Operando XAS of a Bifunctional Gas Diffusion Electrode for Zn-Air Batteries under Realistic Application Conditions
Abstract
:1. Introduction
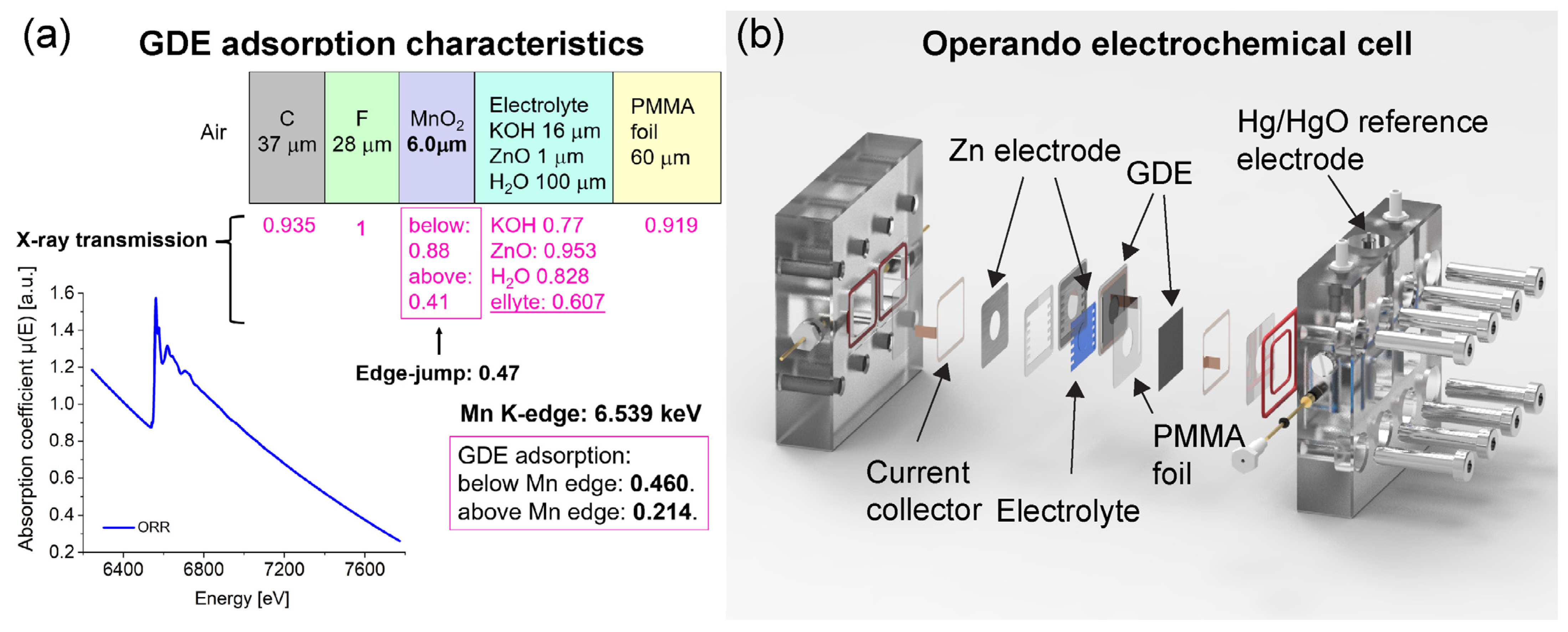
2. Experimental
2.1. Synthesis of α-MnO2
2.2. Fabrication of the GDE
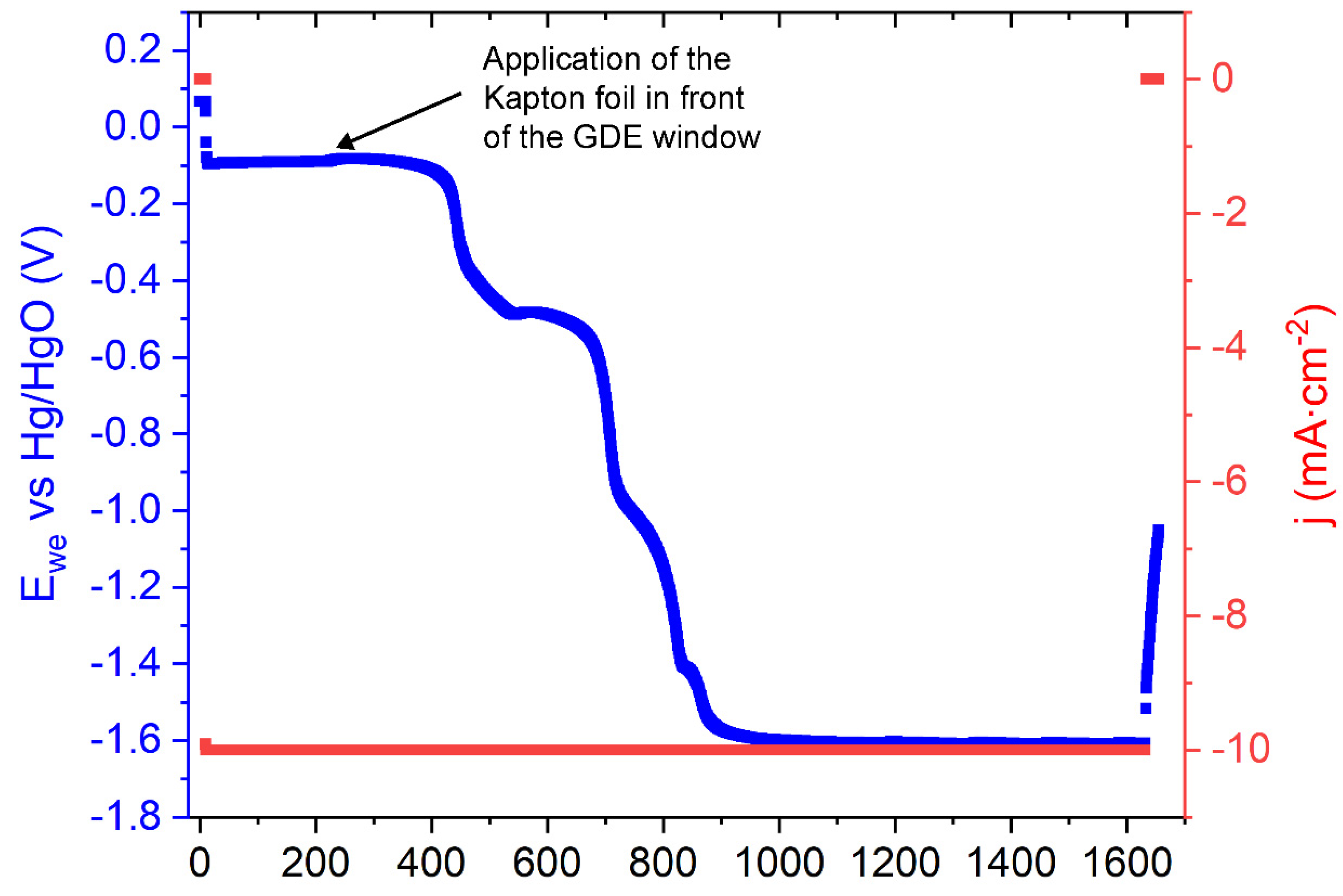
2.3. GDE Testing during Operando XAS
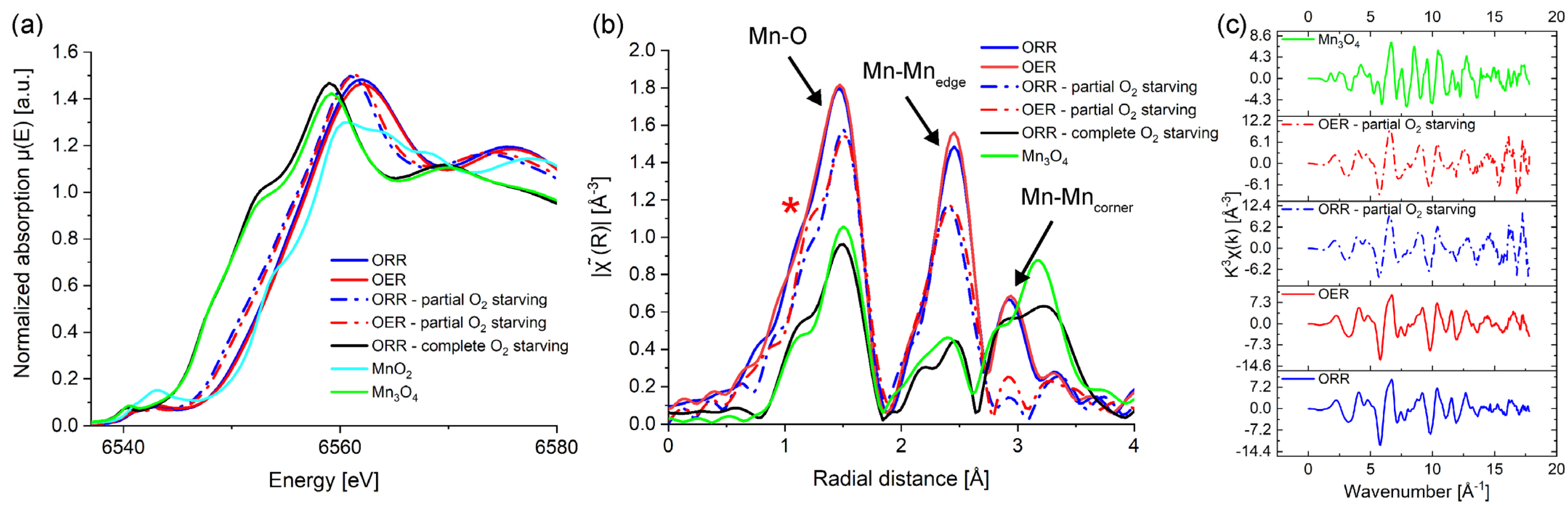
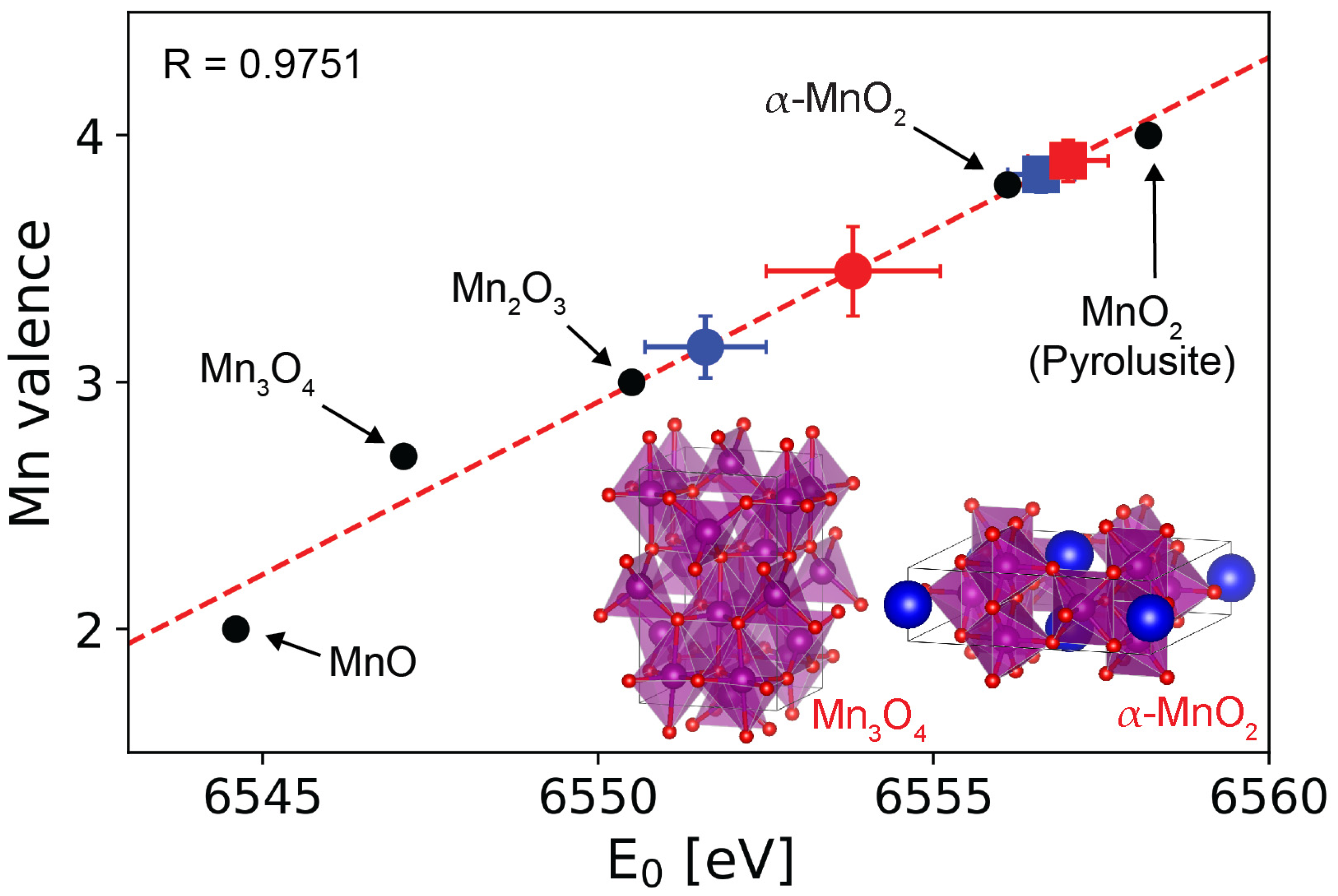
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Risch, M.; Stoerzinger, K.A.; Han, B.; Regier, T.Z.; Peak, D.; Sayed, S.Y.; Wei, C.; Xu, Z. Shao-Horn Y. Redox Processes of Manganese Oxide in Catalyzing Oxygen Evolution and Reduction: An in Situ Soft X-ray Absorption Spectroscopy Study. J. Phys. Chem. C 2017, 121, 17682–17692. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, P. Wood, X-Ray Tomography for Lithium Ion Battery Research: A Practical Guide. V. Annu. Rev. Mater. Res. 2017, 47, 451–479. [Google Scholar] [CrossRef]
- Gorlin, Y.; Lassalle-Kaiser, B.; Benck, J.D.; Gul, S.; Webb, S.M.; Yachandra, V.K.; Yano, J.; Jaramillo, T.F. In Situ X-ray Absorption Spectroscopy Investigation of a Bifunctional Manganese Oxide Catalyst with High Activity for Electrochemical Water Oxidation and Oxygen Reduction. J. Am. Chem. Soc. 2013, 135, 8525–8534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, E.M.; Thorum, M.S.; Vasic, R.; Marinkovic, S.; Frenkel, A.I.; Gewirth, A.A.; Nuzzo, R.G. In Situ Electrochemical X-ray Absorption Spectroscopy of Oxygen Reduction Electrocatalysis with High Oxygen Flux. J. Am. Chem.Soc. 2012, 134, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, K.; Kumar, S.M.S.; Thangamuthu, R.; Ganesan, K.; Murugan, P.; Rajput, P.; Jha, S.N.; Bhattacharyya, D. Physiochemical Investigation of Shape-Designed MnO2 Nanostructures and Their Influence on Oxygen Reduction Reaction Activity in Alkaline Solution. J. Phys. Chem. C 2015, 119, 6604–6618. [Google Scholar] [CrossRef]
- Marini, E.; Ludwig, J.; Sylvain, B. Rational design of a low-cost, durable and efficient bifunctional oxygen electrode for rechargeable metal-air batteries. J. Power Sources 2021, 482, 228900. [Google Scholar] [CrossRef]
- Cicco, A.D.; Aquilanti, G.; Minicucci, M.; Principi, E.; Novello, N.; Cognigni, A.; Olivi, L. Novel XAFS capabilities at ELETTRA synchrotron light source. J. Phys. Conf. Ser. 2009, 190, 012043. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravel, B. Artemis: EXAFS Data Analysis Using Feff with Larch or Ifeffit—Artemis 0.9.26 Documentation. 2016. Available online: https://bruceravel.github.io/demeter/documents/Artemis/index.html (accessed on 1 December 2021).
- The FEFF Project-FEFF. Available online: http://monalisa.phys.washington.edu/feffproject-feff.html (accessed on 1 December 2021).
- Vicat, J.; Fanchon, E.; Strobel, P.; Qui, D.T. The structure of K1.33Mn8O16 and cation ordering in hollandite-type structures. Acta Cryst. 1986, B42, 162–167. [Google Scholar] [CrossRef]
- Lima, F.H.B.; Calegaro, M.L.; Ticianelli, E.A. Electrocatalytic activity of manganese oxides prepared by thermal decomposition for oxygen reduction. Electrochim. Acta 2007, 52, 3732–3738. [Google Scholar] [CrossRef]
- Lee, J.M.; Patil, S.B.; Kang, B.; Lee, S.; Kim, M.G.; Hwang, S.-J. Understanding the crucial role of local crystal order in the electrocatalytic activity of crystalline manganese oxide. J. Mater. Chem. A 2018, 6, 12565–12573. [Google Scholar] [CrossRef]
- Yang, Z.; Ford, D.C.; Park, J.S.; Ren, Y.; Kim, S.; Kim, H.; Fister, T.T.; Chan, M.K.Y.; Thackeray, M.M. Probing the Release and Uptake of Water in α-MnO2·xH2O. Chem. Mater. 2017, 29, 1507–1517. [Google Scholar] [CrossRef]
- Yamamoto, S.; Matsuoka, O.; Fukada, I.; Ashida, Y.; Honda, T.; Yamamoto, N. Using Atomic Force Microscopy to Image the Surface of the Powdered Catalyst KMn8O16. J. Catal. 1996, 159, 401–409. [Google Scholar] [CrossRef]
- Gul, S.; Ng, J.W.D.; Alonso-Mori, R.; Kern, J.; Sokaras, D.; Anzenberg, E.; Lassalle-Kaiser, B.; Gorlin, Y.; Weng, T.-C.; Zwart, P.H.; et al. Simultaneous detection of electronic structure changes from two elements of a bifunctional catalyst using wavelength-dispersive X-ray emission spectroscopy and in situ electrochemistry. Phys. Chem. Chem. Phys. 2015, 17, 8901–8912. [Google Scholar] [CrossRef] [PubMed]
- Calvin, S. XAFS for Everyone, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Momma, K.; Izumi, F. VESTA: A three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 2008, 41, 653–658. [Google Scholar] [CrossRef]
- Nam, K.-W.; Kim, M.G.; Kim, K.-B. In Situ Mn K-edge X-ray Absorption Spectroscopy Studies of Electrodeposited Manganese Oxide Films for Electrochemical Capacitors. J. Phys. Chem. C 2007, 111, 749–758. [Google Scholar] [CrossRef]

| GDE before O2 Starvation | ||||||
|---|---|---|---|---|---|---|
| Condition | Path | R [Å] | CN | σ2 [Å2] | E0 [eV] | R-Factor * |
| ORR | Mn-Oads | 1.620 ± 0.019 | 1.0 | 0.006 ± 5 × 10−4 | 6556.6 ± 0.5 | 0.003 |
| Mn-Olattice | 1.896 ± 0.003 | 7.6 ± 0.3 | ||||
| Mn-Mnedge | 2.878 ± 0.004 | 4.9 | 0.006 ± 3 × 10−4 | |||
| Mn-Mncorner | 3.439 ± 0.007 | 3.7 | ||||
| OER | Mn-Oads | 1.777 ± 0.027 | 1.0 | 0.003 ± 6 × 10−4 | 6557.0 ± 0.5 | 0.004 |
| Mn-Olattice | 1.906 ± 0.004 | 5.7 ± 0.5 | ||||
| Mn-Mnedge | 2.879 ± 0.004 | 4.9 | 0.006 ± 3 × 10−4 | |||
| Mn-Mncorner | 3.442 ± 0.008 | 3.7 | ||||
| GDE after O2 starvation | ||||||
| Condition | Path | R [Å] | CN | σ2 [Å2] | E0 [eV] | R-Factor * |
| ORR | Mn-Oads | 1.699 ± 0.027 | 1.0 | 0.003 ± 8 × 10−4 | 6551.6 ± 0.9 | 0.008 |
| Mn-Olattice | 1.892 ± 0.004 | 5.8 ± 0.5 | ||||
| Mn-Mnedge | 2.869 ± 0.006 | 6.3 | 0.009 ± 4 × 10−4 | |||
| Mn-Mncorner | 3.421 ± 0.081 | 0.5 | ||||
| OER | Mn-Oads | 1.768 ± 0.040 | 1.0 | 0.002 ± 1.1 × 10−3 | 6553.8 ± 1.3 | 0.014 |
| Mn-Olattice | 1.907 ± 0.009 | 4.7 ± 0.9 | ||||
| Mn-Mnedge | 2.875 ± 0.010 | 6.3 | 0.009 ± 5 × 10−4 | |||
| Mn-Mncorner | 3.464 ± 0.097 | 0.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, E.; Oliveira De Souza, D.; Aquilanti, G.; Liebert, M.; Rossi, F.; Bozzini, B. Operando XAS of a Bifunctional Gas Diffusion Electrode for Zn-Air Batteries under Realistic Application Conditions. Appl. Sci. 2021, 11, 11672. https://doi.org/10.3390/app112411672
Marini E, Oliveira De Souza D, Aquilanti G, Liebert M, Rossi F, Bozzini B. Operando XAS of a Bifunctional Gas Diffusion Electrode for Zn-Air Batteries under Realistic Application Conditions. Applied Sciences. 2021; 11(24):11672. https://doi.org/10.3390/app112411672
Chicago/Turabian StyleMarini, Emanuele, Danilo Oliveira De Souza, Giuliana Aquilanti, Michael Liebert, Francesca Rossi, and Benedetto Bozzini. 2021. "Operando XAS of a Bifunctional Gas Diffusion Electrode for Zn-Air Batteries under Realistic Application Conditions" Applied Sciences 11, no. 24: 11672. https://doi.org/10.3390/app112411672
APA StyleMarini, E., Oliveira De Souza, D., Aquilanti, G., Liebert, M., Rossi, F., & Bozzini, B. (2021). Operando XAS of a Bifunctional Gas Diffusion Electrode for Zn-Air Batteries under Realistic Application Conditions. Applied Sciences, 11(24), 11672. https://doi.org/10.3390/app112411672







