Experimental Investigation on the Effects of Ethanol-Enhanced Steam Injection Remediation in Nitrobenzene-Contaminated Heterogeneous Aquifers
Abstract
:1. Introduction
2. Theoretical Background
2.1. Marangoni Effect
2.2. Alcohol-and Heat-Enhanced Marangoni Convection
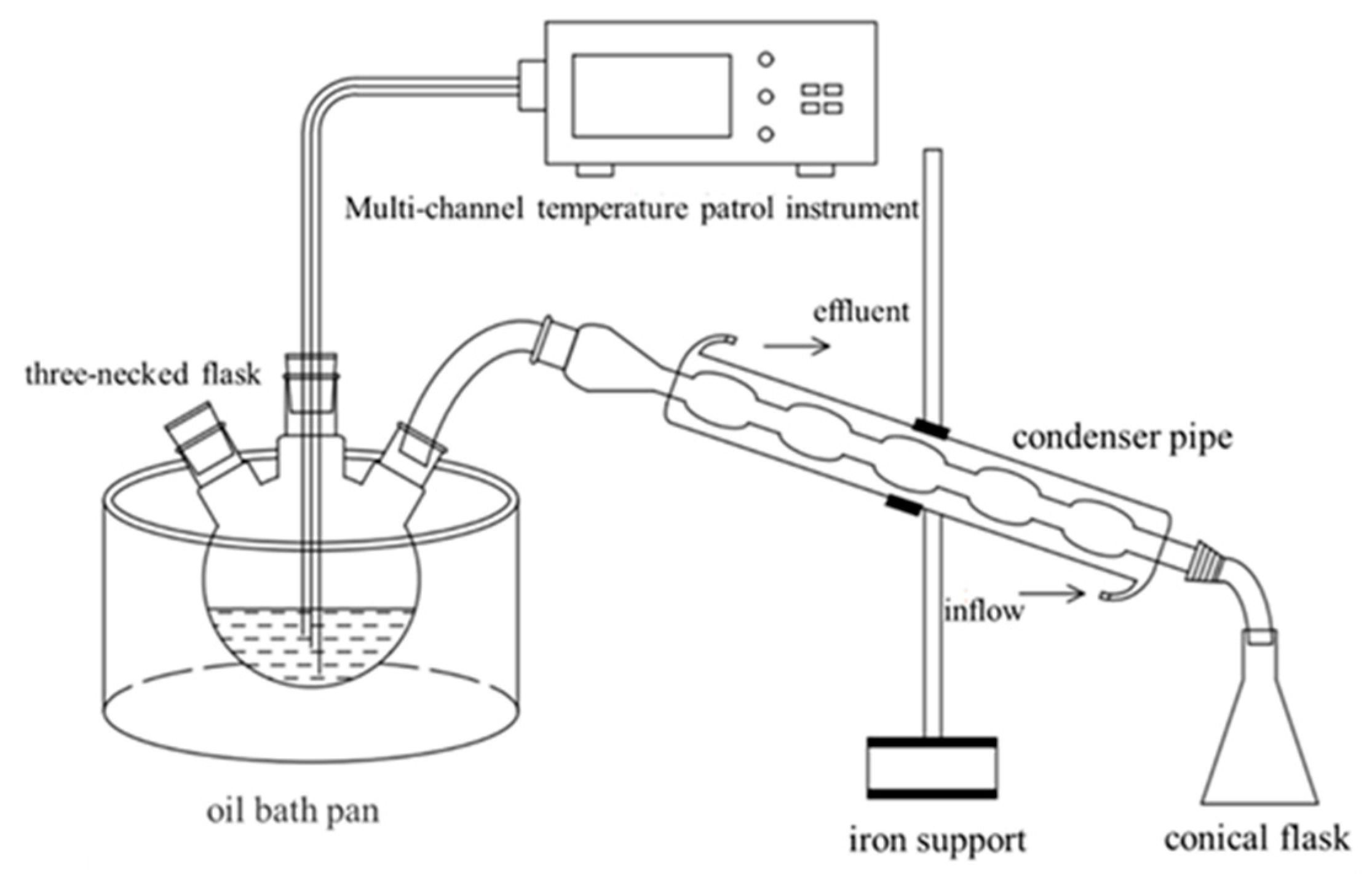
3. Materials and Methods
3.1. Materials
3.2. Experimental Methods
3.2.1. Surface Tension
3.2.2. Effect of Alcohol on Azeotropy
3.2.3. Steam Remediation of Nitrobenzene-Contaminated Heterogeneous Aquifer
3.2.4. Ethanol-Enhanced Steam Remediation of Nitrobenzene-Contaminated Layered Heterogeneous Aquifers
4. Results and Discussion
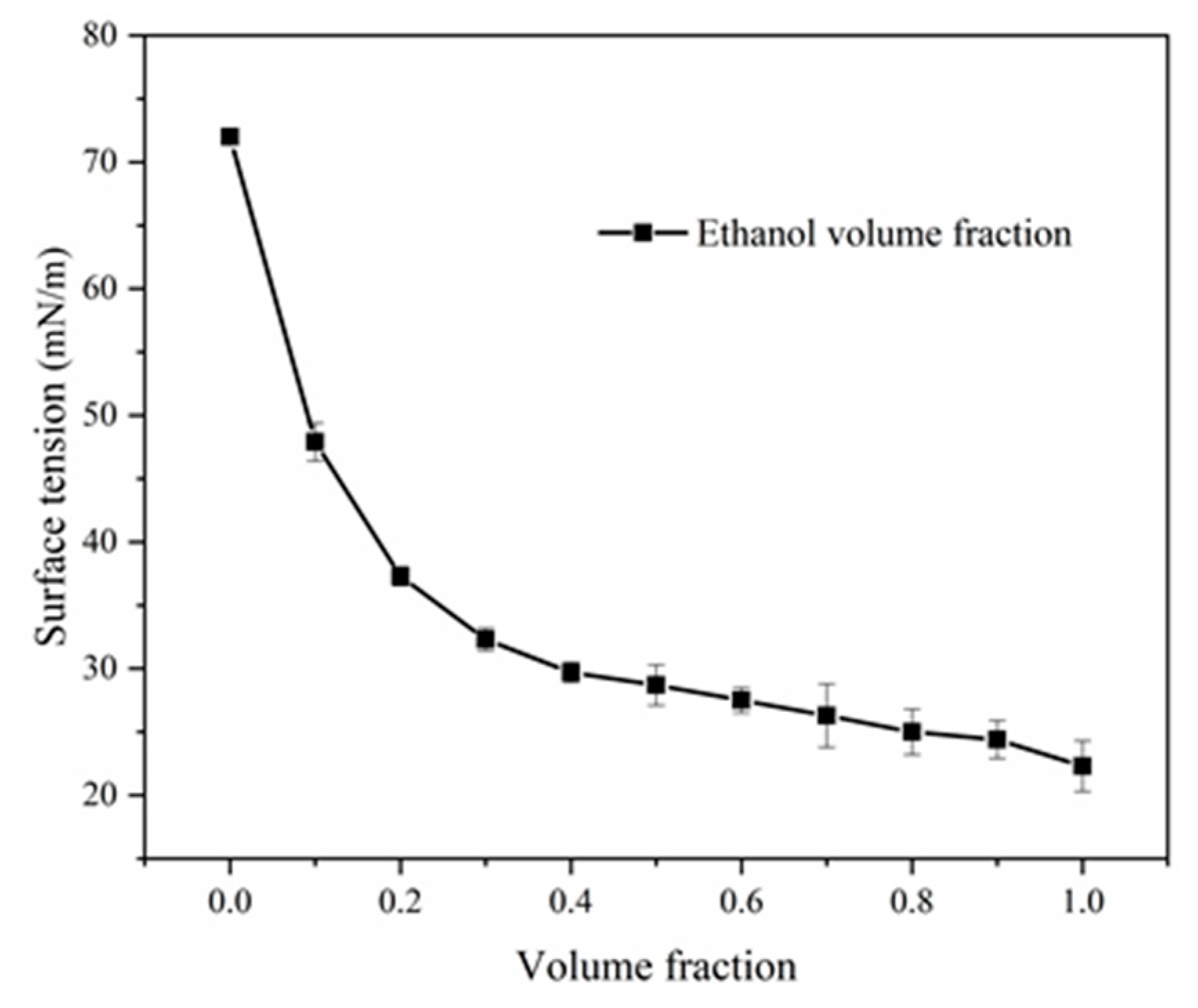
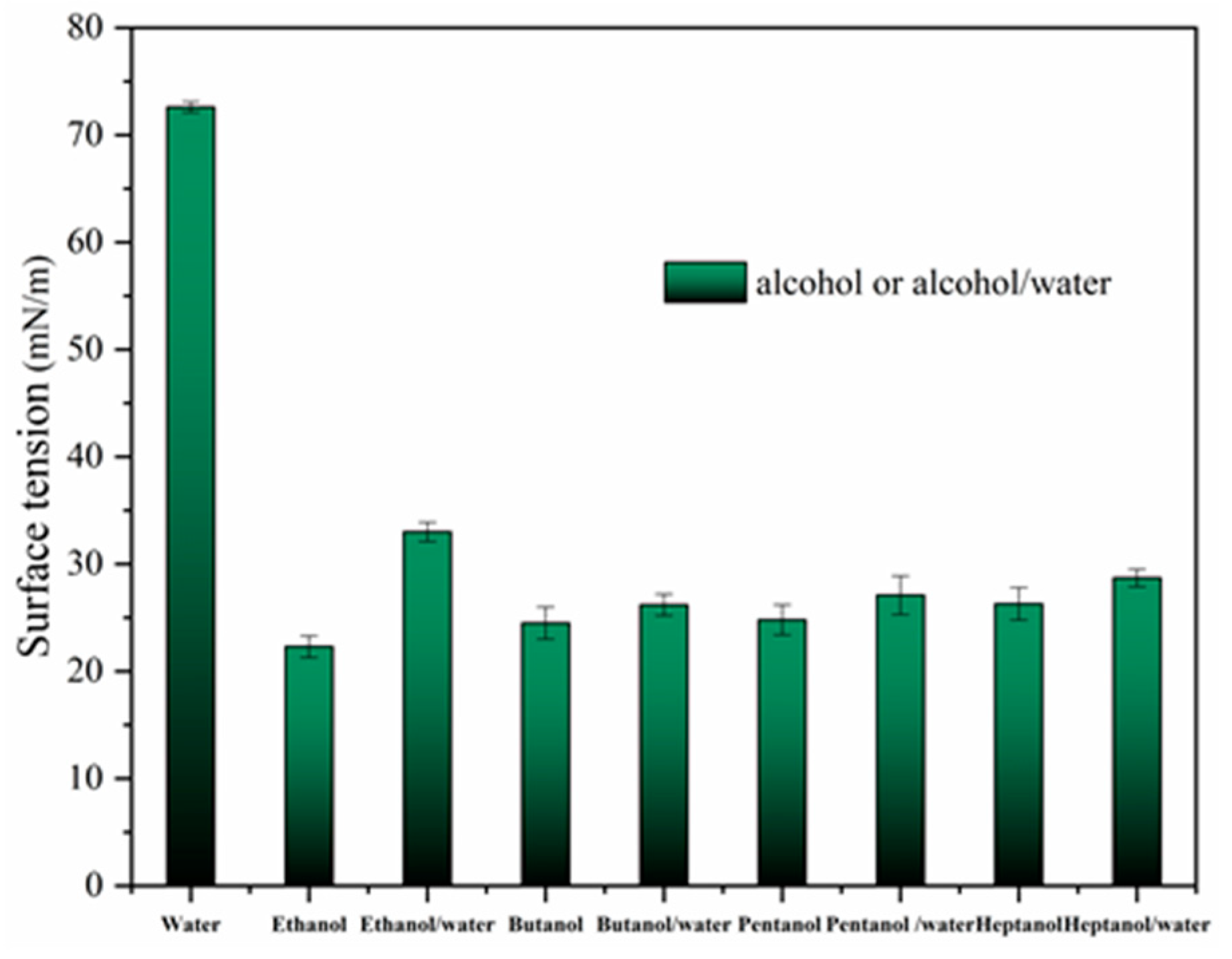
4.1. Effect of Alcohol on Surface Tension
4.2. Effect of Alcohol on Azeotrope
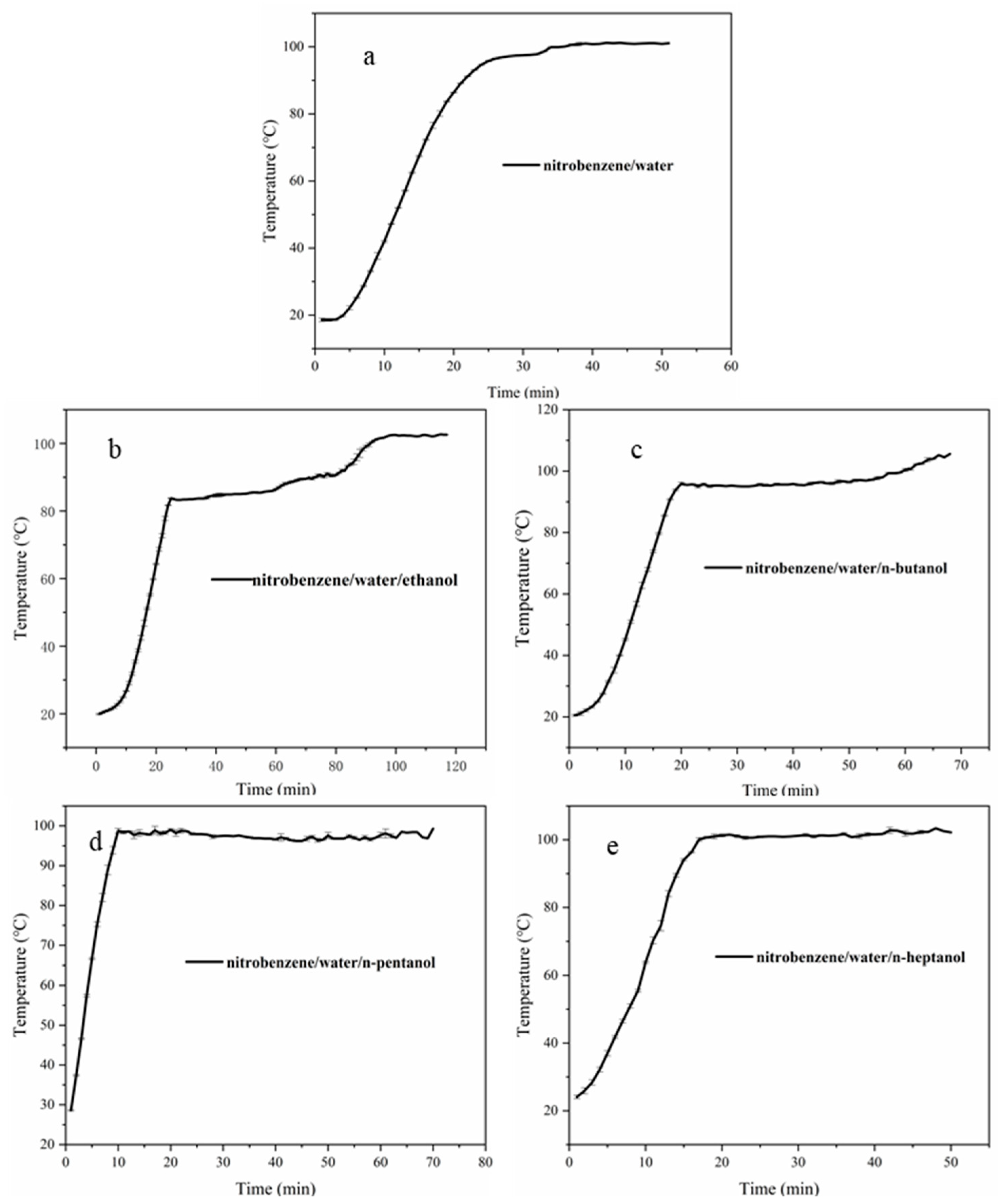
4.2.1. Azeotropic Temperature of Binary Mixture
4.2.2. Azeotropic Temperature of Ternary Mixture
4.3. Steam Remediation of Heterogeneous Aquifers Contaminated by Nitrobenzene
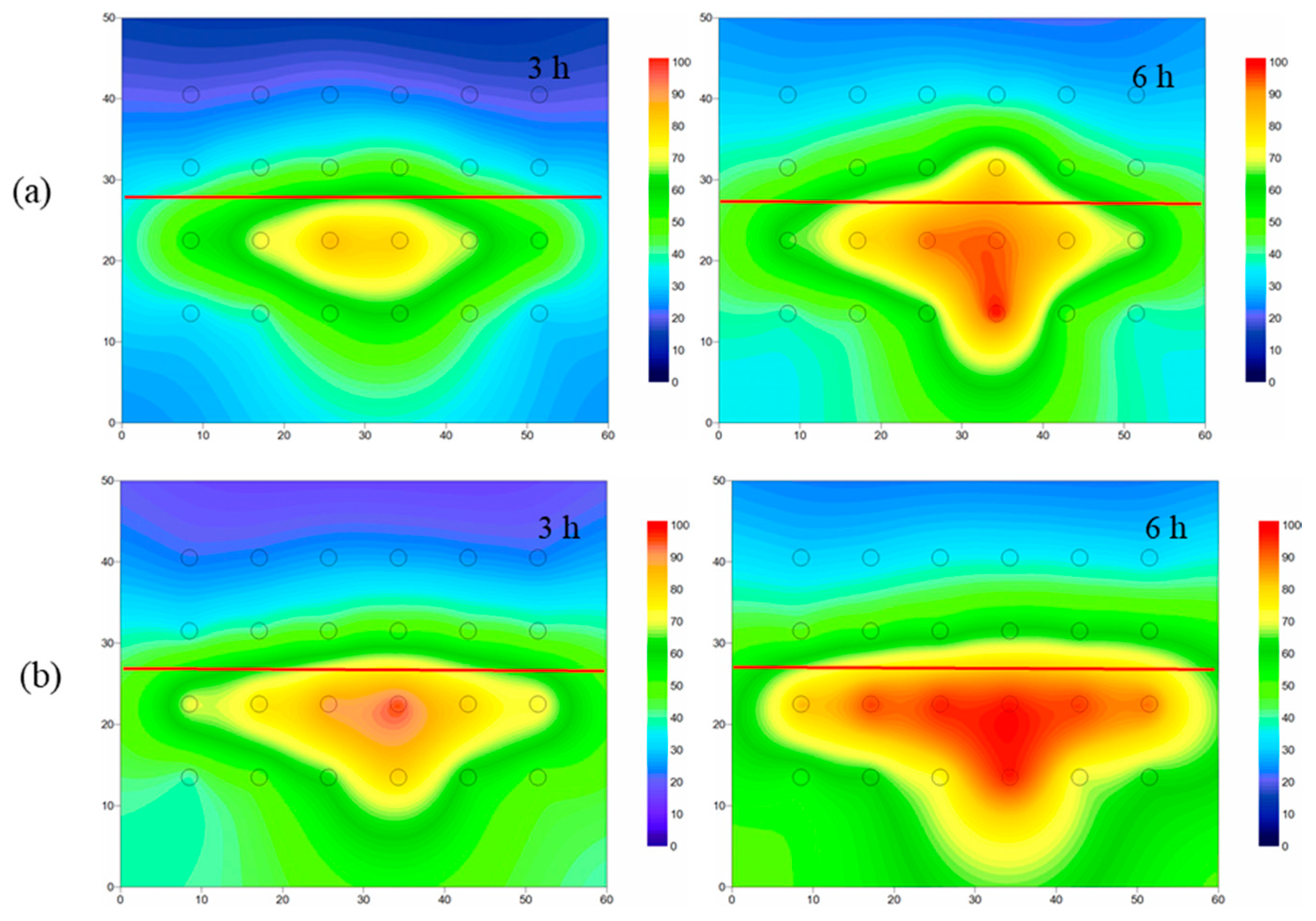
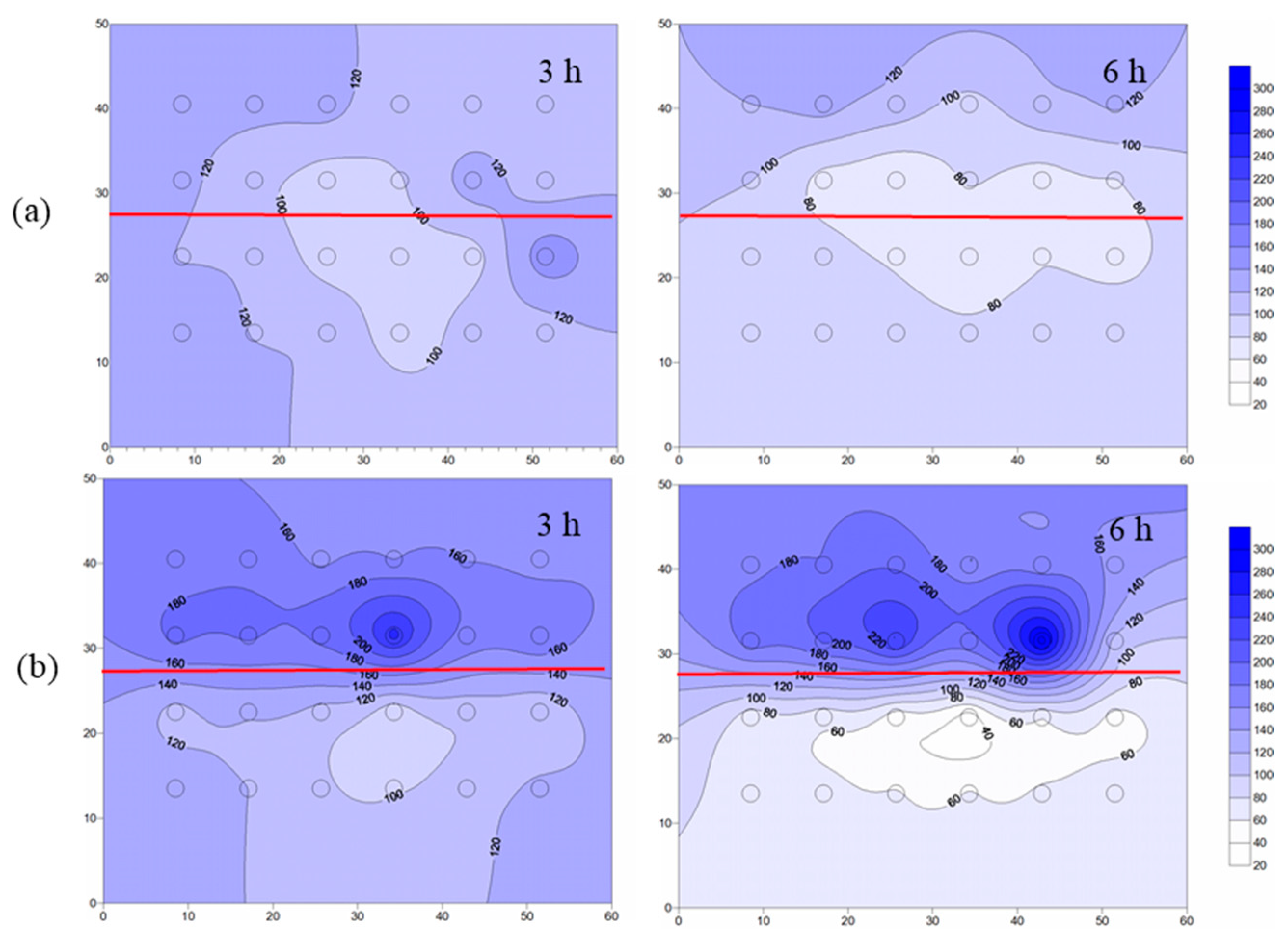
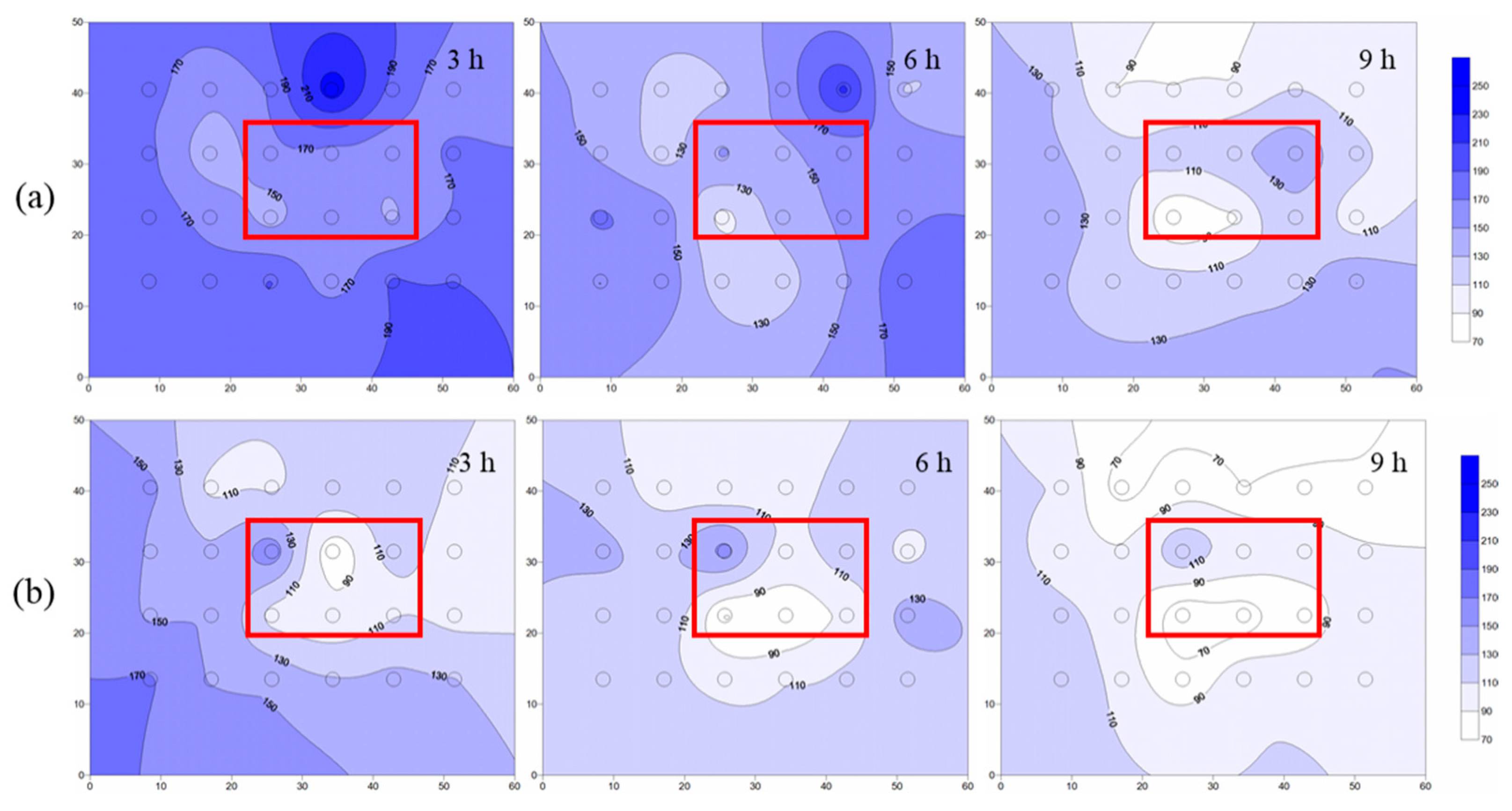
4.3.1. Temperature Distribution of Layered Heterogeneous Aquifer
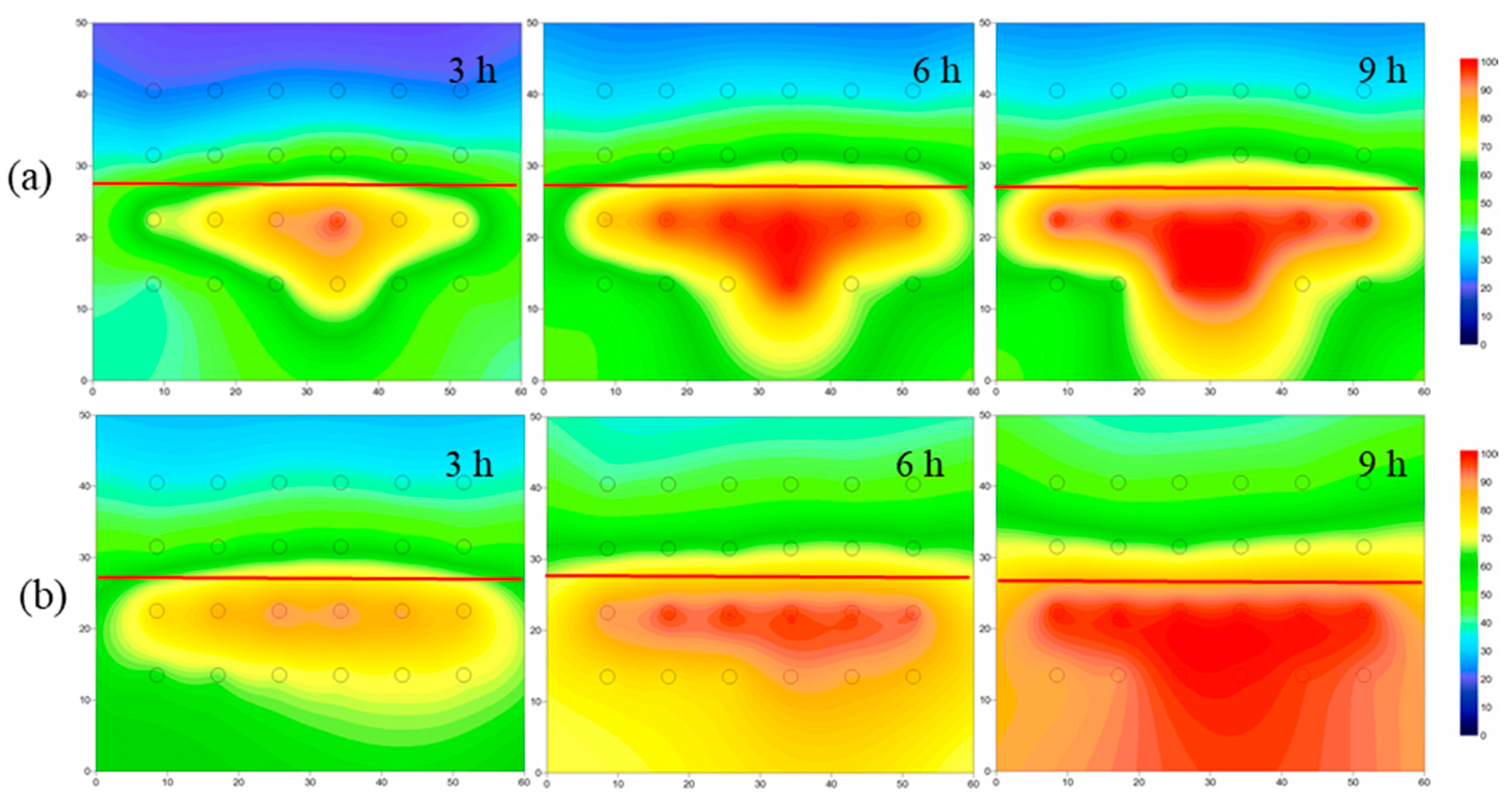
4.3.2. Nitrobenzene Removal in 2-D Layered Heterogeneous Porous Media
4.3.3. Alcohol-Enhanced Steam Remediation of Layered Heterogeneous Aquifer Contaminated by Nitrobenzene
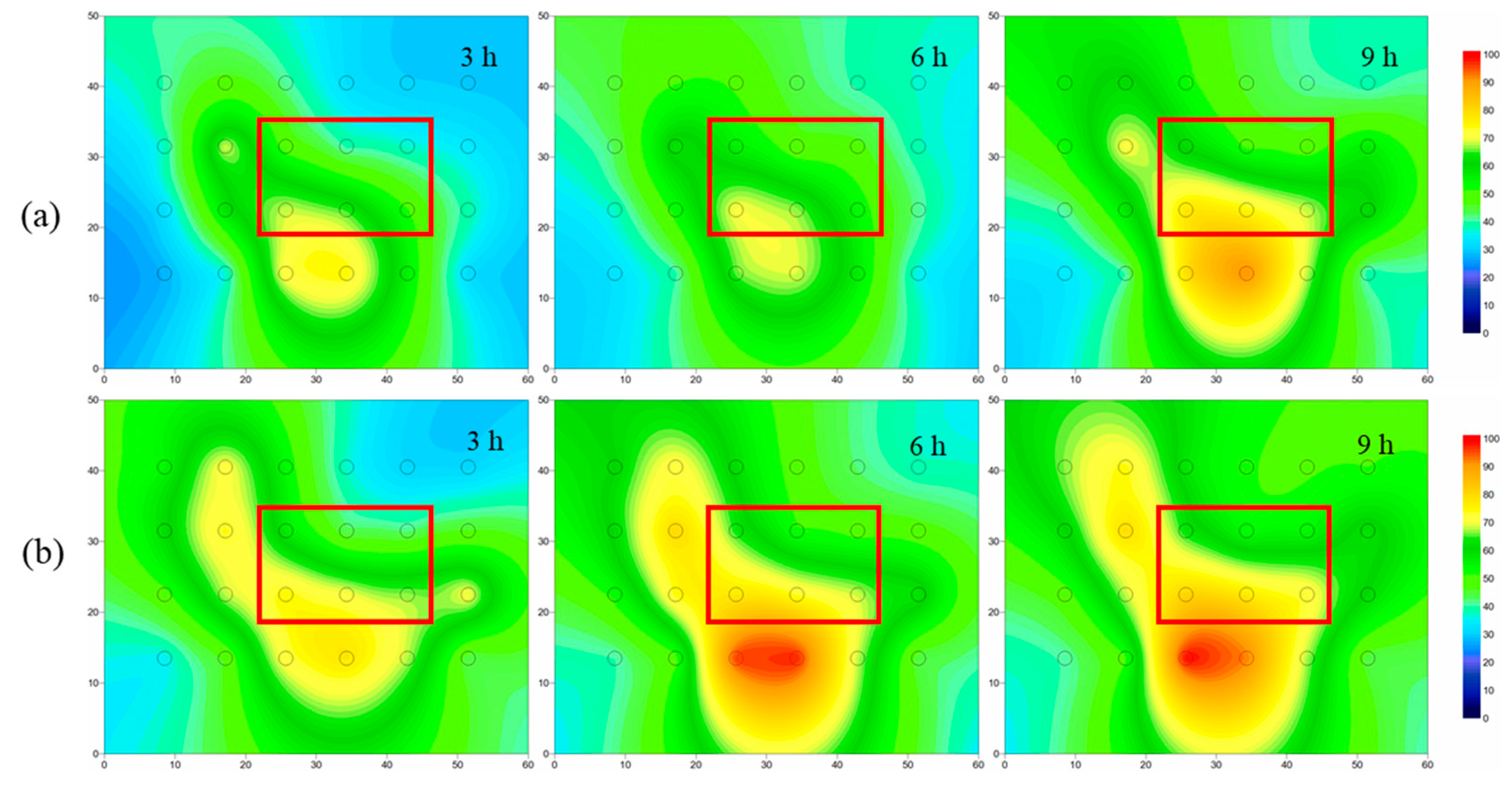
4.3.4. Alcohol-Enhanced Steam Remediation of Lens Heterogeneous Aquifer Contaminated with NB
5. Conclusions
- (1)
- The four alcohols reduced the surface tension of the solution from 72 mN/m to less than 30 mN/m; however, among the four alcohols, only ethanol reduced the azeotropic temperature of NB by 15 °C. Therefore, ethanol can be used as a remediation agent to enhance the steam remediation of heterogeneous aquifers.
- (2)
- When steam entered the layered heterogeneous aquifer, the interface between the low-and high-permeability strata blocked the steam transmission, resulting in poor remediation effect of the low-permeability stratum. After adding ethanol, the average temperature increased, and the area affected by steam increased by 13%, which proved that ethanol addition increased the steam transmission. Moreover, the removal effect of NB was significantly improved, and the average concentration of NB in the simulation tank decreased by 51%.
- (3)
- In the lens heterogeneous aquifer, steam flowed around the lens, forming a preferential flow that resulted in the reduced removal of NB from the lens. After adding ethanol, the steam-affected area increased by 14%, the removal rate of NB increased by 10%, and the NB concentration in the lens decreased by 58%.
- (4)
- Ethanol-enhanced steam injection remediation of heterogeneous aquifers not only enhances the remediation effect and increases the remediation range, but also reduces the remediation costs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Acknowledgments
Conflicts of Interest
References
- Al Yousef, Z.; AlDaif, H.; Al Otaibi, M. An overview of steam injection project in Fractured Carbonate Reservoirs in the Middle East. J. Pet. Sci. Res. 2014, 3, 101–110. [Google Scholar] [CrossRef]
- Davis, E.L. Steam injection for soil and aquifer remediation. Environ. Prot. Agency 1998, 505, 1–16. [Google Scholar]
- Udell, K.S. Application of in situ thermal remediation technologies for DNAPL removal. Groundw. Qual. Remediat. Prot. 1998, 250, 367–374. [Google Scholar]
- Gudbjerg, J.; Sonnenborg, T.O.; Jensen, K.H. Remediation of NAPL below the water table by steam-induced heat conduction. J. Contam. Hydrol. 2004, 72, 207–225. [Google Scholar] [CrossRef]
- Basel, M.D.; Udell, K.S. Effect of Heterogeneities on the Shape of Condensation Fronts in Porous Media; American Society of Mechanical Engineers: New York, NY, USA, 1991; pp. 63–70. [Google Scholar]
- Schmidt, R.; Gudbjerg, J.; Sonnenborg, T.O.; Jensen, K.H. Removal of NAPLs from the unsaturated zone using steam Prevention. J. Contam. Hydrol. 2002, 55, 233–260. [Google Scholar] [CrossRef]
- Heron, G.; Christensen, T.H.; Enfield, C.G. Henry’s law constant for trichloroethylene between 10 and 95 °C. Environ. Sci. Technol. 1998, 32, 1433–1437. [Google Scholar] [CrossRef]
- Schwardt, A.; Dahmke, A.; Köber, R. Henry’s law constants of volatile organic compounds between 0 and 95 °C—Data compilation and complementation in context of urban temperature increases of the subsurface. Chemosphere 2021, 272, 129858. [Google Scholar] [CrossRef]
- Sleep, B.E.; McClure, P.D. The effect of temperature on adsorption of organic compounds to soils. Can. Geotech. J. 2001, 38, 46–52. [Google Scholar] [CrossRef]
- Philippe, N.; Davarzani, H.; Colombano, S.; Dierick, M.; Klein, P.-Y.; Marcoux, M. Experimental study of the temperature effect on two-phase flow properties in highly permeable porous media: Application to the remediation of dense non-aqueous phase liquids (DNAPLs) in polluted soil. Adv. Water Resour. 2020, 146, 103783. [Google Scholar] [CrossRef]
- Tzovolou, D.N.; Aggelopoulos, C.A.; Theodoropoulou, M.A.; Tsakiroglou, C.D. Remediation of the unsaturated zone of NAPL-polluted low permeability soils with steam injection: An experimental study. J. Soils Sediments 2010, 11, 72–81. [Google Scholar] [CrossRef]
- Heron, G.; Vanzutphen, M.; Christensen, H.T.; Enfield, G.C. Soil heating for enhanced remediation of chlorinated solvents_a laboratory study on resistive heating and vapor extraction in a silty, low-permeable soil contaminated with trichloroethylene. Environ. Sci. Technol. 1998, 32, 1474–1481. [Google Scholar] [CrossRef]
- Ochs, S.O.; Class, H.; Färber, A.; Helmig, R. Methods for predicting the spreading of steam below the water table during subsurface remediation. Water Resour. Res. 2010, 46, 1–16. [Google Scholar] [CrossRef]
- Class, H.; Helmig, R. Numerical simulation of non-isothermal multiphase multicomponent processes in porous media. 2. Applications for the injection of steam and air. Adv. Water Resour. 2002, 25, 551–564. [Google Scholar] [CrossRef]
- Nilsson, B.; Tzovolou, D.; Jeczalik, M.; Kasela, T.; Slack, W.; Klint, K.E.; Haeseler, F.; Tsakiroglou, C.D. Combining steam injection with hydraulic fracturing for the in situ remediation of the unsaturated zone of a fractured soil polluted by jet fuel. J. Environ. Manage. 2011, 92, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Akladiss, N.; Hoey, R.; Brandon, B.; Nalipinski, M.; Carroll, S.; Herom, G.; Novakowski, K.; Udell, K. Steam Enhanced Remediation Research for DNAPL in Fractured Rock. EPA 2005, 10, 1–190. [Google Scholar]
- Baker, R.S.; Heron, G. In-Situ Delivery of Heat by Thermal Conduction and Steam Injection for Improved Dnapl Remediation. In Proceedings of the 4th International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, 24–27 May 2004; Battelle Press: Monterey, CA, USA, 2004. [Google Scholar]
- Heron, G.; Carroll, S.; Nielsen, S.G. Full-Scale Removal of DNAPL Constituents Using Steam-Enhanced Extraction and Electrical Resistance Heating. Ground Water Monit. Remediat. 2005, 25, 92–107. [Google Scholar] [CrossRef]
- Chao, H.P.; Hsieh, L.C.; Tran, H.N. Increase in volatilization of organic compounds using air sparging through addition in alcohol in a soil-water system. J. Hazard. Mater. 2018, 344, 942–949. [Google Scholar] [CrossRef]
- Chang, Y.; Yao, M.; Bai, J.; Zhao, Y. Study on the effects of alcohol-enhanced air sparging remediation in a benzene-contaminated aquifer: A new insight. Environ. Sci. Pollut. Res. Int. 2019, 26, 35140–35150. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Azizan, N.A.; Kamaruddin, S.A.; Chelliapan, S.; Mohd Jaini, Z.; Yunus, R.; Rahmat, S.N. Steam-Enhanced Extraction Experiments, Simulations and Field Studies for Dense Non-Aqueous Phase Liquid Removal: A Review. MATEC Web Conf. 2016, 47, 05012. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Herold, K.E. Surface tension of pure water and aqueous lithium bromide with 2-ethyl-hexanol. Appl. Therm. Eng. 2001, 21, 881–897. [Google Scholar] [CrossRef]
- Biscay, F.; Ghoufi, A.; Malfreyt, P. Surface tension of water-alcohol mixtures from Monte Carlo simulations. J. Chem. Phys. 2011, 134, 044709. [Google Scholar] [CrossRef]
- Moraveji, M.K.; Sajjadi, B.; Davarnejad, R. Gas-Liquid Hydrodynamics and Mass Transfer in Aqueous Alcohol Solutions in a Split-Cylinder Airlift Reactor. Chem. Eng. Technol. 2011, 34, 465–474. [Google Scholar] [CrossRef]
- Chen, F.; Freedman, D.L.; Falta, R.W.; Murdoch, L.C. Henry’s law constants of chlorinated solvents at elevated temperatures. Chemosphere 2012, 86, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Koproch, N.; Dahmke, A.; Kober, R. The aqueous solubility of common organic groundwater contaminants as a function of temperature between 5 and 70 °C. Chemosphere 2019, 217, 166–175. [Google Scholar] [CrossRef]
- Komninos, N.P.; Rogdakis, E.D. Geometrical investigation and classification of three-suffix margules binary mixtures including single and double azeotropy. Fluid Phase Equilib. 2019, 494, 212–227. [Google Scholar] [CrossRef]
- Maalem, Y.; Zarfa, A.; Tamene, Y.; Fedali, S.; Madani, H. Prediction of thermodynamic properties of the ternary azeotropic mixtures. Fluid Phase Equilib. 2020, 517, 112613. [Google Scholar] [CrossRef]
- Imaishi, N.; Suzuki, Y.; Hozawa, M.; Fujinawa, K. Interfacial turbulence in gas-liquid mass transfer. Int. Chem. Eng. 1982, 22, 659–665. [Google Scholar] [CrossRef]
- Marangoni, C. Ueber die Ausbreitung der Tropfen einer Flüssigkeit auf der Oberfläche einer anderen. Ann. Der Phys. Und Chem. 1871, 219, 337–354. [Google Scholar] [CrossRef] [Green Version]
- Bénard, H. Les Tourbillons Cellulaires—Dans une Nappe Liquide Propageant de la Chaleur par Convection, en Régime Permanent; Thèse Présentée à la Faculté des Sciences de Paris; Par, M., Bénard, H., Eds.; Gauthier-Villars: Paris, France, 1901. [Google Scholar]
- Rayleigh, L. LIX. On convection currents in a horizontal layer of fluid, when the higher temperature is on the under side. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1916, 32, 529–546. [Google Scholar] [CrossRef] [Green Version]
- Mao, Z.-S.; Chen, J. Numerical simulation of the Marangoni effect on mass transfer to single slowly moving drops in the liquid–liquid system. Chem. Eng. Sci. 2004, 59, 1815–1828. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, G.; Li, H.Z. Note on the mechanism of interfacial mass transfer of absorption processes. Int. J. Heat Mass Transf. 2005, 48, 3454–3460. [Google Scholar] [CrossRef]
- Bodenschatz, E.; de Bruyn, J.R.; Ahlers, G.; Cannell, D.S. Transitions between patterns in thermal convection. Phys. Rev. Lett. 1991, 67, 3078–3081. [Google Scholar] [CrossRef]
- Vidal, A.; Acrivos, A. Effect of Nonlinear Temperature Profiles on Onset of Convection Driven by Surface Tension Gradients. Ind. Eng. Chem. Fundam. 1968, 7, 53–58. [Google Scholar] [CrossRef]
- McTaggart, C.L. Convection driven by concentration- and temperature-dependent surface tension. J. Fluid Mech. 1983, 134, 301–310. [Google Scholar] [CrossRef]
- Buffone, C.; Sefiane, K.; Easson, W. Marangoni-driven instabilities of an evaporating liquid-vapor interface. Phys. Rev. E 2005, 71, 056302. [Google Scholar] [CrossRef]
- Sha, Y.; Chen, H.; Yin, Y.; Tu, S.; Ye, L.; Zheng, Y. Characteristics of the Marangoni Convection Induced in Initial Quiescent Water. Ind. Eng. Chem. Res. 2010, 49, 8770–8777. [Google Scholar] [CrossRef]
- Liu, D.; Tran, T. Vapor-Induced Attraction of Floating Droplets. J. Phys. Chem. Lett. 2018, 9, 4771–4775. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, A.; Gambaryan-Roisman, T.; Stephan, P. Marangoni convection and heat transfer in thin liquid films on heated walls with topography: Experiments and numerical study. Phys. Fluids 2005, 17, 062106. [Google Scholar] [CrossRef]
- Zhang, J.; Oron, A.; Behringer, R.P. Novel pattern forming states for Marangoni convection in volatile binary liquids. Phys. Fluids 2011, 23, 072102. [Google Scholar] [CrossRef]


| Alcohols | Purity | Manufacturer |
|---|---|---|
| Ethanol | AR | Tianjin Huihang Chemical Technology Co. Ltd. |
| N-butanol | Guangdong Guanghua Technology Co. Ltd. | |
| N-pentanol | Beijing Chemical Plant Co. Ltd. | |
| N-heptanol | Beijing Chemical Plant Co. Ltd. |
| Ethanol: Water | Ethanol Volume Fraction | Ethanol (mL) | Water (mL) |
|---|---|---|---|
| 1 | 1 | 40 | — |
| 9:1 | 0.9 | 36 | 4 |
| 8:2 | 0.8 | 32 | 8 |
| 7:3 | 0.7 | 28 | 12 |
| 6:4 | 0.6 | 24 | 16 |
| 5:5 | 0.5 | 20 | 20 |
| 4:6 | 0.4 | 16 | 24 |
| 3:7 | 0.3 | 12 | 28 |
| 2:8 | 0.2 | 8 | 32 |
| 1:9 | 0.1 | 4 | 36 |
| 0 | 0 | — | 40 |
| Test No. | Reagent Composition | Reagent Addition |
|---|---|---|
| 1 | water + NB | 100 mL + 30 mL |
| 2 | water + NB + ethyl alcohol | 25 mL + 30 mL + 100 mL |
| 3 | water + NB + n-butyl alcohol | 40 mL + 30 mL + 80 mL |
| 4 | water + NB + n-amyl alcohol | 60 mL + 30 mL + 60 mL |
| 5 | water + NB + n-heptanol | 90 mL + 30 mL + 30 mL |
| Soil Type | Particle Size (mm) | Hydraulic Conductivity K (cm/s) |
|---|---|---|
| Gravel | 3–5 | 6.1 × 10−1 |
| Coarse sand | 0.4–0.85 | 6.5 × 10−2 |
| Medium sand | 0.2–0.4 | 3.5 × 10−2 |
| Fine sand | 0.1–0.2 | 1.6 × 10−3 |
| Test No. | Packing Configuration | R | |
|---|---|---|---|
| 1 | Upper layer | Medium sand | 17.4 |
| Lower layer | Gravel | ||
| 2 | Upper layer | Fine sand | 381 |
| Lower layer | Gravel |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Yang, X.; Xie, J.; Li, X.; Zhao, Y. Experimental Investigation on the Effects of Ethanol-Enhanced Steam Injection Remediation in Nitrobenzene-Contaminated Heterogeneous Aquifers. Appl. Sci. 2021, 11, 12029. https://doi.org/10.3390/app112412029
Liu R, Yang X, Xie J, Li X, Zhao Y. Experimental Investigation on the Effects of Ethanol-Enhanced Steam Injection Remediation in Nitrobenzene-Contaminated Heterogeneous Aquifers. Applied Sciences. 2021; 11(24):12029. https://doi.org/10.3390/app112412029
Chicago/Turabian StyleLiu, Ruxue, Xinru Yang, Jiayin Xie, Xiaoyu Li, and Yongsheng Zhao. 2021. "Experimental Investigation on the Effects of Ethanol-Enhanced Steam Injection Remediation in Nitrobenzene-Contaminated Heterogeneous Aquifers" Applied Sciences 11, no. 24: 12029. https://doi.org/10.3390/app112412029
APA StyleLiu, R., Yang, X., Xie, J., Li, X., & Zhao, Y. (2021). Experimental Investigation on the Effects of Ethanol-Enhanced Steam Injection Remediation in Nitrobenzene-Contaminated Heterogeneous Aquifers. Applied Sciences, 11(24), 12029. https://doi.org/10.3390/app112412029





