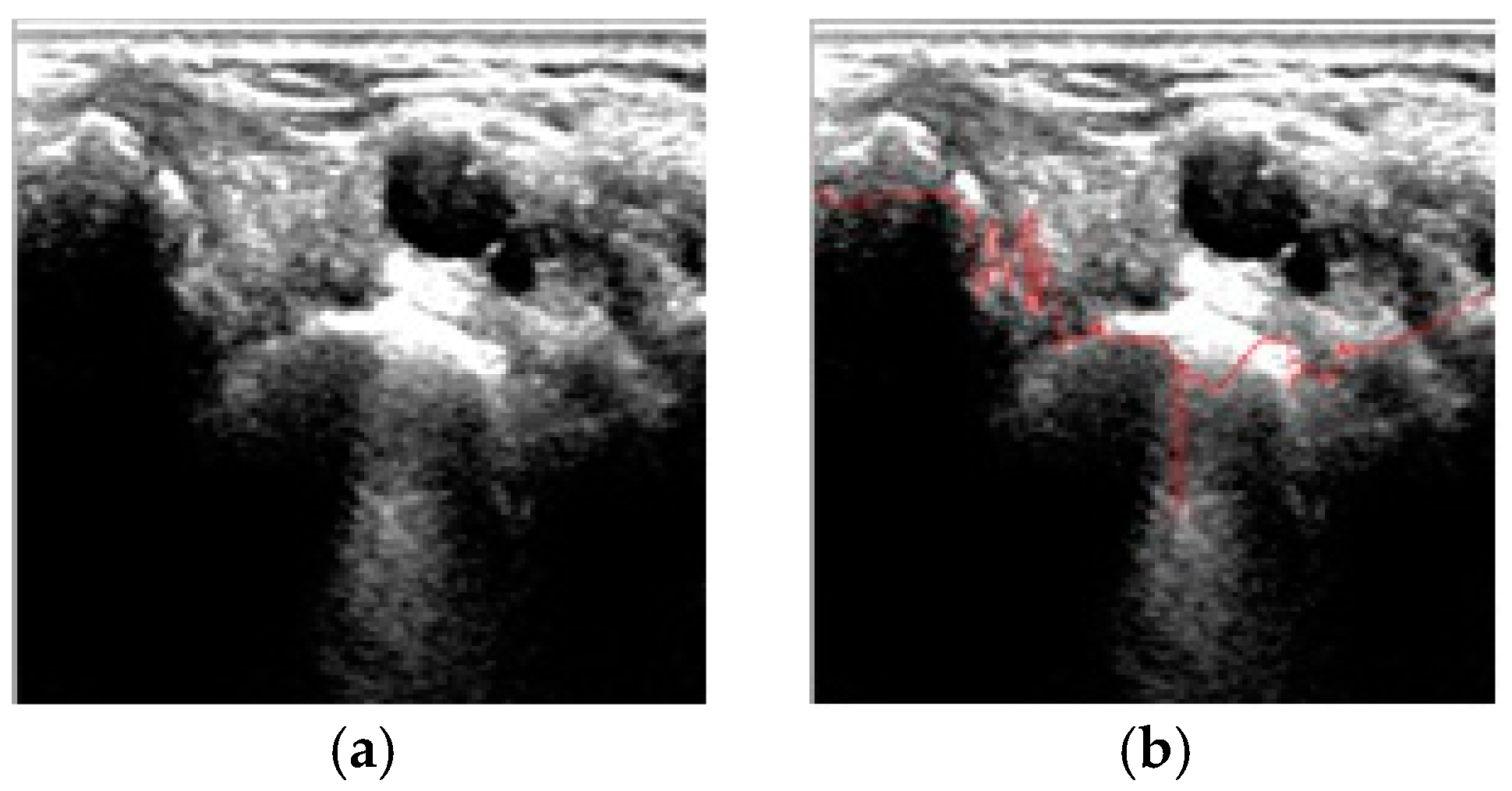
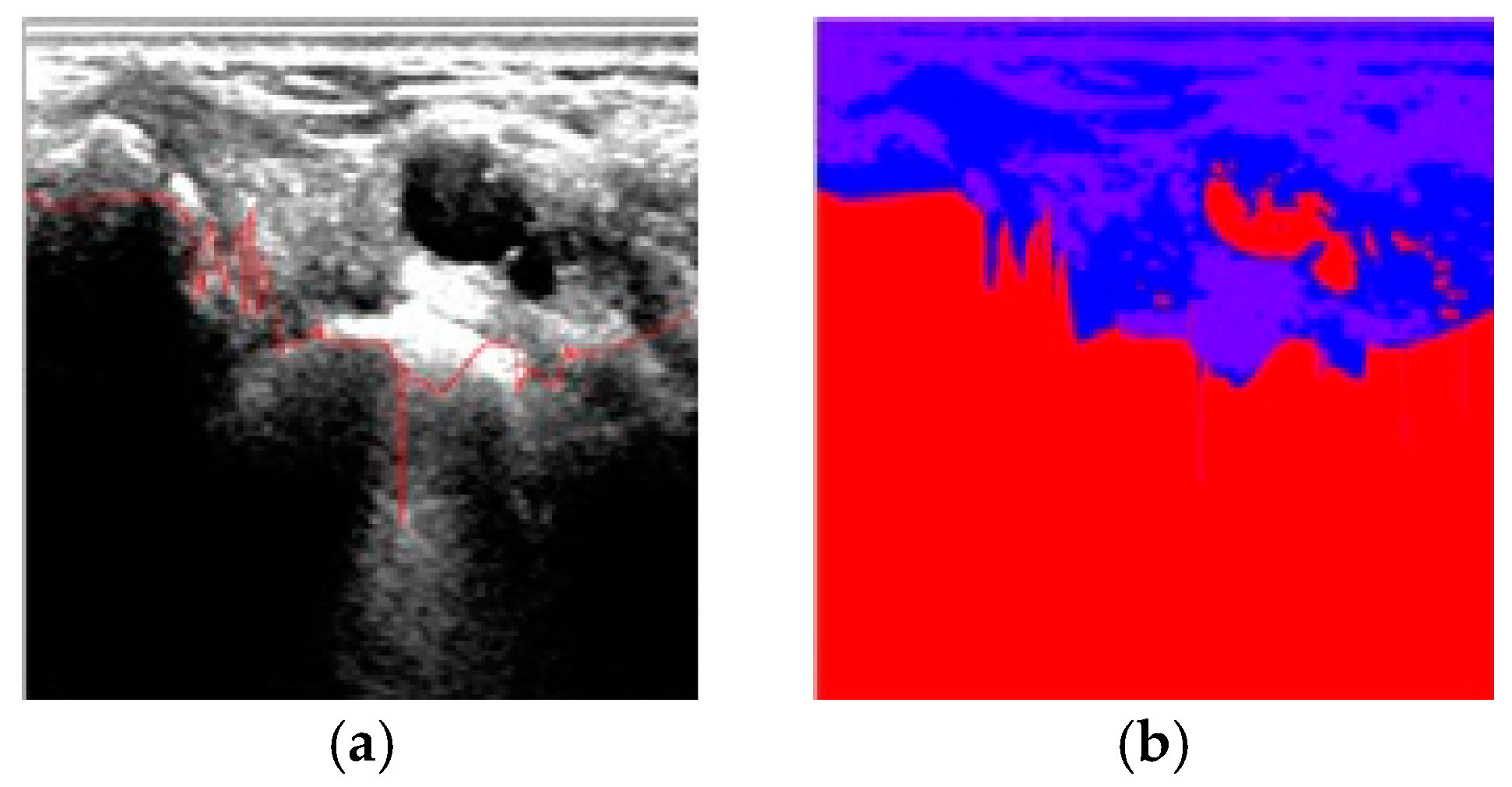
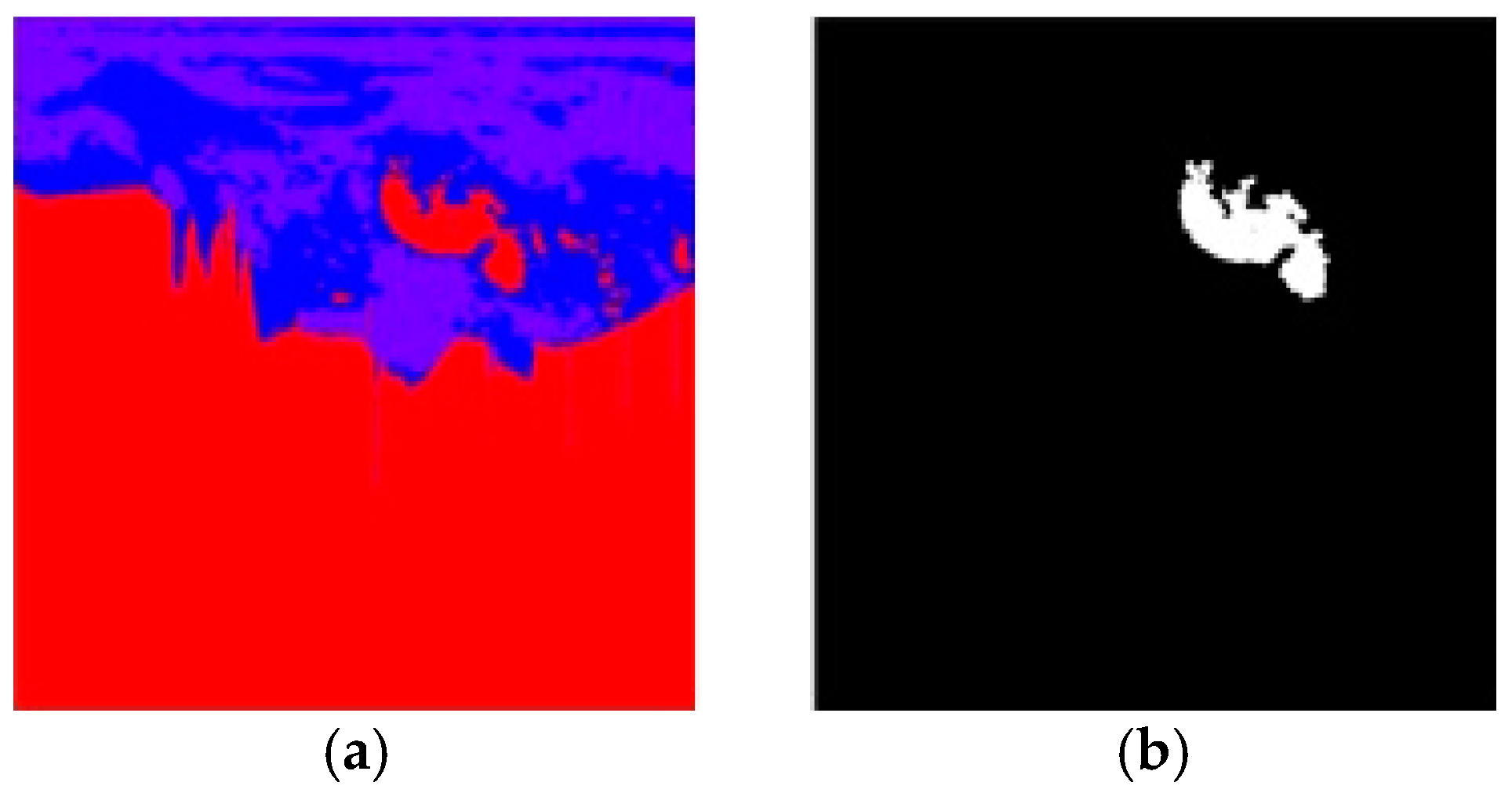
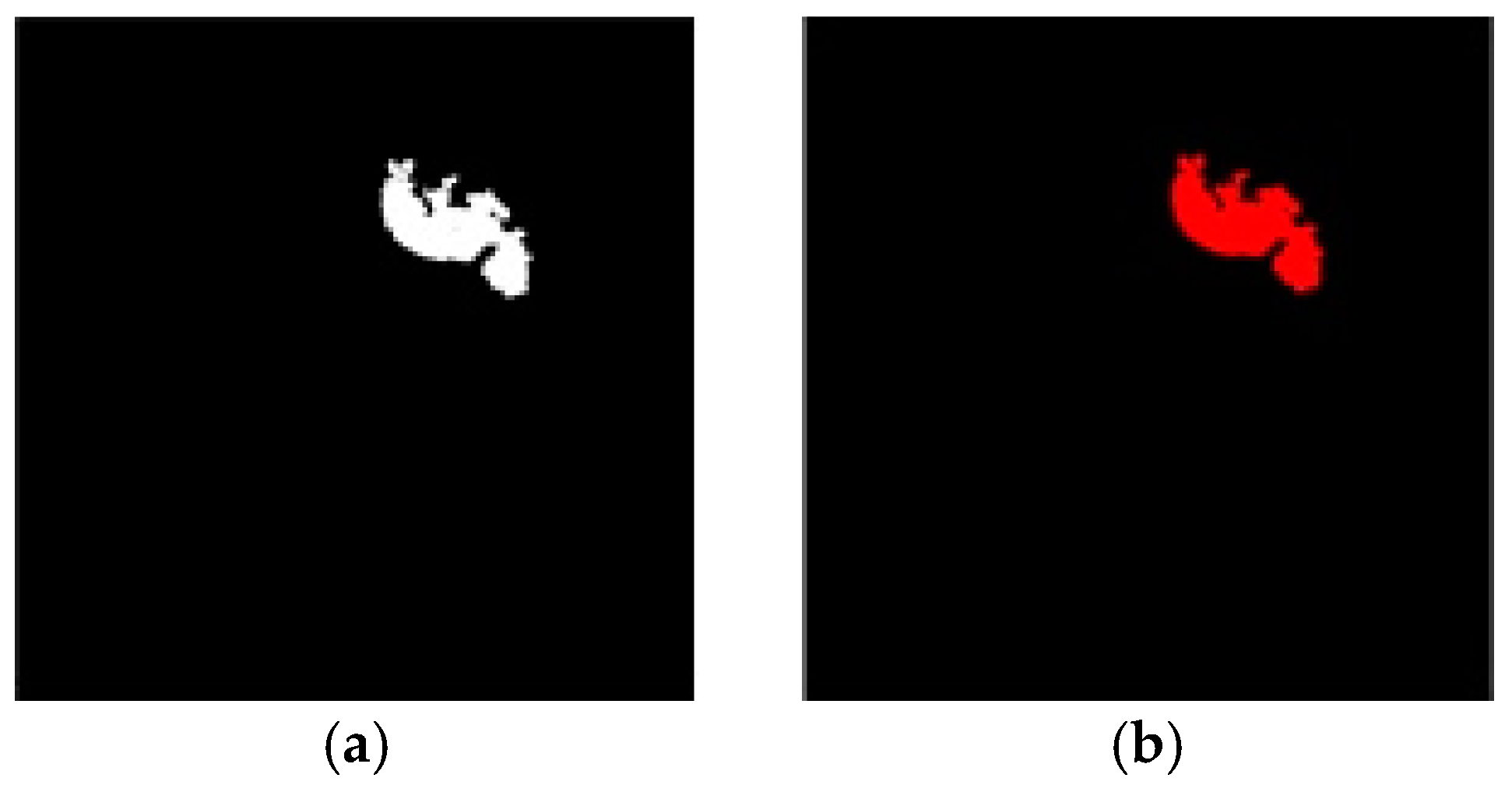
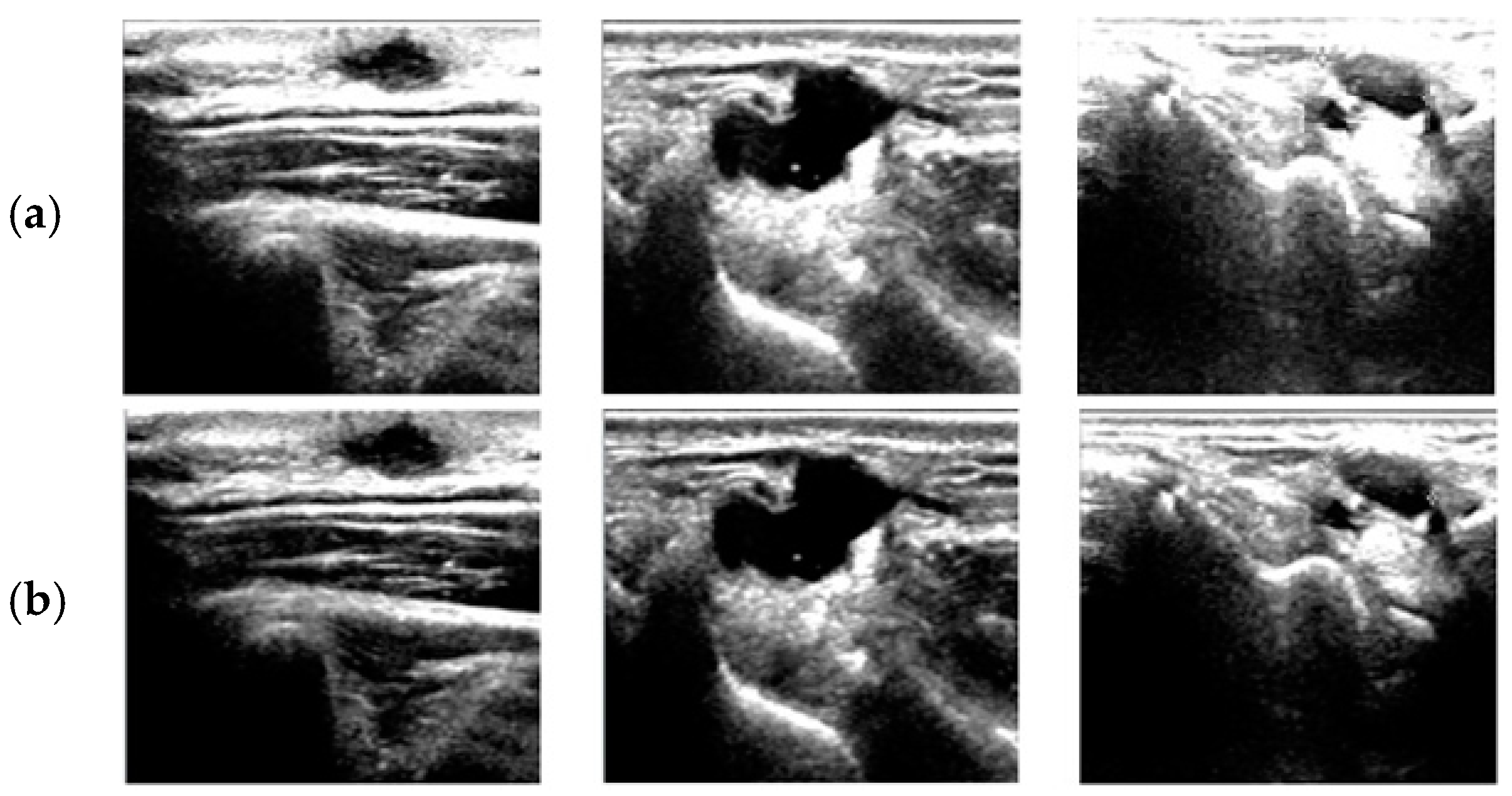
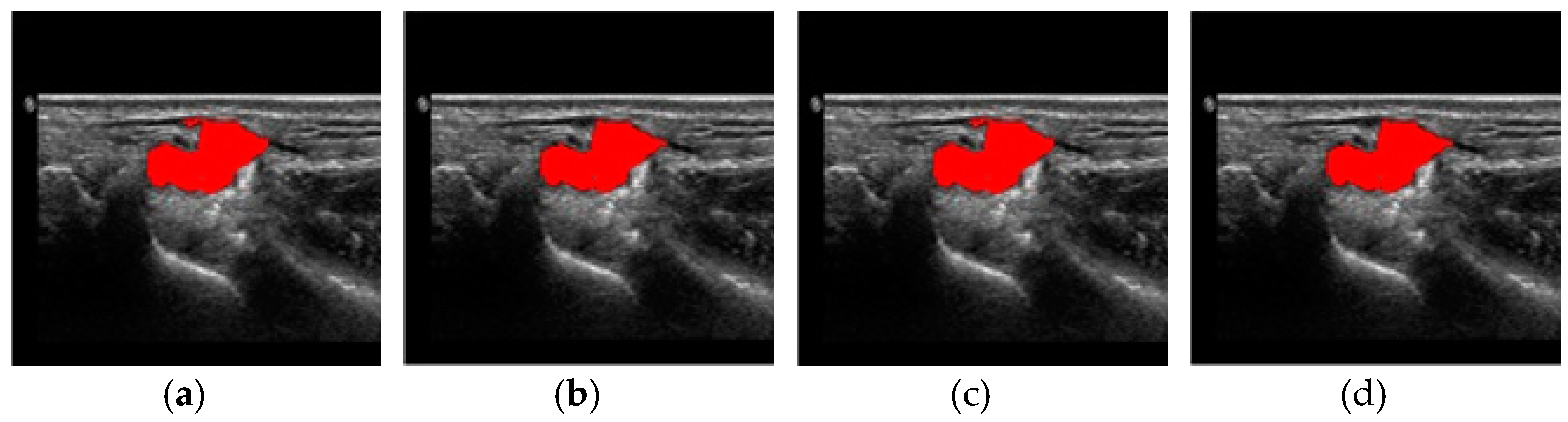
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meena, S.; Gupta, A. Dorsal wrist ganglion: Current review of literature. J. Clin. Orthop. Trauma 2014, 5, 59–64. [Google Scholar] [CrossRef]
- Freire, V.; Guérini, H.; Campagna, R.; Moutounet, L.; Dumontier, C.; Feydy, A.; Drapé, J.L. Imaging of Hand and Wrist Cysts: A Clinical Approach. Am. J. Roentgenol. 2012, 199, W618–W628. [Google Scholar] [CrossRef] [PubMed]
- Gude, W.; Morelli, V. Ganglion Cysts of the Wrist: Pathophysiology, Clinical Picture, and Management. Curr. Rev. Musculoskelet. Med. 2008, 1, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Tophoj, K.; Henriques, U. Ganglion of the wrist—a structure developed from the joint. Acta Orthop. Scand. 1971, 42, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.B. Extracting Ganglion Cysts from Ultrasound Image with Fuzzy Membership Function. J. Korea Inst. Inf. Commun. Eng. 2015, 19, 1296–1300. [Google Scholar] [CrossRef][Green Version]
- Kwon, S.H.; Ryu, K.N.; Park, Y.K.; Jeong, Y.M. Soft tissue masses: Ultrasonographic findings. J. Korean Soc. Med. Ultrasound 2001, 20, 349–355. [Google Scholar]
- Suen, M.; Fung, B.; Lung, C.P. Treatment of Ganglion Cysts. ISRN Orthop. 2013, 2013, 940615. [Google Scholar] [CrossRef]
- Head, L.; Gencarelli, J.R.; Allen, M.; Boyd, K.U. Wrist ganglion treatment: Systematic review and meta-analysis. J. Hand Surg. 2015, 40, 546–553. [Google Scholar] [CrossRef]
- Wang, G.; Jacobson, J.A.; Feng, F.Y.; Girish, G.; Caoili, E.M.; Brandon, C. Sonography of Wrist Ganglion Cysts: Variable and Noncystic Appearances. J. Ultrasound Med. 2007, 26, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Kuliński, S.; Gutkowska, O.; Mizia, S.; Martynkiewicz, J.; Gosk, J. Dorsal and volar wrist ganglions: The results of surgical treatment. Adv. Clin. Exp. Med. 2019, 28, 95–102. [Google Scholar] [CrossRef]
- Gress, F.; Schmitt, C.; Savides, T.; Faigel, D.O.; Catalano, M.; Wassef, W.; Roubein, L.; Nickle, N.; Ciaccia, D.; Bhutani, M.; et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest. Endosc. 2001, 53, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Enikov, E.T.; Anton, R. Image segmentation and analysis of flexion-extension radiographs of cervical spines. J. Med. Eng. 2014, 2014, 976323. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Elamvazuthi, I.; Dass, S.C.; Faye, I.; Vasant, P.; George, J.; Izza, F. Curvelet based automatic segmentation of supraspinatus tendon from ultrasound image: A focused assistive diagnostic method. Biomed. Eng. Online 2014, 13, 157. [Google Scholar] [CrossRef]
- Suryadibrata, A.; Kim, K.B. Ganglion Cyst Region Extraction from Ultrasound Images Using Possibilistic C-Means Clustering Method. J. Inf. Commun. Converg. Eng. 2017, 15, 49–52. [Google Scholar]
- Suryadibrata, A.; Song, D.H.; Kim, K.B. Automatic Ganglion Cyst Detection from Ultrasound Images using Fuzzy C-Means Clustering Method. Information 2017, 20, 2543–2548. [Google Scholar]
- Bezdek, J.C.; Keller, J.; Krisnapuram, R.; Pal, N. Fuzzy Models and Algorithms for Pattern Recognition and Image Processing; Springer Science & Business Media: New York, NY, USA, 1999. [Google Scholar]
- Park, J.; Song, D.H.; Nho, H.; Choi, H.; Kim, K.A.; Park, H.J.; Kim, K.B. Automatic segmentation of brachial artery based on fuzzy C-means pixel clustering from ultrasound images. Int. J. Electr. Comput. Eng. 2018, 8, 638–643. [Google Scholar] [CrossRef][Green Version]
- Pei, H.X.; Zheng, Z.R.; Wang, C.; Li, C.N.; Shao, Y.H. D-FCM: Density based fuzzy c-means clustering algorithm with application in medical image segmentation. Procedia Comput. Sci. 2017, 122, 407–414. [Google Scholar] [CrossRef]
- Huang, H.; Meng, F.; Zhou, S.; Jiang, F.; Manogaran, G. Brain image segmentation based on FCM clustering algorithm and rough set. IEEE Access 2019, 7, 12386–12396. [Google Scholar] [CrossRef]
- Kim, K.B.; Song, D.H. Intelligent automatic extraction of canine cataract object with dynamic controlled fuzzy C-means based quantization. Int. J. Electr. Comput. Eng. 2018, 8, 666–672. [Google Scholar] [CrossRef]
- Rehman, S.N.; Hussain, M.A. Fuzzy C-means algorithm-based satellite image segmentation. Indones. J. Electr. Eng. Comput. Sci. 2018, 9, 332–334. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Bu, H. Fabric defect detection using a hybrid and complementary fractal feature vector and FCM-based novelty detector. Fibres Text. East. Eur. 2017, 25, 46–52. [Google Scholar] [CrossRef]
- Mohammed, K.M.C.; Kumar, S.S.; Prasad, G. Optimized Fuzzy C-means Clustering Methods for Defect Detection on Leather Surface. J. Sci. Ind. Res. 2020, 79, 833–836. [Google Scholar]
- Park, J.; Song, D.H.; Han, S.S.; Lee, S.J.; Kim, K.B. Automatic Extraction of Soft Tissue Tumor from Ultrasonography Using ART2 Based Intelligent Image Analysis. Curr. Med. Imaging Rev. 2017, 13, 447–453. [Google Scholar] [CrossRef]
- Kaur, J.; Choudhary, A. Comparison of Several Contrast Stretching Techniques on Acute Leukemia Images. Int. J. Eng. Innov. Technol. 2012, 2, 332–335. [Google Scholar]
- Al-amri, S.S.; Kalyankarm, N.V.; Khamitkar, S.D. Linear and non-linear contrast enhancement image. Int. J. Comput. Sci. Netw. Secur. 2010, 10, 139–143. [Google Scholar]
- Woo, H.S.; Kim, K.B. Improved Fuzzy Binarization Method with Trapezoid type Membership Function and Adaptive α_cut. J. Korea Inst. Inf. Commun. Eng. 2016, 20, 1852–1859. [Google Scholar] [CrossRef][Green Version]
- Kanth, A.R.; Reddy, Y.N. Cubic spline for a class of singular two-point boundary value problems. Appl. Math. Comput. 2005, 170, 733–740. [Google Scholar]
- Kim, K.B.; Lee, H.J.; Song, D.H.; Woo, Y.W. Extracting fascia and analysis of muscles from ultrasound images with FCM-based quantization technology. Neural Netw. World 2010, 20, 405–416. [Google Scholar]
- Kim, K.B.; Park, H.J.; Song, D.H. Developing Intelligent Health Diagnosis System for Korean Traditional Medicine: Database Construction and Neural Inference. Int. J. Bio-Sci. Bio-Technol. 2014, 6, 1–8. [Google Scholar] [CrossRef]
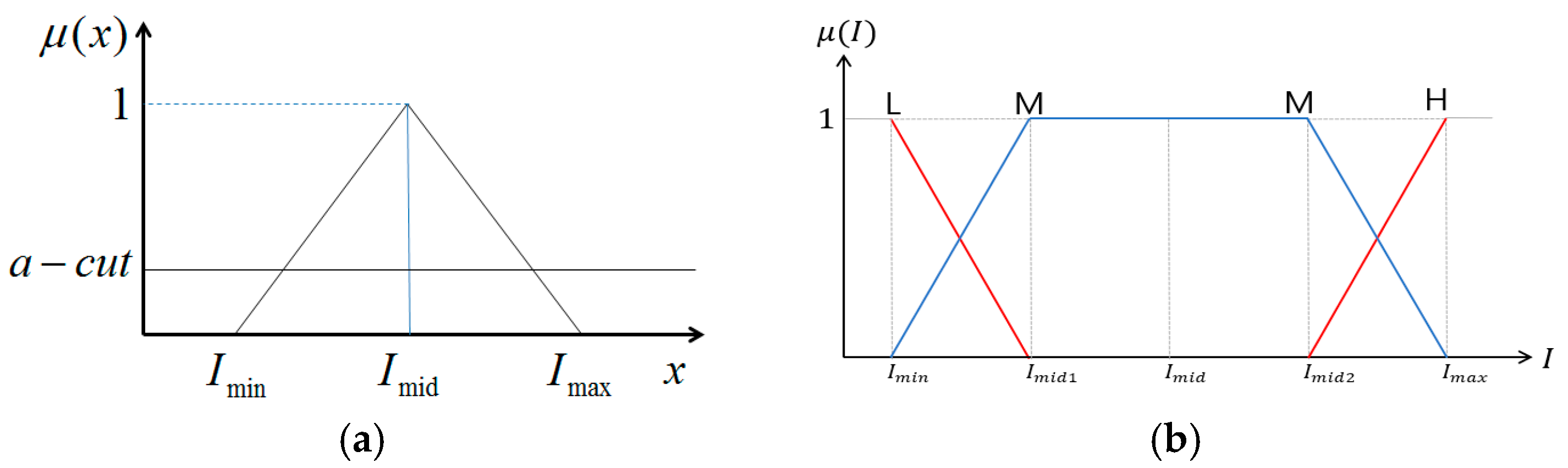
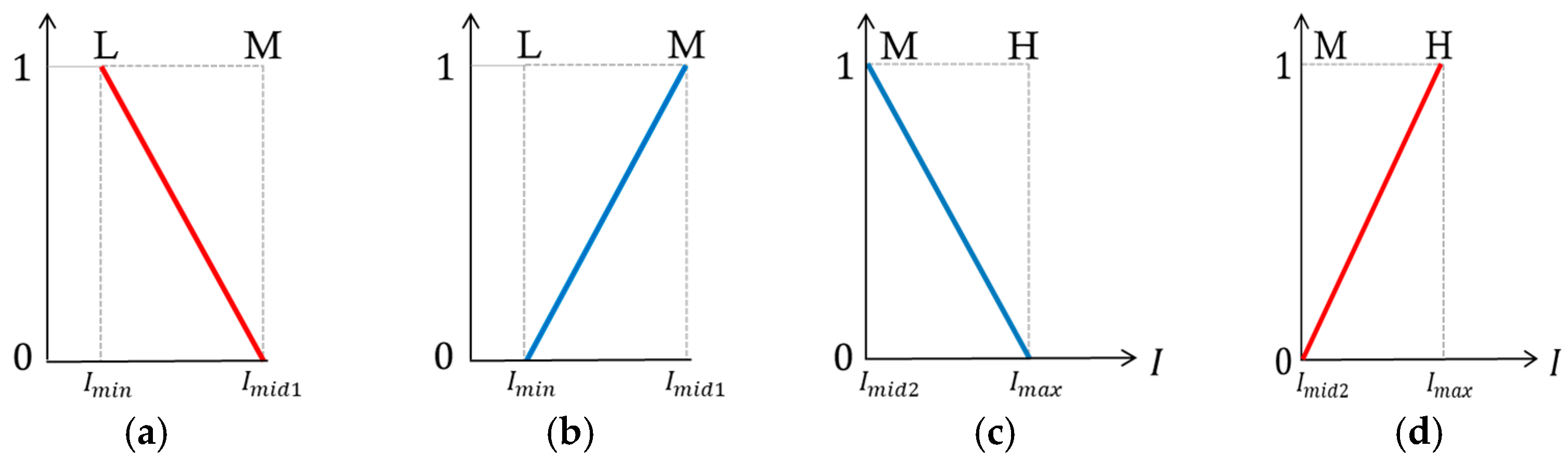

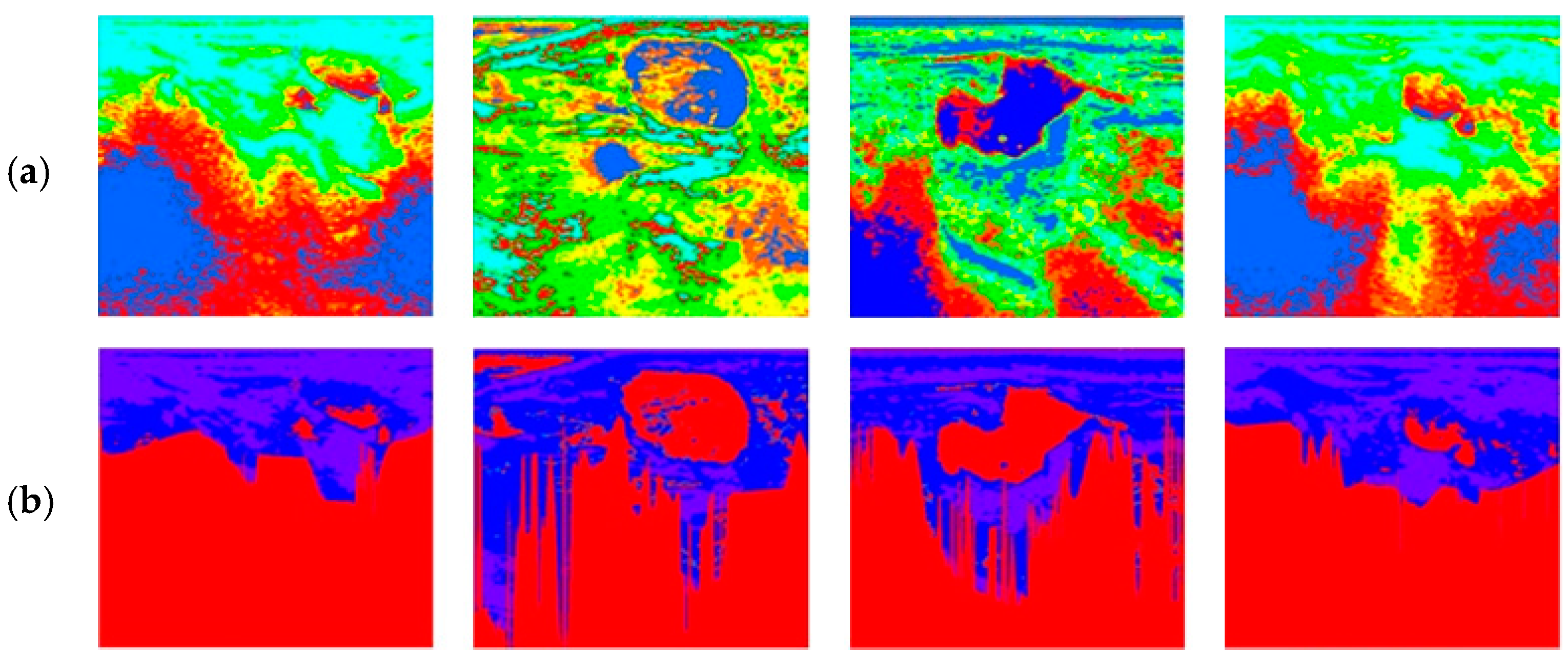
| R1 | If I-L is L and I-U is L, then W is A |
| R2 | If I-L is L and I-U is M, then W is A |
| R3 | If I-L is M and I-U is L, then W is A |
| R4 | If I-L is M and I-U is H, then W is C |
| R5 | If I-L is H and I-U is M, then W is C |
| R6 | If I-L is H and I-U is H, then W is C |
| Method | ART2 | FCM | ||
|---|---|---|---|---|
| # of Images | Vigilance Parameter | # of Clusters | Weight | # of Initial Clusters |
| 90 | 0.1 | 16 | 2 | 10 |
| Method | ART2 [23] | FCM |
|---|---|---|
| Correct | 80 | 86 |
| Incorrect | 10 | 4 |
| Accuracy (%) | 88.9 | 95.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.J.; Song, D.H.; Kim, K.B.; Park, H.J. Efficient Fuzzy Image Stretching for Automatic Ganglion Cyst Extraction Using Fuzzy C-Means Quantization. Appl. Sci. 2021, 11, 12094. https://doi.org/10.3390/app112412094
Lee SJ, Song DH, Kim KB, Park HJ. Efficient Fuzzy Image Stretching for Automatic Ganglion Cyst Extraction Using Fuzzy C-Means Quantization. Applied Sciences. 2021; 11(24):12094. https://doi.org/10.3390/app112412094
Chicago/Turabian StyleLee, Sun Joo, Doo Heon Song, Kwang Baek Kim, and Hyun Jun Park. 2021. "Efficient Fuzzy Image Stretching for Automatic Ganglion Cyst Extraction Using Fuzzy C-Means Quantization" Applied Sciences 11, no. 24: 12094. https://doi.org/10.3390/app112412094
APA StyleLee, S. J., Song, D. H., Kim, K. B., & Park, H. J. (2021). Efficient Fuzzy Image Stretching for Automatic Ganglion Cyst Extraction Using Fuzzy C-Means Quantization. Applied Sciences, 11(24), 12094. https://doi.org/10.3390/app112412094







