Abstract
(1) Background: Considerable evidence indicates that the occurrence of preeclampsia (PE) is associated with a reduced vitamin D (VD) level. Several studies have found that VD deficiency is correlated with disturbed trophoblast invasion, reduced angiogenesis and increased vasoconstriction. Because the vitamin D receptor (VDR) and CYP27B1 and CYP2R1 hydrolases are strongly involved in VD metabolism, the goal of the present study was to evaluate their genes and proteins expression in the placentas from preeclamptic women. (2) Methods: Samples and clinical data were obtained from 100 Polish women (41 women with preeclampsia and 59 healthy pregnant controls). The whole PE group was divided into subgroups according to gestation week of pregnancy ending before and after 34 gestational weeks (early/late-onset preeclampsia (EOPE/LOPE)). However, finally, to reduce confounding by differences in gestational age, the EOPE group was excluded from the analysis of mRNA and protein placental expression, and we focus on the comparison between LOPE and control groups. The placental VDR, CYP27B1 and CYP2R1 mRNA expression was analyzed using RT-PCR, and placental protein levels were determined by ELISA assay. (3) Results. (3.1) Placental gene expression: Expression levels of both genes, CYP27B1 (1.17 vs. 1.05 in controls, p = 0.006) and CYP2R1 (2.01 vs. 1.89 in controls, p = 0.039), were significantly higher in preeclamptic placentas than in the control group. Interestingly, VDR expression was significantly lower in placentas from the PE group (1.15 vs. 1.20 in controls, p = 0.030). After dividing all preeclamptic women into subgroups only for the CYP27B1 gene, a significantly higher placental expression in the LOPE subgroup than the healthy controls was observed (padj = 0.038). (3.2) Placental protein expression: The results revealed that protein expression levels of CYP27B1 in the preeclamptic group were similar (5.32 vs. 5.23 in controls, p = 0.530). There was a significant difference in median VDR and CYP2R1 protein levels between studied groups (VDR: 2.56 vs. 3.32 in controls, p < 0.001; CYP2R1: 1.32 vs. 1.43 in controls, p = 0.019). After stratification of preeclamptic women into subgroups, a significant difference was observed only in the VDR protein level. The medians in the LOPE subgroups were significantly lower compared to the healthy control group. In the whole study group, the placental VDR protein level was inversely correlated with systolic and diastolic blood pressure (all p < 0.001), and positively correlated with gestational age (p < 0.001) and infant birth weight (p = 0.014). (4) Conclusions: Lower mRNA and protein expression of VDR in preeclamptic placentas, and also VDR protein expression, could play a pivotal role in preeclampsia development. Additionally, the higher mRNA expression of both CYP27B1 and CYP2R1 hydrolase genes in placentas from preeclamptic women could indicate the compensatory role of these enzymes in preeclampsia etiology. Our results also indicate that placental VDR protein level could be one of the factors modulating blood pressure in pregnant women, as well as influencing gestational age and infant birth weight. Considering the importance of these findings, future studies are warranted.
1. Introduction
Preeclampsia (PE) is a specific disease in pregnancy which can have serious implications, being a leading cause of fetal and maternal morbidity and mortality [1,2]. According to a World Health Organization (WHO) systematic analysis performed in 2014, hypertensive disorders in pregnancy were the second cause of maternal death after hemorrhage, accounting for 14% of such cases worldwide [3]. In mothers with acute renal and liver dysfunction, pulmonary edema and brain edema could be observed [4].
The etiology of preeclampsia is complex and not fully understood. Currently, it is assumed that this disorder is based on processes that lead to a disturbed process of placentation [5]. Many studies have shown that the occurrence of PE is associated with a reduced vitamin D (VD) level [6]. Moreover, most scientific societies recommend VD supplementation during pregnancy due to its widespread deficiency in the population of pregnant women [7,8,9] and the potential relationship of VD deficiency with occurrence of obstetric complications [10,11].
In the placenta, especially in the syncytiotrophoblast, the expression of genes related to the vitamin D pathway, such as 25-hydroxylase (CYP2R1), 1α-hydroxylase (CYP27B1), and 24-hydroxylase (CYP24A1), has been described, which guarantees efficient VD metabolism in pregnant women. These hydroxylases play an important role in the conversion of cholecalciferol to calcitriol, which is the active form of vitamin D (1,25(OH)2D3, 1,25-D3). Alterations in expression and activity of hydroxylases could disturb the circulating VD level. The plasma concentration of calcitriol increases 2–3 times in pregnant women compared to the level before pregnancy, starting at 10–12 weeks of gestation and continuing until the end of pregnancy [12]. The VD augmentation is independent of the parathyroid hormone, the level of which remains unchanged throughout pregnancy [13].
In humans, vitamin D manifests a pleiotropic function and acts on target organs, specifically binding to the vitamin D receptor (VDR). The important action of vitamin D in pregnancy has a combined influence in both mother and fetus. Experimental studies have shown that both the 1,25(OH)2D3 form and its precursor 25(OH)D increase trophoblast invasion in vitro [14]. In the human syncytiotrophoblast, calcitriol in an autocrine manner regulates the synthesis of human chorionic gonadotropin (hCG) [15], human placental lactogen (hPL) [16], estradiol, and progesterone [17].
The vitamin D receptor (VDR) belongs to a family of nuclear steroid receptors and, as a nuclear transcription factor, binds to the retinoid X receptor (RXR). This complex recognizes the vitamin D responsive elements (VDREs) in the DNA promoter sequence of genes regulated directly by vitamin D [18]. The expression of VDR is influenced by co-activators and co-repressors, which enhance or inhibit gene expression, respectively. Co-receptors bind to the VDR and block its activity in the absence of a ligand. According to the multipotential action of vitamin D, even VDRs have been located in many tissues of the human body [19,20].
It was demonstrated conclusively that the proper VD level during pregnancy and appropriate placental expression of genes linked to the VD pathway are crucial for the growth and function of the placenta [21]. On the other hand, several studies have shown that vitamin D deficiency could be strongly associated with preeclampsia development [22,23,24,25]. This fact may be related to changes in VDR and hydrolase placental expression, which disturb VD metabolism in preeclamptic women. Additionally, the abnormal trophoblast invasion in early pregnancy and disturbing placental angiogenesis are connected with the development of PE. Considering the above observations, we hypothesized that expression of VDR and several hydrolase genes, as well as proteins, in preeclamptic placentas would differ from those in physiological pregnancies [5]. Thus, the goal of the present study was to evaluate the VDR, CYP27B1 and CYP2R1 gene and protein expression levels in the placentas from preeclamptic women.
2. Materials and Methods
2.1. Patients
Samples and clinical data were obtained from 100 women (41 women with preeclampsia and 59 controls) living in west-central Poland. All women were patients at the Division of Perinatology and Women’s Diseases, Poznan University of Medical Sciences, between December 2018 and March 2021, where they underwent obstetric examination and had a hospital delivery. Informed consent was obtained from each mother before delivery, and the study protocol was approved by the Local Bioethical Committee at Poznan University of Medical Sciences (No. 1129/18). Preeclampsia was defined, according to ACOG criteria, as systolic ≥ 140 mmHg and diastolic ≥ 90 mmHg blood pressure confirmed by two measurements, with other signs of disease [26]. All patients were with singleton pregnancy, of Polish origin. Women with chronic hypertension, diabetes mellitus, renal or endocrinological disease, multiple pregnancy, age younger than 19 or older than 35, as well as habitual smokers, were excluded from the study. Healthy, pregnant, normotensive women were enrolled in the control group. The whole group with preeclampsia was divided into subgroups according to gestation week of pregnancy ending before and after gestation week 34 (early/late-onset PE (EOPE/LOPE)) [26,27,28]. However, finally, to reduce confounding by differences in gestational age, the EOPE group was excluded from the analysis of mRNA and protein placental expression, and we focused on the comparison between LOPE and control groups.
The fragments of the placenta were obtained immediately after vaginal delivery or caesarean section. Samples approximately 1 cm3 in size were obtained from the maternal side in order to obtain villous cytotrophoblasts and decidua (central and marginal part of placental disc). Following removal of the maternal and fetal surfaces, the sample was washed twice in cold PBS (phosphate-buffered saline), placed in liquid nitrogen, and transported to the laboratory for storage at −80 °C.
2.2. RNA Extraction and cDNA Synthesis
Total cellular RNA was isolated from the placenta tissue using TriPure Isolation Reagent (Roche, Basel, Switzerland) according to the manufacturer’s protocol. The concentrations and the purity of RNA were determined using a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). RNA samples were stored at −80 °C. Complementary DNA was synthesized from 2 µg of total RNA in a total volume of 20 µL using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). The obtained transcripts were stored at −20 °C or used directly for the real-time quantitative PCR (RT-PCR).
2.3. Real-Time PCR
The level of mRNA expression was analyzed using the RT-PCR method. All primer sequences were synthesized by TIB Molbiol (TIB Molbiol GmbH, Berlin, Germany) and are summarized in Table 1. Amplicon size and reaction specificity were confirmed by agarose gel electrophoresis and melting curve analysis. RT-PCR was carried out using a LightCycler 96 Instrument (Roche, Basel, Switzerland) and a LightCycler 96 SYBR Green I Master (Roche, Basel, Switzerland) according to the manufacturer’s protocol. GAPDH was used as a housekeeping gene for normalization (endogenous internal standard). The PCR program was initiated with activation at 95 °C for 10 min. Each PCR cycle comprised a denaturation step at 95 °C, an annealing step at a specific temperature, and an extension step at 72 °C. The quantitative PCR was monitored by measuring the increase in fluorescence by the binding of SYBR Green I dye to the generated double-stranded cDNA. All samples were run in duplicate using the LightCycler 96 Instrument, and the melting curves were analyzed using the LightCycler 96 Basic Software.

Table 1.
Sequences of primers used for real-time PCR.
2.4. Enzyme-Linked Immunosorbent Assay
The Human VDR (Vitamin D3 receptor) ELISA Kit (sensitivity: 0.375 ng/mL; AssayGenie, Dublin, Ireland), Human 25-hydroxyvitamin D-1 alpha hydroxylase (CYP27B1) ELISA Kit (sensitivity: 0.33 ng/mL; MyBioSource, San Diego, CA, USA), and Human Vitamin D 25-Hydroxylase (CYP2R1) ELISA Kit (sensitivity: 0.1 ng/mL; MyBioSource, San Diego, CA, USA) were employed to evaluate the concentrations of VDR, CYP27B1 and CYP2R1 from placenta tissue homogenates according to the manufacturers’ protocols. The reaction was blocked, and the absorbance was measured on a microplate reader (Infinite 200, TECAN, Männedorf, Switzerland). The concentrations of VDR, CYP27B1 and CYP2R1 were determined by interpolation of the standard curve using linear regression analysis.
2.5. Statistical Analysis
All statistical analyses were carried out using R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria, accessed on 12 October 2021) [33] and the ggstatsplot package [34]. The normal distribution of the data was tested using the Shapiro–Wilk test. Quantitative variables with Gaussian distribution were expressed as means ± standard deviation (SD), and in the absence of normal distribution as median and interquartile range. Clinical characteristics between groups were compared using Student’s t-test for normally distributed data and Fisher’s test for nominal variables. The Mann–Whitney U test was used for nonparametric gene and protein expression data. The correlation analyses were performed using the Spearman rank method. Holm method adjusted p-values of <0.05 were considered statistically significant.
3. Results
3.1. Clinical Characteristics
The baseline clinical characteristics of 41 preeclamptic women and 59 healthy controls are summarized in Table 2. The women of both study groups were of similar age (mean ± SD was 30.73 ± 5.00 in PE women vs. 30.98 ± 4.54 years in controls, p = 0.7988). Mean values of maternal blood pressure and pre- and post-pregnancy BMI were higher in the group of women with preeclampsia than in the control group. The gestational age was significantly lower in the group of women with PE (33.78 ± 3.24 vs. 39.02 ± 1.05 gestation weeks in controls, p < 0.001). In the group of women with preeclampsia, all pregnancies ended with caesarean births, while there was no statistically significant difference in the prevalence of primiparous women compared to the control group (p = 0.2223). Mean birth weight, Apgar score, and placenta weight of infants from mothers with PE were significantly lower than in normotensive mothers. Twenty women (48.78%) from the study group developed preeclampsia before 34 weeks of gestation (EOPE), and in 21 women (51.22%) after 34 weeks of pregnancy (LOPE). The mean gestational age at delivery was 38.29 ± 1.31 in LOPE and 29.30 ± 1.72 weeks in the EOPE group (p < 0.001). Comparing the gestational age at delivery between controls and PE subgroups, it differed significantly for EOPE, (p < 0.001) but not for the LOPE group (p = 0.068).

Table 2.
Basic characteristics of preeclampsia cases and normotensive controls.
Blood chemistry tests were performed only in the PE group. When comparing their results for groups with EOPE and LOPE, statistically significant differences were observed for proteinuria (mean 24-h protein measurements: 6.07 in EOPE vs. 2.82 g/24 h in LOPE, p = 0.031, and in the single sample: 353.68 in EOPE vs. 163.69 mg/dL in LOPE, p = 0.002, respectively).
3.2. Placental Gene Expression
The mRNA expression of VDR, CYP27B1 and CYP2R1 was studied in 100 human placentas using quantitative real-time PCR. The expression levels of both genes—CYP27B1 (1.17 vs. 1.05 in controls, p = 0.006) and CYP2R1 (2.01 vs. 1.89 in controls, p = 0.039)—were significantly higher in preeclamptic placentas compared to the control group. Interestingly, VDR expression was significantly lower in placentas from the PE group (1.15 vs. 1.20 in controls, p = 0.030) (Table 3). After dividing all preeclamptic women into subgroups according to delivery before and after the 34th week of gestation, significantly higher placental expression in the LOPE subgroup compared to healthy controls was observed only for the CYP27B1 gene (p = 0.012) (Figure 1).

Table 3.
Expression of mRNA VDR, CYP27B1 and CYP2R1 in placentas of preeclamptic and healthy women.
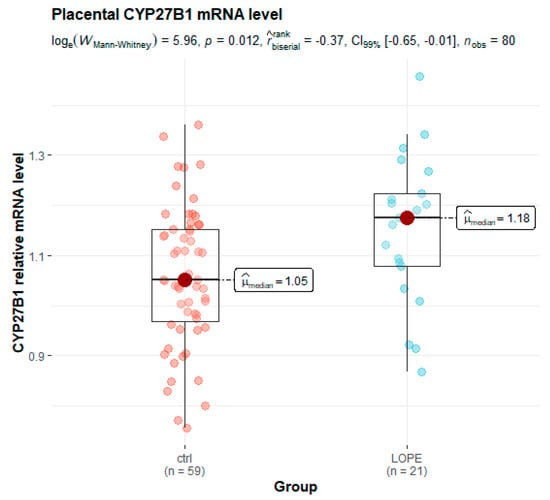
Figure 1.
Box and whisker plot of placental relative levels of CYP27B1 mRNA in the late-onset preeclampsia (LOPE) subgroup and healthy controls.
3.3. Placental Protein Expression
Concentrations of placental proteins were measured using enzyme-linked immunosorbent assay. The results (Table 4) revealed that protein expression levels of CYP27B1 in the preeclamptic group were similar (5.32 vs. 5.23 in controls, p = 0.530). There was a significant difference in median VDR and CYP2R1 protein levels between studied groups (VDR: 2.56 vs. 3.32 in controls, p < 0.001; CYP2R1: 1.32 vs. 1.43 in controls, p = 0.019). After stratification of preeclamptic women into early- and late-onset subgroups, statistically significant differences were observed only in the VDR protein level. The medians (25th, 75th percentile) were as follows: 2.56 (1.90–2.77) ng/mL in the LOPE, and 3.32 (2.78–3.86) in the controls (Figure 2).

Table 4.
Concentrations of VDR, CYP27B1 and CYP2R1 protein from placenta tissue homogenates (ELISA).
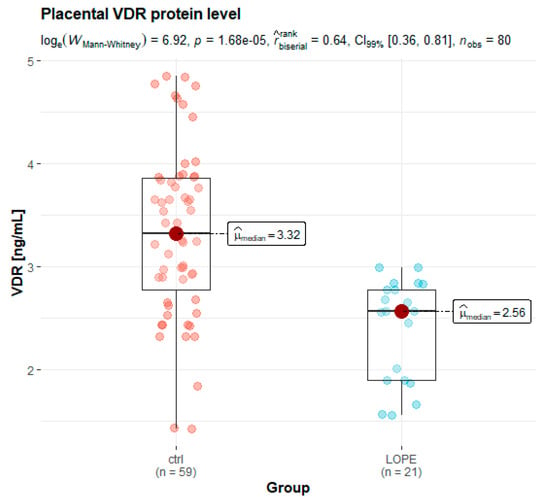
Figure 2.
Box and whisker plots of placental levels of VDR protein in the LOPE group and healthy controls.
3.4. Correlations Analysis between Placental Gene/Protein Expression and Clinical Parameters
Correlation analysis was conducted in order to identify associations between expression levels of studied placental genes and proteins. In the total 100 analyzed women, positive correlations were found for mRNA and protein levels of all studied genes. The highest correlation was observed between CYP27B1 mRNA and CYP2R1 mRNA (rho = 0.61, 95% CI: 0.47–0.72, p < 0.001), and the lowest between CYP2R1 mRNA and VDR protein levels (rho = 0.07, 95% CI: 0.13–0.27, p = 0.493).
In the group of women with PE, the most statistically significant correlations were found between the VDR mRNA level and CYP2R1 mRNA (p < 0.001), VDR mRNA (p = 0.029), and CYP27B1 protein (p < 0.001). Moreover, in this group, CYP2R1 mRNA positively correlated with CYP2R1 protein (p = 0.017) and CYP27B1 mRNA (p < 0.001).
In the controls, CYP27B1 mRNA significantly correlated with CYP2R1 mRNA (p = 0.005), VDR mRNA (p = 0.013) and VDR protein (p < 0.001). Moreover, VDR mRNA was correlated with VDR and CYP27B1 proteins (p = 0.025 and 0.014, respectively), and for CYP2R1, the protein level correlated with mRNA (p < 0.001). More detailed information on the correlations between protein and mRNA levels of the studied genes in the preeclamptic women and healthy controls is presented in Figure 3.
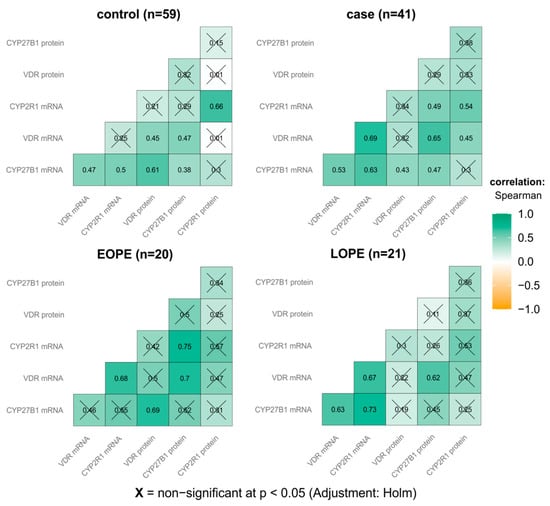
Figure 3.
Correlation matrix of placental VDR, CYP27B1 and CYP2R1 mRNA and protein levels in preeclamptic women and healthy controls (inside each group).
The correlation was also analyzed between gene and protein expression levels and various mother and offspring clinical factors. In the whole study group, placental VDR protein level was inversely correlated with systolic and diastolic blood pressure (all p < 0.001) and positively with gestational age (p < 0.001) and infant birth weight (p = 0.014).
4. Discussion
The vitamin D endocrine system plays a pivotal role in the control of bone turnover and calcium hemostasis, as well as modulation of the immunological system [35]. From this point of view it is not surprising that vitamin D is linked to many diseases, including diabetes, cardiovascular disease, and cancer, osteoarthritis, and neurodegenerative disorders [36,37,38]. Recently it has been reported that vitamin D may be involved in hypertensive disturbances during pregnancy. Subsequently, several studies showed that PE is characterized by changes in the systemic and placental metabolism of vitamin D in comparison with physiological pregnancies [39,40]. Moreover, preeclampsia was shown to be associated with decreased activation, increased catabolism, and impaired placental uptake of vitamin D [41]. These findings are supported by demonstration of VDR and RXR alpha expression in trophoblastic villi, decidua and smooth muscle cells of the placental vessels [42]. The presence of VDR expression on placental smooth muscle cells could indicate that VD is also involved in remodeling of placental vessels, such as growth and migration of vascular smooth muscle cells [43].
4.1. VDR mRNA Expression
In our study we analyzed VDR, CYP27B1 and CYP2R1 genes involved in vitamin D metabolism. The most interesting observation was the lower VDR mRNA expression (p = 0.030) in placentas of preeclamptic women. This finding was maintained after stratification of the preeclamptic group into early- and late-onset subgroups, both of which differed significantly from the control group.
It was also noteworthy that in placentas of the PE group higher mRNA expression of CYP27B1 (p = 0.006) and CYP2R1 (p = 0.039) genes compared to the healthy pregnant women was observed. In addition, a reduced placental level of CYP2R1 protein in the PE group (CYP2R1 median 1.32 vs. 1.43 in controls, p = 0.019) compared to healthy women was noted. This difference was also observed after dividing the whole PE group into early- and late-onset preeclampsia.
Numerous studies have assessed the gene expression in placenta from preeclamptic women. Our results are consistent with several previous analyses performed in women with preeclampsia and fetal growth restriction (FGR).
In an interesting study, Ma et al. analyzed the expression of proteins related to VD metabolism—vitamin D binding protein (VDBP), 25-hydroxylase (CYP2R1), 1α-hydroxylase (CYP27B1), 24-hydroxylase (CYP24A1), and vitamin D receptor (VDR)—in placenta of preeclamptic women and healthy pregnant controls. The expression of VDR and CYP2R1 proteins was lower, while CYP27B1 and CYP24A1 were higher, in the group of women with PE. The authors explain these inconsistent results as an effect of oxidative stress, which could have disturbed the expression of genes related to VD metabolism in the placenta of women with PE [21].
Less encouraging results were obtained in a study analyzing both methylation and expression of genes related to VD metabolism: VDR, CYP27B1, and RXR. Hypermethylation of DNA for VDR, CYP27B1 and RXR was observed in the placenta of women with PE, assuming that hypermethylation reduces gene expression. Only decreased expression of RXR was observed in the group of preeclamptic women, without statistically significant differences for other proteins [44].
In contrast to the above-mentioned studies, Xiao et al. observed higher placenta VDR mRNA expression and lower VD maternal concentration in women with preeclampsia compared to healthy controls. The authors stated that this may be due to the placental compensation mechanism in conditions of vitamin D deficiency [45].
No less exciting results were obtained in pregnant women with FGR, which is a condition associated with impaired invasion of the placenta and repeatedly occurs in preeclampsia. In the study by Nguyen et al., the VDR mRNA expression in placenta was reduced in FGR pregnancies compared to the healthy control group. In addition, this study also attempted to assess the influence of VDR mRNA expression on the trophoblast function. For this purpose, BeWo cell lines were used, where VDR mRNA inactivation led to increased levels of markers for cell differentiation, adhesion and apoptosis, and in this way to uncontrolled premature maturation and trophoblast apoptosis [46]. Further studies by Nguyen et al. showed that decreased VDR mRNA expression in the placenta may lead to disturbed expression of genes regulating the cell cycle and directly lead to placenta insufficiency with FGR and PE development [47]. Furthermore, it was found that vitamin D deficiency in the mother may be connected with lower VDR mRNA expression in the placenta in FGR and PE women, which could be associated with the trophoblast persistence capacity in these disorders [48].
4.2. VDR Protein Level in Placentas
Second interesting observation was the lower VDR protein level (p < 0.001) in placentas of preeclamptic women. Numerous studies have assessed the protein level of VDR and hydrolases involved in the VD pathway in placenta from preeclamptic women. Our obtained results are consistent with several previous analyses performed in women with preeclampsia and fetal growth restriction (FGR). In the study by Nguyen et al., the VDR protein level in placenta was reduced in FGR pregnancies compared to the healthy control group [46].
Moreover, in our research was to find in the whole studied group a positive correlation between placental VDR protein level and gestational age (p < 0.001) as well as infant birth weight (p = 0.014). This issue has been discussed in several aspects in recent analysis.
It is also noteworthy that in our whole study group the VDR protein level in placentas was inversely correlated with systolic and diastolic blood pressures (all p < 0.001). In animal studies it was proposed that vitamin D serum level is involved in the pathogenesis of cardiovascular diseases as well as arterial hypertension. In correlation with the blood pressure level, vitamin D shows antihypertensive properties through reducing renin-angiotensin-aldosterone system activity and improves vasodilatation [49,50]. Following this information it is believed that the best antihypertensive vitamin D effect is noted in patients with elevated blood pressure and, additionally, VD deficiency [51,52].
Nema et al. in an animal model noted reduced blood pressure after VD supplementation during pregnancy. In addition, the authors observed improvement of placental angiogenesis by normalizing the VEGF and Flt-1 placental levels, without influencing the levels of PlGF and Hif1α [53].
Despite these observations, the human clinical research reveals inconsistent results in this field. Shahid et al. observed a significantly lower VD level in the PE group compared to healthy normotensive women (p < 0.001), as well as a negative correlation of VD level with systolic (p < 0.001) and diastolic blood pressure (p < 0.001) [54]. Zeng et al. reported that low VD level could be a risk factor for early- and late-onset PE occurrence [55]. Interestingly, Forde et al. did not detect any effect of VD intake on maternal blood pressure in healthy pregnant women [56].
Budhwar et al. reported that VD deficiency disturbed homeostasis of the inflammatory response in the placenta, activating the cytokine network and followed by spontaneous premature preterm delivery [57]. At the same time, Dutra et al. observed a correlation between maternal VD levels and VDR gene polymorphisms, as well as the risk of premature birth [58]. No less interesting was the observation that maternal VD serum level was associated with infant birthweight among both white and black women [59,60].
Because the VD metabolism is closely linked with VDR protein activity, it is clear that placental VDR gene and protein expression could play a significant role in regulation of blood pressure in healthy pregnant as well as preeclamptic women [61,62] and have a significant impact on preterm birth occurrence and newborn birthweight.
4.3. Study Limitation
Our study assessed the placental expression of three genes related to the VD pathway—VDR, CYP27B1 and CYP2R1—which could elucidate the significance of disturbances of VD metabolism in preeclamptic women. The presented analysis covers a relatively small sample size of material; however, the patients were carefully enrolled into the study based on the presence of severe preeclampsia, and subsequently divided into early- and late-onset preeclampsia. Moreover, in the performed analysis, the concentration of vitamin D in women with preeclampsia was not determined.
However, on the other hand, the most pivotal results, such as placental expression levels, were correlated with important baseline clinical and laboratory data, which provided important results such as the inverse correlation of placental VDR protein level with systolic and diastolic blood pressure, as well as the positive correlation with gestational age and infant birth weight in the whole studied group.
Unfortunately, we did not have the opportunity to compare mRNA and protein expression in placentas between the EOPE group and a gestational-age-appropriate group of women.
Furthermore, it should be remarked that some additional factors influence VD metabolism, such as other polymorphism variants of candidate genes of VD metabolism, life style, and another environmental agents.
5. Conclusions
To our best knowledge, this is the first study investigating whether the placental expression of genes and proteins involved in vitamin D metabolism is linked to preeclampsia occurrence in Polish women. We found that the disturbed, lower mRNA and protein expression of VDR in preeclamptic placentas, and furthermore, VDR protein expression, could play a pivotal role in preeclampsia development.
Additionally, the higher mRNA expression of both CYP27B1 and CYP2R1 hydrolase genes in placentas from preeclamptic women could indicate the compensatory role of these enzymes in preeclampsia etiology. Our results also indicate that placental VDR protein level could be one of the factors modulating blood pressure in pregnant women, as well as influencing gestational age and infant birth weight. Considering the importance of the obtained results, this analysis merits future studies.
Author Contributions
Conceptualization, J.M.-S. and A.S.-M.; methodology, G.K., A.B. and J.M.-S.; software, G.K.; validation, G.K., A.B., M.W. and J.M.-S.; formal analysis, K.D.; investigation, J.M.-S., A.B. and M.W.; resources, J.M.-S. and M.O.; data curation, T.M.K., H.W. and J.M.-S.; writing—original draft preparation, J.M.-S.; writing—review and editing, M.O. and T.M.K.; visualization, G.K. and J.M.-S.; supervision, A.S.-M.; project administration, A.S.-M., H.W. and K.D.; funding acquisition, K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Local Bioethical Committee at Poznan University of Medical Sciences (Decision No. 1129/18).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Basso, O.; Rasmussen, S.; Weinberg, C.R.; Wilcox, A.J.; Irgens, L.M.; Skjaerven, R. Trends in fetal and infant survival following preeclampsia. JAMA 2006, 296, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Odegård, R.A.; Vatten, L.J.; Nilsen, S.T.; Salvesen, K.A.; Austgulen, R. Preeclampsia and fetal growth. Obstet. Gynecol. 2000, 96, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Metin-Gulmezoglu, A.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Hutcheon, J.A.; Lisonkova, S.; Joseph, K.S. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best. Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 391–403. [Google Scholar] [CrossRef]
- Brosens, I.; Puttemans, P.; Benagiano, G. Placental bed research: I. The placental bed: From spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019, 221, 437–456. [Google Scholar] [CrossRef]
- Akbari, S.; Khodadadi, B.; Ahmadi, S.A.Y.; Abbaszadeh, S.; Shahsavar, F. Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: A systematic review and updated meta-analysis. Taiwan J. Obstet. Gynecol. 2018, 57, 241–247. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2016, 7, CD008873. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, CD008873. [Google Scholar] [CrossRef]
- Amouzegar, A.; Azizi, F.; Ashrafivand, S.; Ahi, Z.; Saleh, M.; Mohaghegh, S.; Gargari, S.S. Prevalence of calcium and vitamin D deficiency and their association with feto-maternal outcomes in a sample of Iranian pregnant women. Hum. Antib. 2020, 28, 305–312. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynecologists. Vitamin D in Pregnancy; Scientific Impact Paper; Royal College of Obstetricians and Gynecologists: London, UK, 2014; pp. 1–11. [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 495: Vitamin D: Screening and Supplementation during Pregnancy. Obstet. Gynecol. 2011, 118, 197–198. [Google Scholar] [CrossRef]
- Weisman, Y. Maternal, fetal and neonatal vitamin D and calcium metabolism during pregnancy and lactation. Vitam. Rickets. 2003, 6, 34–49. [Google Scholar] [CrossRef]
- Díaz, L.; Sánchez, I.; Avila, E.; Halhali, A.; Vilchis, F.; Larrea, F. Identification of a 25-hydroxyvitamin D3 1alpha-hydroxylase gene transcription product in cultures of human syncytiotrophoblast cells. J. Clin. Endocrinol. Metab. 2000, 85, 2543–2549. [Google Scholar] [CrossRef][Green Version]
- Chan, S.Y.; Susarla, R.; Canovas, D.; Vasilopoulou, E.; Ohizua, O.; McCabe, C.J.; Hewison, M.; Kilby, M.D. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta 2015, 36, 403–409. [Google Scholar] [CrossRef]
- Barrera, D.; Avila, E.; Hernández, G.; Méndez, I.; González, L.; Halhali, A.; Larrea, F.; Morale, S.A.; Díaz, L. Calcitriol affects hCG gene transcription in cultured human syncytiotrophoblasts. Reprod. Biol. Endocrinol. 2008, 6, 3. [Google Scholar] [CrossRef]
- Stephanou, A.; Ross, R.; Handwerger, S. Regulation of human placental lactogen expression by 1,25-dihydroxyvitamin D3. Endocrinology 1994, 135, 2651–2656. [Google Scholar] [CrossRef]
- Barrera, D.; Avila, E.; Hernández, G.; Halhali, A.; Biruete, B.; Larrea, F.; Diaz, L. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J. Steroid. Biochem. Mol. Biol. 2007, 103, 529–532. [Google Scholar] [CrossRef]
- Baker, A.R.; McDonnell, D.P.; Hughes, M.; Crisp, T.M.; Mangelsdorf, D.J.; Haussler, M.R.; Pike, J.W.; Shine, J.; O’Malley, B.W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc. Natl. Acad. Sci. USA 1988, 85, 3294–3298. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Norman, A.W. The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci. Signal. 2009, 2, re4. [Google Scholar] [CrossRef]
- Ma, R.; Gu, Y.; Zhao, S.; Sun, J.; Groome, L.J.; Wang, Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am. J. Physiol. Endocrinol. Metab. 2012, 303, 928–935. [Google Scholar] [CrossRef]
- Vestergaard, A.L.; Justesen, S.; Volqvartz, T.; Aagaard, S.K.; Andreasen, M.F.; Lesnikova, I.; Uldbjerg, N.; Larsen, A.; Bor, P. Vitamin D insufficiency among Danish pregnant women-Prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet. Gynecol. Scand. 2021, 100, 480–488. [Google Scholar] [CrossRef]
- Yue, C.Y.; Gao, J.P.; Zhang, C.Y.; Ying, C.M. Is serum vitamin D deficiency before gestational 20 weeks a risk factor for preeclampsia? Clin. Nutr. 2021, 40, 4430–4435. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obstet. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef]
- Magielda-Stola, J.; Drews, K.; Wolski, H.; Seremak-Mrozikiewicz, A. Vitamin D3 and its receptor in selected obstetrical complications. Ginekol. Pol. 2021, 92, 460–465. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists and Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists task force on hypertension in pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Poon, L.C.; Nicolaides, K.H. Early prediction of preeclampsia. Obstet. Gynecol. Int. 2014, 2014, 297397. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Brown, M.A.; Zeeman, G.G.; Dekker, G.; Sibai, B.M. The definition of severe and early-onset preeclampsia. Statements from the international society for the study of hypertension in pregnancy (ISSHP). Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2013, 3, 44–47. [Google Scholar] [CrossRef]
- McCurdy, R.D.; McGrath, J.J.; Mackay-Sim, A. Validation of the comparative quantification method of real-time PCR analysis and a cautionary tale of housekeeping gene selection. Gene Ther. Mol. Biol. 2008, 12, 15–24. [Google Scholar]
- Van der Meijden, K.; van Essen, H.W.; Bloemers, F.W.; Schulten, E.A.; Lips, P.; Bravenboer, N. Regulation of CYP27B1 mRNA expression in primary human osteoblasts. Calcif. Tissue Int. 2016, 99, 164–173. [Google Scholar] [CrossRef]
- Liu, K.; Meng, H.; Hou, J. Activity of 25-hydroxylase in human gingival fibroblasts and periodontal ligament cells. PLoS ONE 2012, 7, e52053. [Google Scholar] [CrossRef]
- Amatoril, S.; Persico, G.; Fanelli, M. Real-time quantitative PCR array to study drug-induced changes of gene expression in tumor cell lines. J. Cancer Metastasis Treat. 2017, 3, 90–99. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Res. Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Khammissa, R.A.G.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The biological activities of vitamin D and its receptor in relation to calcium and bone homeostasis, cancer, immune and cardiovascular systems, skin biology, and oral health. BioMed Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346–353. [Google Scholar] [CrossRef]
- Di Somma, C.; Elisabetta, S.; Luigi, B.; Volha, Z.V.; Silvia, S.; Chiara, M.; Massimo, S.; Gianluca, A.; Annamaria, C.; Paolo, M. Vitamin D and neurological diseases: An endocrine view. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef]
- Annweiler, C.; Llewellyn, D.J.; Beauchet, O. Low serum vitamin D concentrations in Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2013, 33, 659–674. [Google Scholar] [CrossRef]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory effects of vitamin D in pregnancy and beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef]
- Knabl, J.; Vattai, A.; Ye, Y.; Jueckstock, J.; Hutter, S.; Kainer, F.; Mahner, S.; Jeschke, U. Role of placental VDR expression and function in common late pregnancy disorders. Int. J. Mol. Sci. 2017, 18, 2340. [Google Scholar] [CrossRef]
- Tamblyn, J.A.; Susarla, R.; Jenkinson, C.; Jeffery, L.E.; Ohizua, O.; Chun, R.F.; Chan, S.Y.; Kilby, M.D.; Hewison, M. Dysregulation of maternal and placental vitamin D metabolism in preeclampsia. Placenta 2017, 50, 70–77. [Google Scholar] [CrossRef]
- Pospechova, K.; Rozehnal, V.; Stejskalova, L.; Vrzal, R.; Pospisilova, N.; Jamborova, G.; May, K.; Siegmund, W.; Dvorak, Z.; Nachtigal, P.; et al. Expression and activity of vitamin D receptor in the human placenta and in choriocarcinoma BeWo and JEG-3 cell lines. Mol. Cell Endocrinol. 2009, 299, 178–187. [Google Scholar] [CrossRef]
- Rebsamen, M.C.; Sun, J.; Norman, A.W.; Liao, J.K. 1alpha,25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ. Res. 2002, 91, 17–24. [Google Scholar] [CrossRef]
- Anderson, C.M.; Ralph, J.L.; Johnson, L.; Scheett, A.; Wright, M.L.; Taylor, J.Y.; Ohm, J.E.; Uthus, E. First trimester vitamin D status and placental epigenomics in preeclampsia among Northern Plains primiparas. Life Sci. 2015, 129, 10–15. [Google Scholar] [CrossRef]
- Xiao, J.P.; Lv, J.X.; Yin, Y.X.; Jiang, L.; Li, W.S.; Tao, T.; Liao, X.P.; Xu, Z.C. Lower maternal and fetal vitamin D status and higher placental and umbilical vitamin D receptor expression in preeclamptic pregnancies. Int. J. Clin. Exp. Pathol. 2017, 10, 10841–10851. [Google Scholar]
- Nguyen, T.P.; Yong, H.E.; Chollangi, T.; Borg, A.J.; Brennecke, S.P.; Murthi, P. Placental vitamin D receptor expression is decreased in human idiopathic fetal growth restriction. J. Mol. Med. 2015, 93, 795–805. [Google Scholar] [CrossRef]
- Nguyen, T.P.H.; Yong, H.E.J.; Chollangi, T.; Brennecke, S.P.; Fisher, S.J.; Wallace, E.M.; Ebeling, P.R.; Murthi, P. Altered downstream target gene expression of the placental Vitamin D receptor in human idiopathic fetal growth restriction. Cell Cycle 2018, 17, 182–190. [Google Scholar] [CrossRef]
- Hutabarat, M.; Wibowo, N.; Obermayer-Pietsch, B.; Huppertz, B. Impact of vitamin D and vitamin D receptor on the trophoblast survival capacity in preeclampsia. PLoS ONE 2018, 13, e0206725. [Google Scholar] [CrossRef]
- Adamczak, M.; Surma, S.; Więcek, A. Vitamin D and Arterial Hypertension: Facts and Myths. Curr. Hypertens. Rep. 2020, 22, 57. [Google Scholar] [CrossRef]
- McMullan, C.J.; Borgi, L.; Curhan, G.C.; Fisher, N.; Forman, J.P. The effect of vitamin D on renin-angiotensin system activation and blood pressure: A randomized control trial. J. Hypertens. 2017, 35, 822–829. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Ritz, E.; Pieber, T.R. Vitamin D status and arterial hypertension: A systematic review. Nat. Rev. Cardiol. 2009, 6, 621–630. [Google Scholar] [CrossRef]
- Tamez, H.; Kalim, S.; Thadhani, R.I. Does vitamin D modulate blood pressure? Curr. Opin. Nephrol. Hypertens. 2013, 22, 204–209. [Google Scholar] [CrossRef]
- Nema, J.; Sundrani, D.; Joshi, S. Prenatal vitamin D supplementation reduces blood pressure and improves placental angiogenesis in an animal model of preeclampsia. Food Funct. 2020, 11, 10413–10422. [Google Scholar] [CrossRef]
- Shahid, S.; Ladak, A.; Fatima, S.S.; Zaidi, F.A.; Farhat, S. Association of vitamin D levels with preeclampsia. J. Pak. Med. Assoc. 2020, 70, 2390–2393. [Google Scholar] [CrossRef]
- Zeng, S.; Cheng, X.; Chen, R.; Wu, J.; Zhou, J. Low level of vitamin D is a risk factor for the occurrence of early and late onset pre-eclampsia in pregnant women. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Forde, H.; Crowley, R.K.; McKenna, M.J.; Kilbane, M.T.; Conway, M.; McDonnell, C.M.; Twomey, P.J.; McAuliffe, F.M. No effect of calcium and vitamin D intake on maternal blood pressure in a healthy pregnant population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 8–14. [Google Scholar] [CrossRef]
- Budhwar, S.; Verma, P.; Verma, R.; Gupta, S.; Rai, S.; Rajender, S.; Singh, K. Altered cord serum 25-hydroxyvitamin D signaling and placental inflammation is associated with pre-term birth. Am. J. Reprod. Immunol. 2020, 83, e13201. [Google Scholar] [CrossRef]
- Dutra, L.V.; Affonso-Kaufman, F.A.; Cafeo, F.R.; Kassai, M.S.; Barbosa, C.P.; Santos Figueiredo, F.W.; Suano-Souza, F.I.; Bianco, B. Association between vitamin D plasma concentrations and VDR gene variants and the risk of premature birth. BMC Pregnancy Childbirth 2019, 20, 3. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Zmuda, J.M.; Cooper, M.E.; Parrott, M.S.; Roberts, J.M.; Marazita, M.L.; Simhan, H.N. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J. Nutr. 2010, 140, 999–1006. [Google Scholar] [CrossRef]
- Swamy, G.K.; Garrett, M.E.; Miranda, M.L.; Ashley-Koch, A.E. Maternal vitamin D receptor genetic variation contributes to infant birthweight among black mothers. Am. J. Med. Genet. Part A 2011, 155A, 1264–1271. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Shakiba, M.; Rezavand, N.; Rahimi, Z.; Vaisi-Raygani, A.; Rahimi, Z.; Shakiba, E. Gene variants and haplotypes of Vitamin D biosynthesis, transport, and function in preeclampsia. Hypertens. Pregnancy 2021, 40, 1–8. [Google Scholar] [CrossRef]
- Rezavand, N.; Tabarok, S.; Rahimi, Z.; Vaisi-Raygani, A.; Mohammadi, E.; Rahimi, Z. The effect of VDR gene polymorphisms and vitamin D level on blood pressure, risk of preeclampsia, gestational age, and body mass index. J. Cell. Biochem. 2019, 120, 6441–6448. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).