The Impact of Fast-Rise-Time Electromagnetic Field and Pressure on the Aggregation of Peroxidase upon Its Adsorption onto Mica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Protein
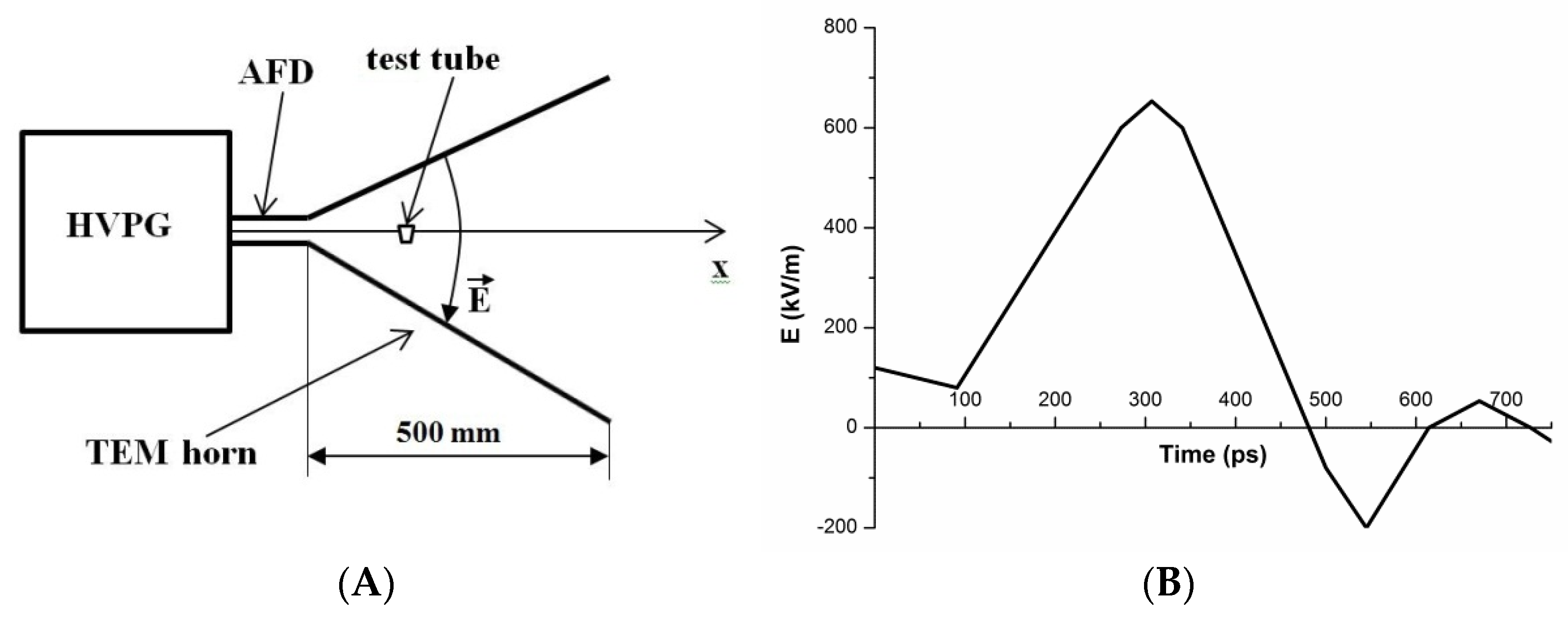
2.2. Experimental Setup for the Study of the Effect of the PEMF
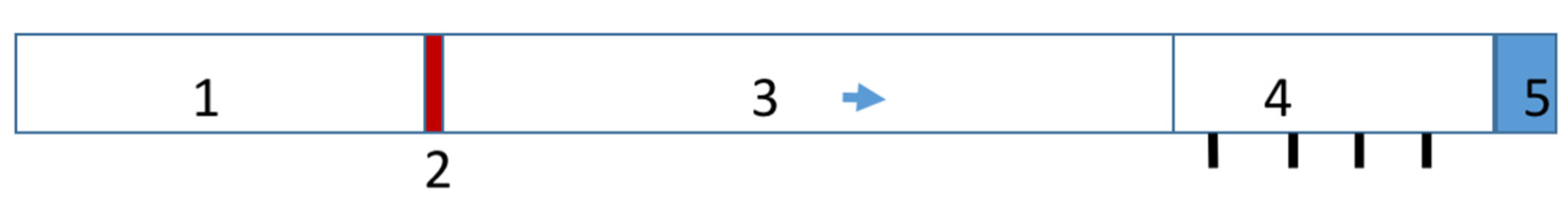
2.3. Experimental Setup for the Study of the Effect of the Shock Wave
2.4. AFM Experiments and Sample Preparation
2.5. Estimation of HRP Enzymatic Activity
3. Results
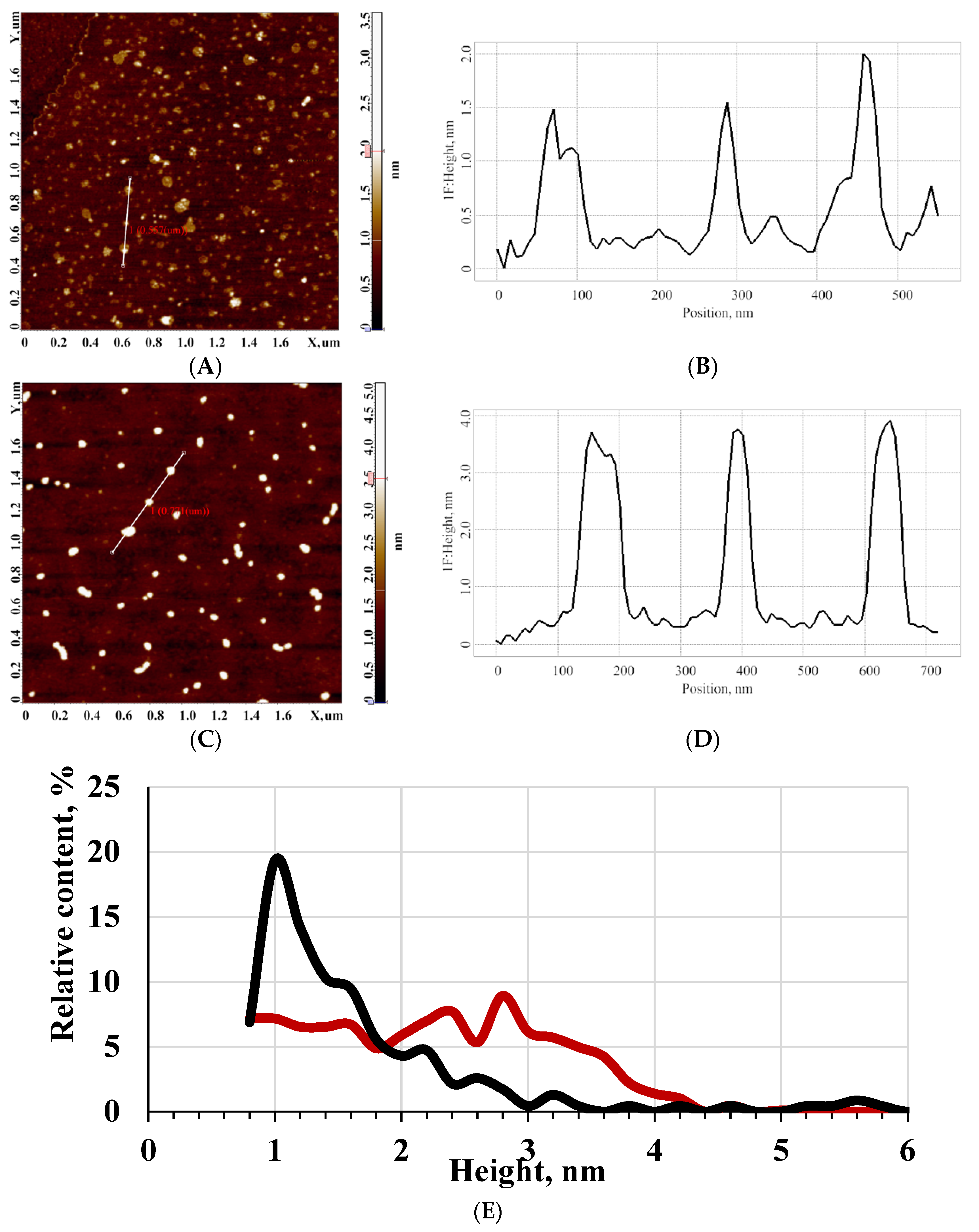
3.1. AFM Study of the Effect of Pulsed Electromagnetic Field
3.2. AFM Study of the Effect of Pulsed Pressure
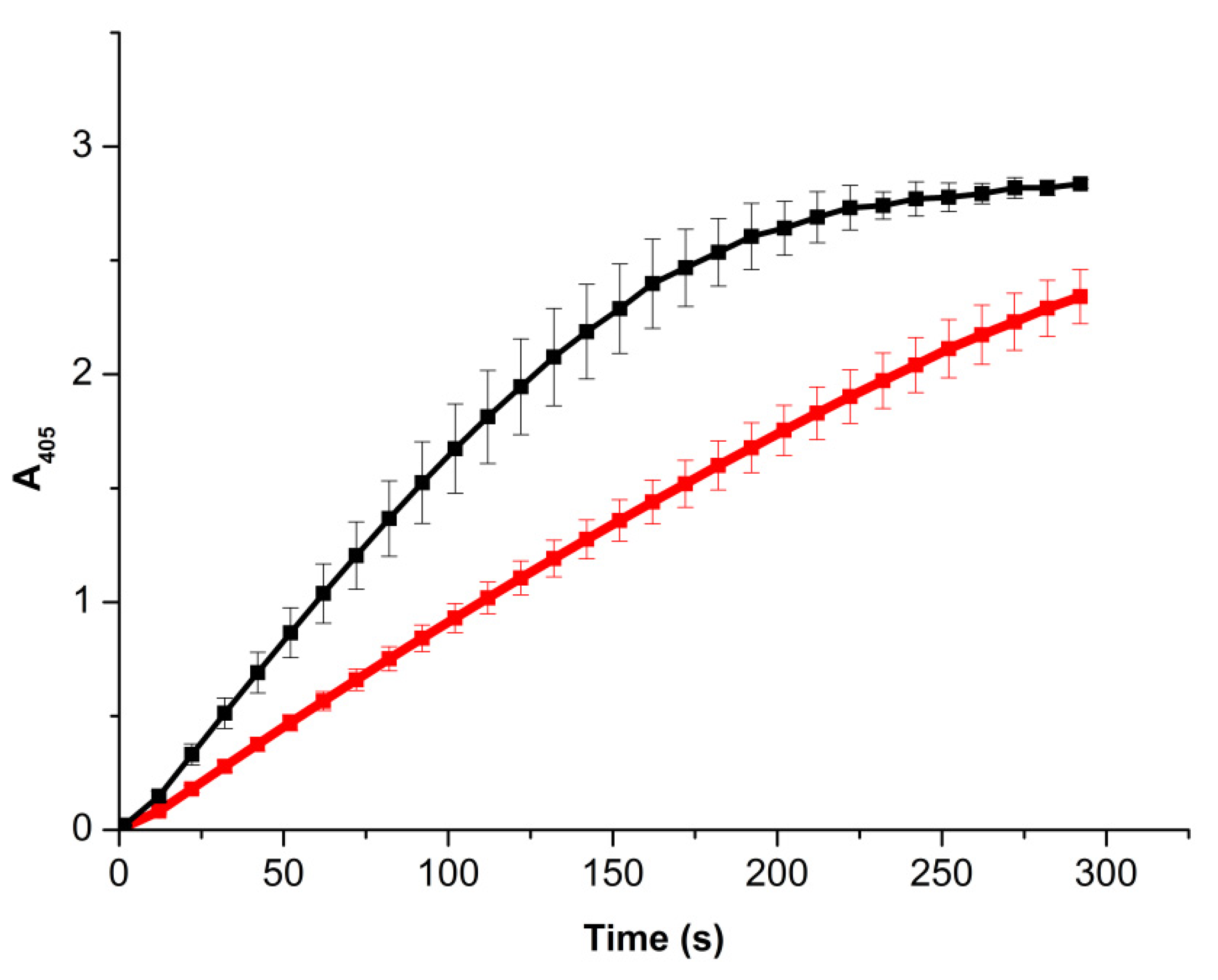
3.3. Spectrophotometry-Based Estimation of the Effect of Pulsed Electromagnetic and Presssure Field on the Enzymatic Activity of HRP
4. Discussion
- (1)
- HRP monomer—HRP monomer;
- (2)
- HRP monomer—mica surface;
- (3)
- HRP monomer—solvent (buffer solution).
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warille, A.A.; Altun, G.; Elamin, A.A.; Kaplan, A.A.; Mohamed, H.; Yurt, K.K.; Elhaj, A.E. Skeptical approaches concerning the effect of exposure to electromagnetic fields on brain hormones and enzyme activities. J. Microsc. Ultrastruct. 2017, 5, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Moloney, B.M.; McAnena, P.F.; Abd Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Glynn, C.; O’Connell, A.M.; Ennis, R.; Lowery, A.J.; et al. Microwave Imaging in Breast Cancer–Results from the First-In-Human Clinical Investigation of the Wavelia System. Acad. Radiol. 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Zinoviev, S.V.; Evdokimov, A.N.; Sakharov, K.Y.; Turkin, V.A.; Aleshko, A.I.; Ivanov, A.V. Determination of therapeutic value of ultra-wideband pulsed electromagnetic microwave radiation on models of experimental oncology. Meditsinskaya Fiz. Med. Phys. 2015, 3, 62–67. Available online: https://www.researchgate.net/publication/282975692_Determination_of_Therapeutic_Value_of_Ultra-Wideband_Pulsed_Electromagnetic_Microwave_Radiation_on_Models_of_Experimental_Oncology (accessed on 29 November 2021).
- Jumaat, H.; Ping, K.H.; Abd Rahman, N.H.; Yon, H.; Redzwan, F.N.M.; Awang, R.A. A compact modified wideband antenna with CBCPW, stubline and notch-staircase for breast cancer microwave imaging application. AEU-Int. J. Electron. Commun. 2021, 129, 153492. [Google Scholar] [CrossRef]
- Karam, S.A.S.; O’Loughlin, D.; Oliveira, B.L.; O’Halloran, M.; Asl, B.M. Weighted delay-and-sum beamformer for breast cancer detection using microwave imaging. Measurement 2021, 177, 109283. [Google Scholar] [CrossRef]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. High Pressure Effects on Protein Structure and Function. PROTEINS Struct. Funct. Genet. 1996, 24, 81–91. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Larionov, D.I.; Repnikov, V.V.; Ivanova, N.D.; et al. AFM and FTIR Investigation of the Effect of Water Flow on Horseradish Peroxidase. Molecules 2021, 26, 306. [Google Scholar] [CrossRef] [PubMed]
- Gavrilenko, T.I.; Ryzhkova, N.A.; Parkhomenko, A.N. Myeloperoxidase and its role in development of ischemic heart disease. Ukr. J. Cardiol. 2014, 4, 119–126. [Google Scholar]
- Teng, N.; Maghzal, G.J.; Talib, J.; Rashid, I.; Lau, A.K.; Stocker, R. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2017, 22, 51–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobson, G.A.; Yee, K.C.; Ng, C.H. Elevated plasma glutathione peroxidase concentration in acute severe asthma: Comparison with plasma glutathione peroxidase activity, selenium and malondialdehyde. Scand. J. Clin. Lab. Investig. 2007, 67, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.; Xia, K.; Colón, W.; Vieira, S.I.; Ribeiro, F. Protein aggregation, cardiovascular diseases, and exercise training: Where do we stand? Ageing Res. Rev. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Reumers, J.; Couceiro, J.R.; De Smet, F.; Gallardo, R.; Rudyak, S.; Cornelis, A.; Rozenski, J.; Zwolinska, A.; Marine, J.C.; et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011, 7, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Direito, I.; Monteiro, L.; Melo, T.; Figueira, D.; Lobo, J.; Enes, V.; Moura, G.; Henrique, R.; Santos, M.A.S.; Jerónimo, C.; et al. Protein Aggregation Patterns Inform about Breast Cancer Response to Antiestrogens and Reveal the RNA Ligase RTCB as Mediator of Acquired Tamoxifen Resistance. Cancers 2021, 13, 3195. [Google Scholar] [CrossRef]
- Levy, C.B.; Stumbo, A.C.; Ano Bom, A.P.; Portari, E.A.; Cordeiro, Y.; Carneiro, Y.; Silva, J.L.; De Moura-Gallo, C.V. Co-localization of mutant p53 and amyloid-like protein aggregates in breast tumors. Int. J. Biochem. Cell Biol. 2011, 43, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Yang-Hartwich, Y.; Bingham, J.; Garofalo, F.; Alvero, A.B.; Mor, G. Detection of p53 Protein Aggregation in Cancer Cell Lines and Tumor Samples. In Apoptosis and Cancer. Methods in Molecular Biology; Mor, G., Alvero, A., Eds.; Humana Press: New York, NY, USA, 2015; Volume 1219. [Google Scholar] [CrossRef]
- Sopova, J.V.; Koshel, E.I.; Belashova, T.A.; Zadorsky, S.P.; Sergeeva, A.V.; Siniukova, V.A.; Shenfeld, A.A.; Velizhanina, M.E.; Volkov, K.V.; Nizhnikov, A.A.; et al. RNA-binding protein FXR1 is presented in rat brain in amyloid form. Sci. Rep. 2019, 9, 18983. [Google Scholar] [CrossRef] [Green Version]
- Benseny-Cases, N.; Álvarez-Marimon, E.; Aso, E.; Carmona, M.; Klementieva, O.; Appelhans, D.; Ferrer, I.; Cladera, J. In situ structural characterization of early amyloid aggregates in Alzheimer’s disease transgenic mice and Octodon degus. Sci. Rep. 2020, 10, 5888. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Fu, Z.; Meng, L.; He, M.; Zhang, Z. The early events that initiate β-amyloid aggregation in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.; Ravera, S.; Panfoli, I.; Pepe, I.M. Effects of extremely low frequency electromagnetic fields on membrane-associated enzymes. Arch. Biochem. Biophys. 2005, 441, 191–198. [Google Scholar] [CrossRef]
- Thumm, S.; Löschinger, M.; Glock, S.; Hämmerle, H.; Rodemann, H.P. Induction of cAMP-dependent protein kinase A activity in human skin fibroblasts and rat osteoblasts by extremely low-frequency electromagnetic fields. Radiat. Env. Biophys. 1999, 38, 195–199. [Google Scholar] [CrossRef]
- Caliga, R.; Maniu, C.L.; Mihăşan, M. ELF-EMF exposure decreases the peroxidase catalytic efficiency in vitro. Open Life Sci. 2016, 11, 71–77. [Google Scholar] [CrossRef]
- Hamedi, N.; Saviz, M.; Banaei, A.; Shabani, S.; Qafary, M.; Moosavi-Movahedi, A.A.; Faraji-Dana, R. Effects of circularly polarized electromagnetic fields on solvated hemoglobin structure. J. Mol. Liq. 2020, 312, 113283. [Google Scholar] [CrossRef]
- Lopes, L.C.; Barreto, M.T.; Gonçalves, K.M.; Alvarez, H.M.; Heredia, M.F.; De Souza, R.O.M.; Cordeiro, Y.; Dariva, C.; Fricks, A.T. Stability and structural changes of horseradish peroxidase: Microwave versus conventional heating treatment. Enzym. Microb. Technol. 2015, 69, 10–18. [Google Scholar] [CrossRef]
- Latorre, M.E.; Bonelli, P.R.; Rojas, A.M.; Gerschenson, L.N. Microwave inactivation of red beet (Beta vulgaris L. var. conditiva) peroxidase and polyphenoloxidase and the effect of radiation on vegetable tissue quality. J. Food Eng. 2012, 109, 676–684. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. The influence of rotating magnetic field on bio-catalytic dye degradation using the horseradish peroxidase. Biochem. Eng. J. 2019, 147, 81–88. [Google Scholar] [CrossRef]
- Emamdadi, N.; Gholizadeh, M.; Housaindokht, M.R. Investigation of static magnetic field effect on horseradish peroxidase enzyme activity and stability in enzymatic oxidation process. Int. J. Biol. Macromol. 2021, 170, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xie, Y.; Wang, S.; Shang, S.; Zhao, J.; Lu, X. A wideband picosecond pulsed electric fields (psPEF) exposure system for the nanoporation of biological cells. Bioelectrochemistry 2021, 140, 107790. [Google Scholar] [CrossRef] [PubMed]
- Perelmuter, V.M.; Cha, V.A.; Chuprikova, E.M. Medical and Biological Aspects of Interaction of Electromagnetic Waves with the Body; Tomsk Polytechnic University: Tomsk, Russia, 2009. [Google Scholar]
- Pleshakova, T.O.; Malsagova, K.A.; Kaysheva, A.L.; Kopylov, A.T.; Tatur, V.Y.; Ziborov, V.S.; Kanashenko, S.L.; Galiullin, R.A.; Ivanov, Y.D. Highly sensitive protein detection by biospecific AFM-based fishing with pulsed electrical stimulation. FEBS Open Biol. 2017, 7, 1186–1195. [Google Scholar] [CrossRef]
- Hui Bon Hoa, G.; Douzou, P.; Dahan, N.; Balny, C. High-pressure spectrometry at subzero temperatures. Anal. Biochem. 1982, 120, 125–135. [Google Scholar] [CrossRef]
- Eisenmenger, M.J.; Reyes-De-Corcuera, J.I. High pressure enhancement of enzymes: A review. Enzym. Microb. Technol. 2009, 45, 331–347. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Katsaros, G.; Taoukis, P. Comparison of the application of high pressure and pulsed electric fields technologies on the selective inactivation of endogenous enzymes in tomato products. Innov. Food Sci. Emerg. Technol. 2016, 38, 349–355. [Google Scholar] [CrossRef]
- Akazawa, S.I.; Tokuyama, H.; Sato, S.; Watanabe, T.; Shida, Y.; Ogasawara, W. High-pressure tolerance of earthworm fibrinolytic and digestive enzymes. J. Biosci. Bioeng. 2018, 125, 155–159. [Google Scholar] [CrossRef]
- Metzler, D.E. Biochemistry, the Chemical Reactions of Living Cells, 1st ed.; Academic Press: Cambridge, UK, 1977. [Google Scholar]
- Davies, P.F.; Rennke, H.G.; Cotran, R.S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J. Cell Sci. 1981, 49, 69–86. [Google Scholar] [CrossRef]
- Welinder, K.G. Amino acid sequence studies of horseradish peroxidase. amino and carboxyl termini, cyanogen bromide and tryptic fragments, the complete sequence, and some structural characteristics of horseradish peroxidase. Cent. Eur. J. Biochem. 1979, 96, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Ignatenko, O.V.; Sjölander, A.; Hushpulian, D.M.; Kazakov, S.V.; Ouporov, I.V.; Chubar, T.A.; Poloznikov, A.A.; Ruzgas, T.; Tishkov, V.I.; Gorton, L.; et al. Electrochemistry of chemically trapped dimeric and monomeric recombinant horseradish peroxidase. Adv. Biosens. Bioelectron. 2013, 2, 25–34. [Google Scholar]
- Rogozhin, V.V.; Kutuzova, G.D.; Ugarova, N.N. Inhibition of horseradish peroxidase by N-ethylamide of o-sulfobenzoylacetic acid. Russ. J. Bioorganic. Chem. 2000, 26, 138–141. [Google Scholar] [CrossRef]
- Veitch, N.C.; Smith, A.T. Horseradish peroxidase. Adv. Inorg. Chem. 2000, 51, 107–162. [Google Scholar]
- Shannon, L.M.; Kay, E.; Lew, J.Y. Peroxidase isozymes from horseradish roots I. Isolation and physical properties. J. Biol. Chem. 1966, 241, 2166–2172. [Google Scholar] [CrossRef]
- Tams, J.W.; Welinder, K.G. Mild chemical deglycosylation of horseradish peroxidase yields a fully active, homogeneous enzyme. Anal. Biochem. 1995, 228, 48–55. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Bukharina, N.S.; Archakov, A.I.; Ivanov, Y.D. Atomic force microscopy for protein detection and their physicochemical characterization. Int. J. Mol. Sci. 2018, 19, 1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufrêne, Y.F.; Ando, T.; Garcia, R.; Alsteens, D.; Martinez-Martin, D.; Engel, A.; Gerber, C.; Müller, D.J. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat. Nanotechnol. 2017, 12, 295–307. [Google Scholar] [CrossRef]
- Uvarov, V.Y.; Ivanov, Y.D.; Romanov, A.N.; Gallyamov, M.O.; Kiselyova, O.I.; Yaminsky, I.V. Scanning tunneling microscopy study of cytochrome P450 2B4 incorporated in proteoliposomes. Biochimie 1996, 78, 780–784. [Google Scholar] [CrossRef]
- Kiselyova, O.I.; Yaminsky, I.V.; Ivanov, Y.D.; Kanaeva, I.P.; Kuznetsov, V.Y.; Archakov, A.I. AFM study of membrane proteins, cytochrome P450 2B4, and NADPH–Cytochrome P450 reductase and their complex formation. Arch. Biochem. Biophys. 1999, 371, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, V.Y.; Ivanov, Y.D.; Bykov, V.A.; Saunin, S.A.; Fedorov, I.A.; Lemeshko, S.V.; Hoa, H.B.; Archakov, A.I. Atomic force microscopy detection of molecular complexes in multiprotein P450cam containing monooxygenase system. Proteomics 2002, 2, 1699–1705. [Google Scholar] [CrossRef]
- Archakov, A.I.; Ivanov, Y.D. Optical biosensor and scanning probe microscopy studies of cytochrome P450 interactions with redox partners and phospholipid layers. Methods Enzymol. 2002, 357, 94–103. [Google Scholar] [CrossRef]
- Kuznetsov, V.Y.; Ivanov, Y.D.; Archakov, A.I. Atomic force microscopy revelation of molecular complexes in the multiprotein cytochrome P450 2B4-containing system. Proteomics 2004, 4, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Ramacviciene, A.; Snitka, V.; Mieliauskiene, R.; Ramanavicius, A. AFM-study of complement system assembly initiated by antigen-antibody complex. Cent. Eur. J. Chem. 2006, 4, 194–206. [Google Scholar] [CrossRef]
- Archakov, A.I.; Ivanov, Y.D. Application of AFM and optical biosensor for investigation of complexes formed in P450-containing monooxygenase systems. Biochim. Biophys. Acta-Proteins Proteom. 2011, 1814, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Pleshakova, T.; Kaysheva, A.; Bayzyanova, J.; Anashkina, A.; Uchaikin, V.; Ziborov, V.; Konev, V.; Archakov, A.; Ivanov, Y. The detection of hepatitis C virus core antigen using AFM chips with immobolized aptamers. J. Virol. Methods 2018, 251, 99–105. [Google Scholar] [CrossRef]
- Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Ziborov, V.S.; Bayzyanova, J.M.; Konev, V.A.; Uchaikin, V.F.; Archakov, A.I.; Ivanov, Y.D. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines 2019, 10, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaysheva, A.L.; Pleshakova, T.O.; Stepanov, A.A.; Ziborov, V.S.; Saravanabhavan, S.S.; Natesan, B.; Archakov, A.I.; Ivanov, Y.D. Immuno-MALDI MS dataset for improved detection of HCVcoreAg in sera. Data Brief. 2019, 25, 104240. [Google Scholar] [CrossRef] [PubMed]
- Usychenko, V.G.; Sorokin, L.N.; Usychenko, A.S. Penetration of Electromagnetic Radiation Energy into a Semiconductor Element Base of Technical Means without Specialized Receiving Antennas. J. Commun. Technol. Electron. 2020, 65, 1448–1456. [Google Scholar] [CrossRef]
- Li, C.; Arakawa, T. Feasibility of circular dichroism to study protein structure at extreme concentrations. Int. J. Biol. Macromol. 2019, 132, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.A.; Sakharov, K.Y.; Mikheev, O.V.; Turkin, V.A.; Aleshko, A.I. Radiators of ultrashort electromagnetic pulses. In Proceedings of the Third International Conference on Ultrawideband and Ultrashort Impulse Signals, Sevastopol, Ukraine, 18–22 September 2006; pp. 203–205. [Google Scholar] [CrossRef]
- Podosenov, S.A.; Sakharov, K.Y.; Svekis, Y.G.; Sokolov, A.A. Linear two-wire transmission line coupling to an external electromagnetic field, Part II: Specific cases, experiment. IEEE Trans. Electromagn. Compat. 1995, 37, 566–574. [Google Scholar] [CrossRef]
- Dobrotvorsky, M.I.; Sakharov, K.Y.; Mikheev, O.V.; Turkin, V.A.; Aleshko, A.I.; Dnischenko, V.N. Measuring instruments of powerful UWB EMP parameters. In Proceedings of the Third International Conference on Ultrawideband and Ultrashort Impulse Signals, Sevastopol, Ukraine, 18–22 September 2006; pp. 373–375. [Google Scholar] [CrossRef]
- Sakharov, K.Y.; Turkin, V.A.; Mikheev, O.V.; Dobrotvorskii, M.I.; Sukhov, A.V. A picosecond pulsed electric field strength measuring transducer. Meas. Tech. 2014, 57, 201–205. [Google Scholar] [CrossRef]
- Gaydon, A.G.; Hurle, I.R. The Shock Tube in High-Temperature Chemical Physics; Reinhold Pub: Corp, NY, USA, 1963. [Google Scholar]
- Ziborov, V.; Galiullin, R.; Efremov, V.; Shumova, V.; Fortov, V. Application of laser schlieren method for measurment of shock front structure in helium with small admixture of heavy moltcules. Bull. Mosc. Reg. State Univ. Ser. Phys. Math. 2014, 4, 105–109. [Google Scholar]
- Thomson, N.H. Imaging the substructure of antibodies with tapping-mode AFM in air: The importance of a water layer on mica. J. Microsc. 2005, 217, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Keller, D. Reconstruction of STM and AFM images distorted by finite-size tips. Surface Sci. 1991, 253, 353–364. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Smelov, M.V.; Ivanova, N.D.; Ziborov, V.S. AFM imaging of protein aggregation in studying the impact of knotted electromagnetic field on a peroxidase. Sci. Rep. 2020, 10, 9022. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Romanova, T.S.; Valueva, A.A.; Tatur, V.Y.; Stepanov, I.N.; Ziborov, V.S. Investigation of the Influence of Liquid Motion in a Flow-based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Water Flow. Appl. Sci. 2020, 10, 4560. [Google Scholar] [CrossRef]
- Sanders, S.A.; Bray, R.C.; Smith, A.T. pH-dependent properties of a mutant horseradish peroxidase isoenzyme C in which Arg38 has been replaced with lysine. Eur. J. Biochem. 1994, 224, 1029–1037. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Bukharina, N.S.; Frantsuzov, P.A.; Pleshakova, T.O.; Kanashenko, S.L.; Medvedeva, N.V.; Argentova, V.V.; Zgoda, V.G.; Munro, A.W.; Archakov, A.I. AFM study of cytochrome CYP102A1 oligomeric state. Soft Matter 2012, 8, 4602–4608. [Google Scholar] [CrossRef]
- Chu, J.W.; Kimura, T. Studies on Adrenal Steroid Hydroxylases Molecular and catalytic properties of adrenodoxin reductase (a flavoprotein). J. Biol. Chem. 1973, 248, 2089–2094. [Google Scholar] [CrossRef]
- Davydov, D.R.; Hui, B.; Hoa, G.; Peterson, J.A. Dynamics of protein-bound water in the heme domain of P450BM3 studied by high-pressure spectroscopy: Comparison with P450cam and P450 2B4. Biochemistry 1999, 38, 751–761. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mazumdar, S. Structural and conformational stability of horseradish peroxidase: Effect of temperature and pH. Biochemistry 2000, 39, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Sono, M.; Perera, R.; Jin, S.; Makris, T.M.; Sligar, S.G.; Bryson, T.A.; Dawson, J.H. The influence of substrate on the spectral properties of oxyferrous wild-type and T252A cytochrome P450-CAM. Arch. Biochem. Biophys. 2005, 436, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Strickland, E.H. Circular dichroism of horseradish peroxidase and its enzyme-substrate compounds. Biochim. Biophys. Acta (BBA)-Enzymol. 1968, 151, 70–75. [Google Scholar] [CrossRef]
- Murugan, R.; Mazumdar, S. Role of substrate on the conformational stability of the heme active site of cytochrome P450cam: Effect of temperature and low concentrations of denaturants. J. Biol. Inorg. Chem. 2004, 9, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Efimov, A.V. Chirality and Handedness of Protein Structures. Biochemistry 2018, 83, S103–S110. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziborov, V.S.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Larionov, D.I.; Evdokimov, A.N.; Tatur, V.Y.; et al. The Impact of Fast-Rise-Time Electromagnetic Field and Pressure on the Aggregation of Peroxidase upon Its Adsorption onto Mica. Appl. Sci. 2021, 11, 11677. https://doi.org/10.3390/app112411677
Ziborov VS, Pleshakova TO, Shumov ID, Kozlov AF, Valueva AA, Ivanova IA, Ershova MO, Larionov DI, Evdokimov AN, Tatur VY, et al. The Impact of Fast-Rise-Time Electromagnetic Field and Pressure on the Aggregation of Peroxidase upon Its Adsorption onto Mica. Applied Sciences. 2021; 11(24):11677. https://doi.org/10.3390/app112411677
Chicago/Turabian StyleZiborov, Vadim S., Tatyana O. Pleshakova, Ivan D. Shumov, Andrey F. Kozlov, Anastasia A. Valueva, Irina A. Ivanova, Maria O. Ershova, Dmitry I. Larionov, Alexey N. Evdokimov, Vadim Yu. Tatur, and et al. 2021. "The Impact of Fast-Rise-Time Electromagnetic Field and Pressure on the Aggregation of Peroxidase upon Its Adsorption onto Mica" Applied Sciences 11, no. 24: 11677. https://doi.org/10.3390/app112411677
APA StyleZiborov, V. S., Pleshakova, T. O., Shumov, I. D., Kozlov, A. F., Valueva, A. A., Ivanova, I. A., Ershova, M. O., Larionov, D. I., Evdokimov, A. N., Tatur, V. Y., Aleshko, A. I., Sakharov, K. Y., Dolgoborodov, A. Y., Fortov, V. E., Archakov, A. I., & Ivanov, Y. D. (2021). The Impact of Fast-Rise-Time Electromagnetic Field and Pressure on the Aggregation of Peroxidase upon Its Adsorption onto Mica. Applied Sciences, 11(24), 11677. https://doi.org/10.3390/app112411677





