Environmental Impacts and Immobilization Mechanisms of Cadmium, Lead and Zinc in Geotechnical Composites Made from Contaminated Soil and Paper-Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
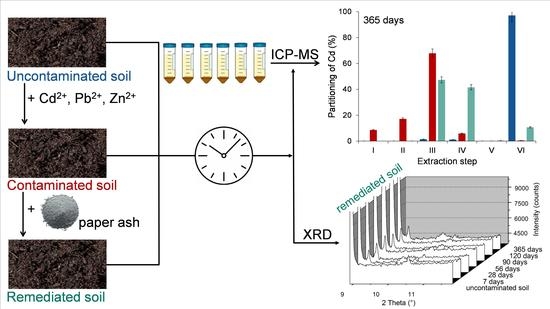
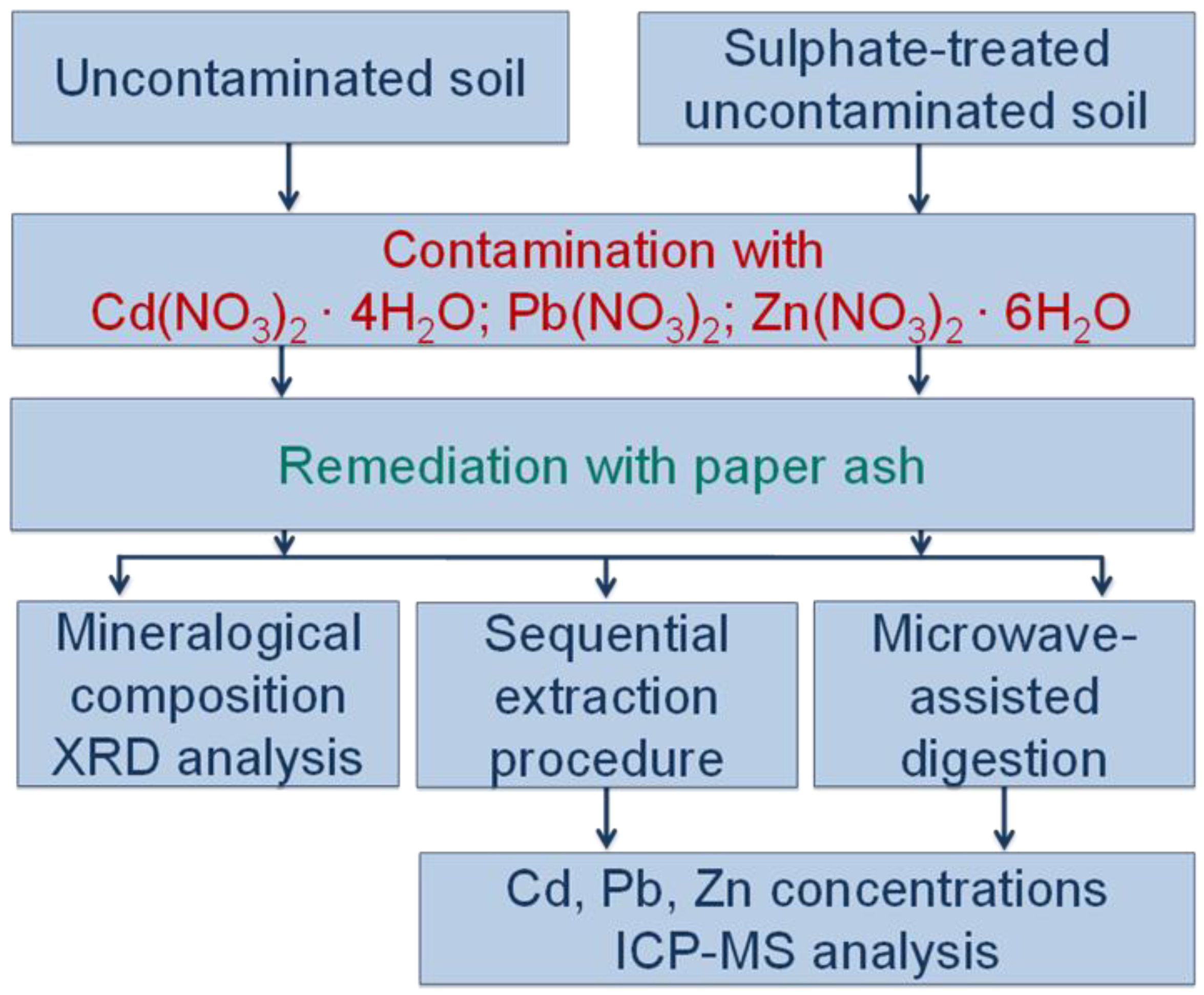
2.2. Experimental Design
2.3. Apparatus
2.4. Determination of the Total Metal Content in the Soil Samples
2.5. Sequential Extraction Procedure
3. Results and Discussion
3.1. Quality Control of the Analytical Data
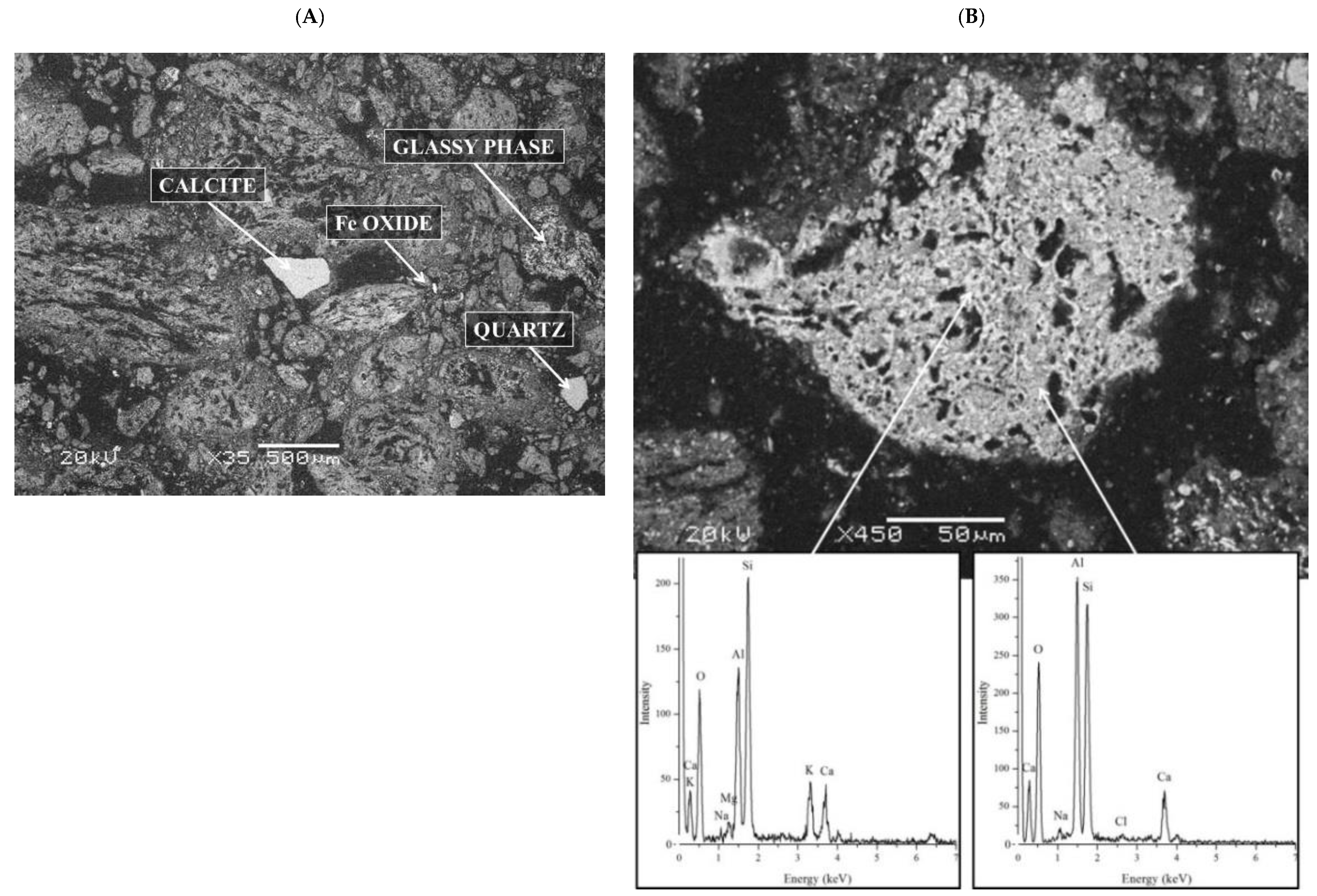
3.2. Characteristics of the Soil and Paper-Ash
3.3. Investigation of the Immobilization Mechanisms, Stability of the Mineral Phases of Cd, Pb, and Zn after the Remediation, and the Environmental Impacts of Soil Remediation
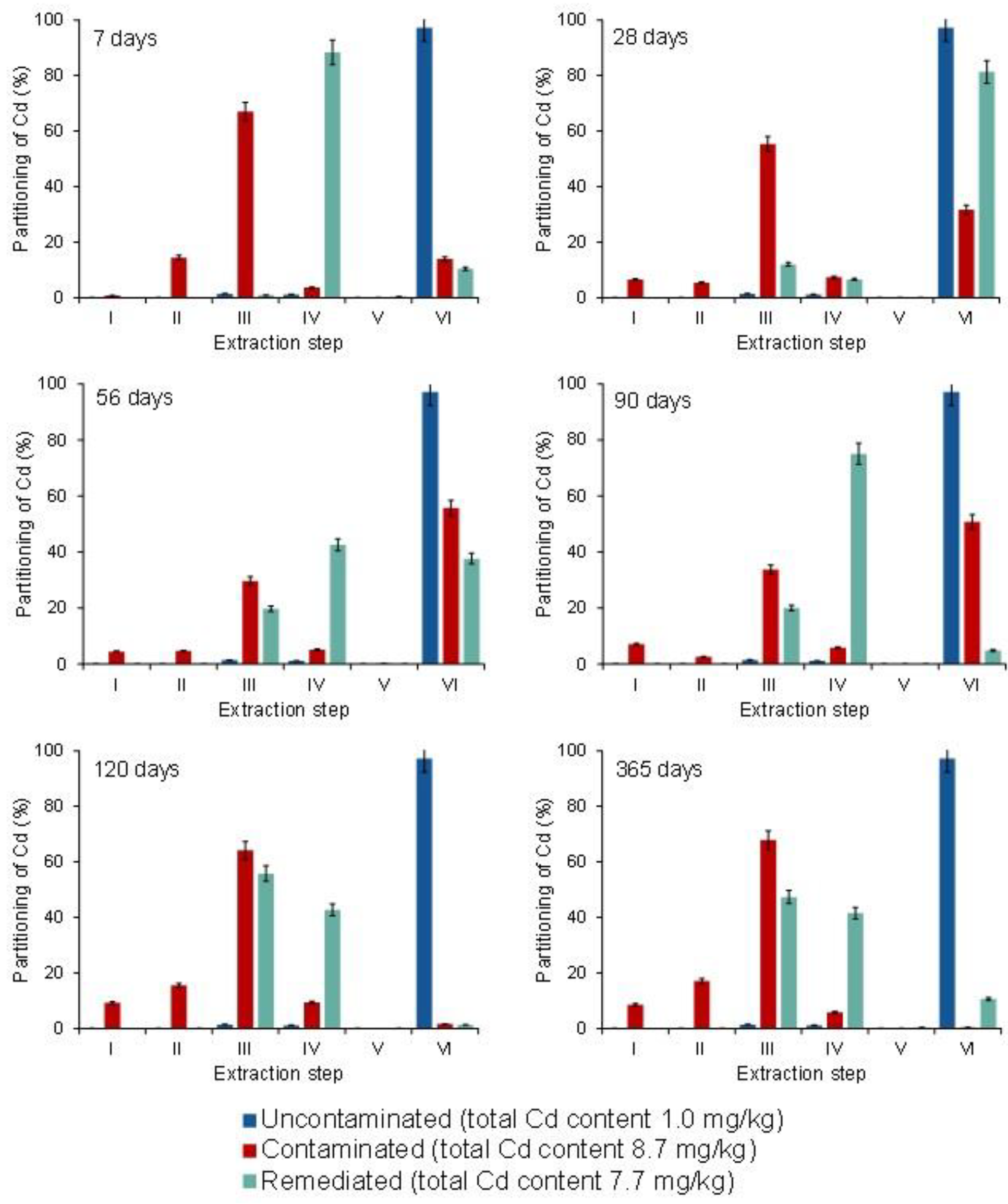
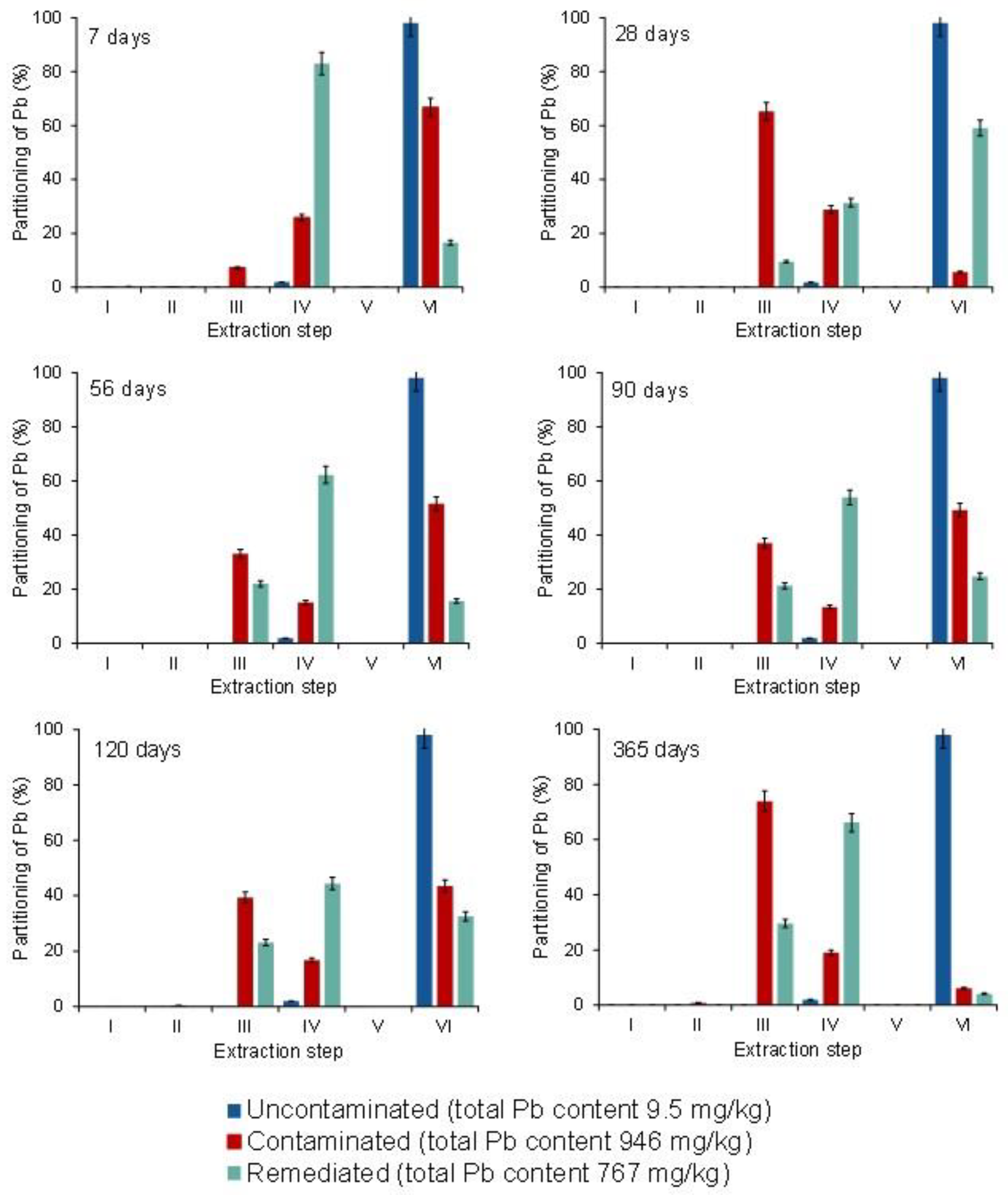
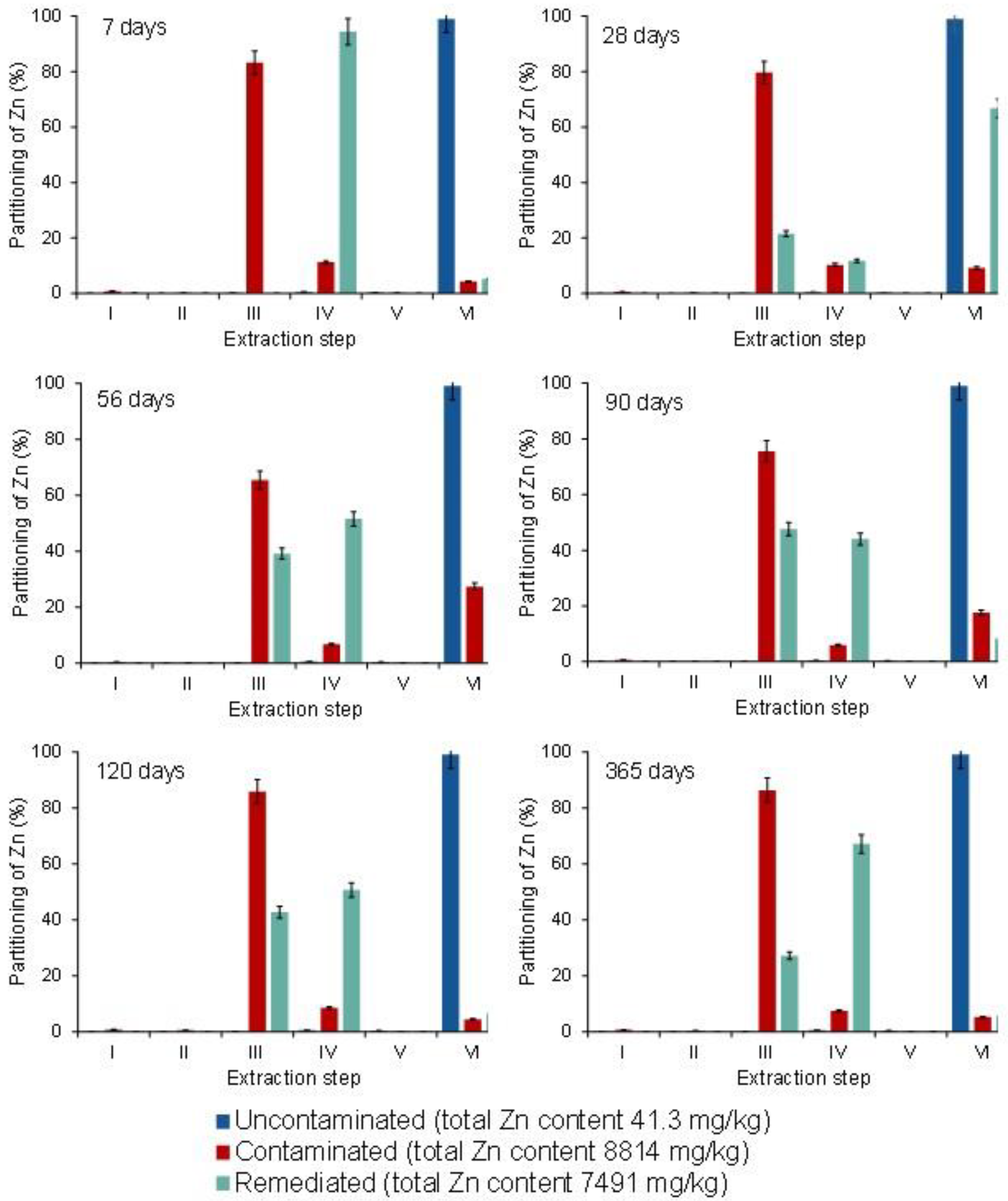
3.4. Partitioning of Cd, Pb, and Zn in Uncontaminated, Contaminated, and Remediated Soils
3.5. Investigation of the Mineralogical Composition of the Remediated Soils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; FAO: Rome, Italy, 2018; Volume 142, ISBN 978-92-5-130505-8. [Google Scholar]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in the soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef] [Green Version]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of soil pollution by heavy metals and their accumulation in vegetables: A review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef] [Green Version]
- Pérez, A.P.; Eugenio, N.R. Status of Local Soil Contamination in Europe: Revision of the Indicator Progress in the Management Contaminated Sites in Europe; EUR 29124 EN; Publications Office of the European Union: Luxembourg, 2018; p. JRC107508. ISBN 978-92-79-80072-6. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. B 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Nejad, Z.D.; Jung, M.C.; Kim, K. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 27–953. [Google Scholar] [CrossRef]
- Pandey, A.; Rabbani, A. Soil stabilisation using cement. Int. J. Civ. Eng. Technol. 2017, 8, 316–322. [Google Scholar]
- Yi, X.; Xuefeng, L.; Yingming, X.; Xu, Q.; Qingqing, H.; Lin, W.; Yuebing, S. Remediation of heavy metal-polluted agricultural soils using clay minerals: A review. Pedosphere 2017, 27, 193–204. [Google Scholar] [CrossRef]
- Radziemska, M.; Wyszkowski, M.; Bęś, A.; Mazur, Z.; Jeznach, J.; Brtnický, M. The applicability of compost, zeolite and calcium oxide in assisted remediation of acidic soil contaminated with Cr(III) and Cr(VI). Environ. Sci. Pollut. Res. 2019, 26, 21351–21362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhu, Y.; Cheng, L.; Anderson, B.; Zhao, X.; Wang, D.; Ding, A. Review on utilization of biochar for metal-contaminated soil and sediment remediation. J. Environ. Sci. 2018, 63, 156–173. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, G.; Mao, R. Effects of particle sizes of rock phosphate on immobilizing heavy metals in lead zinc mine soils. J. Soil Sci. Plant Nutr. 2014, 14, 258–266. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bułkowska, K.; Klik, B. Feasibility of using humic substances from compost to remove heavy metals (Cd, Cu, Ni, Pb, Zn) from contaminated soil aged for different periods of time. J. Hazard. Mater. 2015, 300, 882–891. [Google Scholar] [CrossRef]
- Leelarungroj, K.; Likitlersuang, S.; Chompoorat, T. Leaching mechanisms of heavy metals from fly ash stabilised soils. Waste Manag. Res. 2018, 36, 616–623. [Google Scholar] [CrossRef]
- Mavroulidou, M. Use of waste paper sludge ash as a calcium-based stabiliser for clay soils. Waste Manag. Res. 2018, 36, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Oprčkal, P.; Mladenovič, A.; Zupančič, N.; Ščančar, J.; Milačič, R.; Zalar Serjun, V. Remediation of contaminated soil by red mud and paper-ash. J. Clean. Prod. 2020, 256, 120440. [Google Scholar] [CrossRef]
- Cappai, G.; Cara, S.; Muntoni, A.; Piredda, M. Application of accelerated carbonation on MSW combustion APC residues for metal immobilization and CO2 sequestration. J. Hazard. Mater. 2012, 207–208, 159–164. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and of the Council. EN 1744-3: Tests for Chemical Properties of Aggregates Part 3: Preparation of Eluates by Leaching of Aggregates; Slovinsky Institute of Standardization: Ljubljana, Slovenia, 2002. [Google Scholar]
- Zhang, H. Probing into the speciation of trace metals and research methods, in Behaviors of trace metals in environment. In Behaviors of Trace Metals in Environment; Springer Nature: Singapore, 2020; pp. 299–342. [Google Scholar] [CrossRef]
- Okoro, H.K.; Fatoki1, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G. A review of sequential extraction procedures for heavy metals speciation in soil and sediments. Open Acc. Sci. Rep. 2012, 1, 1–9. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Hursthouse, A.; Cuthbert, S. The potential of sequential extraction in the characterisation and management of wastes from steel processing: A prospective review. Int. J. Environ. Res. Public Health 2015, 12, 11724–11755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Milačič, R.; Zuliani, T.; Ščančar, J. Environmental impact of toxic elements in red mud studied by fractionation and speciation procedures. Sci. Total Environ. 2012, 426, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Sakan, S.M.; Sakan, N.M.; Đorđević, D.S. Trace element study in Tisa River and Danube alluvial sediment in Serbia. Int. J. Sed. Res. 2013, 28, 234–245. [Google Scholar] [CrossRef]
- Sungurac, A.; Soylakb, M.; Ozcana, H. Investigation of heavy metal mobility and availability by the BCR sequential extraction procedure: Relationship between soil properties and heavy metals availability. Chem. Spec. Bioavail. 2014, 26, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Duan, M.; Li, Y.; Nwankwegu, A.S.; Ji, Y.; Zhang, J. Concentration and pollution assessment of heavy metals within surface sediments of the Raohe Basin, China. Sci. Rep. 2019, 9, 13100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ščančar, J.; Milačič, R.; Burica, O.; Stražar, M. Water and acetic acid leachable Cd, Cr, Cu, Fe, Ni, Pb and Zn in lime-treated sewage sludge. Ann. Chim. 2001, 91, 375–379. [Google Scholar]
- Janoš, P.; Vávrová, J.; Herzogová, L.; Pilařová, V. Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma 2010, 159, 335–341. [Google Scholar] [CrossRef]
- Ashrafi, M.; Mohamad, S.; Yusoff, I.; Hamid, F.S. Immobilization of Pb, Cd, and Zn in a contaminated soil using eggshell and banana stem amendments: Metal leachability and a sequential extraction study. Environ. Sci. Pollut. Res. 2015, 22, 223–230. [Google Scholar] [CrossRef]
- Wang, L.; Cho, D.-W.; Tsanga, D.C.W.; Caoc, X.; Houd, D.; Shend, Z.; Alessie, D.S.; Okf, Y.S.; Poon, C.S. Green remediation of As and Pb contaminated soil using cement-free clay based stabilization/solidification. Environ. Internet 2019, 126, 336–345. [Google Scholar] [CrossRef]
- Moretti, L.; Natali, S.; Tiberi, A.; D’Andrea, A. Proposal for a methodology based on XRD and SEM-EDS to proposal for a methodology based on XRD and SEM-EDS to monitor effects of lime-treatment on clayey soils. Appl. Sci. 2020, 10, 2569. [Google Scholar] [CrossRef] [Green Version]
- Fazle Bari, A.S.M.; Lamb, D.; Choppala, G.; Bolan, N.; Seshadri, B.; Rahman, A.; Rahman, M.M. Geochemical fractionation and mineralogy of metal(loid)s in abandoned mine soils: Insights into arsenic behaviour and implications to remediation. J. Hazard. Mater. 2020, 399, 123029. [Google Scholar] [CrossRef]
- Nejad, Z.D.; Jung, M.C. The effects of biochar and inorganic amendments on soil remediation in the presence of hyperaccumulator plant. Int. J. Energy Environ. Eng. 2017, 8, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Vidmar, J.; Zuliani, T.; Novak, P.; Drinčić, A.; Ščančar, J.; Milačič, R. Elements in water, suspended particulate matter and sediments of the Sava River. J. Soils Sed. 2017, 17, 1917–1927. [Google Scholar] [CrossRef] [Green Version]
- Official Gazette of Republic Slovenia, Decree on Waste Landfill, Nos. 2020, 10/14, 54/15, 36/16, 37/18 and 129/20. Available online: https://www.ecolex.org/details/legislation/decree-on-the-landfill-of-waste-lex-faoc130542/ (accessed on 16 November 2021).
- Official Gazette of EU. Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste (2018). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32018L0851 (accessed on 16 November 2021).
- IUPAC-NIST Solubility Database, Version 1.1 NIST Standard Reference Database 106 Last Update to Data Content. 2012. Available online: https://srdata.nist.gov/solubility/ (accessed on 16 November 2021). [CrossRef]

| Step | Soil Fraction | Extracting Solution | Mode of Extraction |
|---|---|---|---|
| I | Water-soluble | Water (20 mL) | Shaking (16 h) |
| II | Exchangeable | 1 M NaCH3COO (20 mL, pH 8.2) | Shaking (1 h) |
| III | Bound to carbonates | 1 M NaCH3COO (20 mL, pH 5) | Shaking (5 h) |
| IV | Bound to Fe/Mn oxides and hydroxides | 0.04 M NH2OH HCl in 25% v/v CH3COOH (20 mL, pH 2) | Extracted at 95 °C (6 h) |
| V | Bound to organic matter | 156.25 mL of 30% H2O2 + 93.75 mL of 0.02 M HNO3 (20 mL, pH 2) | Extracted at 85 °C (2 h) |
| VI | Bound to silicate lattice | 0.2 g of dried residual sample | Microwave-assisted digestion |
| Compound | Solubility (g/100 mL) |
|---|---|
| CdCO3 | 3.932 × 10−5 |
| Cd(OH)2 | 2.697 × 10−4 |
| PbCO3 | 7.269 × 10−5 |
| Pb(OH)2 | 1.615 × 10−4 |
| ZnCO3 | 4.692 × 10−5 |
| * Zn(OH)2 | 1 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đurić, M.; Oprčkal, P.; Zalar Serjun, V.; Pranjić, A.M.; Ščančar, J.; Milačič, R.; Mladenovič, A. Environmental Impacts and Immobilization Mechanisms of Cadmium, Lead and Zinc in Geotechnical Composites Made from Contaminated Soil and Paper-Ash. Appl. Sci. 2021, 11, 11822. https://doi.org/10.3390/app112411822
Đurić M, Oprčkal P, Zalar Serjun V, Pranjić AM, Ščančar J, Milačič R, Mladenovič A. Environmental Impacts and Immobilization Mechanisms of Cadmium, Lead and Zinc in Geotechnical Composites Made from Contaminated Soil and Paper-Ash. Applied Sciences. 2021; 11(24):11822. https://doi.org/10.3390/app112411822
Chicago/Turabian StyleĐurić, Marija, Primož Oprčkal, Vesna Zalar Serjun, Alenka Mauko Pranjić, Janez Ščančar, Radmila Milačič, and Ana Mladenovič. 2021. "Environmental Impacts and Immobilization Mechanisms of Cadmium, Lead and Zinc in Geotechnical Composites Made from Contaminated Soil and Paper-Ash" Applied Sciences 11, no. 24: 11822. https://doi.org/10.3390/app112411822
APA StyleĐurić, M., Oprčkal, P., Zalar Serjun, V., Pranjić, A. M., Ščančar, J., Milačič, R., & Mladenovič, A. (2021). Environmental Impacts and Immobilization Mechanisms of Cadmium, Lead and Zinc in Geotechnical Composites Made from Contaminated Soil and Paper-Ash. Applied Sciences, 11(24), 11822. https://doi.org/10.3390/app112411822