Evaluation of Active Layer Thickness Influence in Long-Term Stability and Degradation Mechanisms in CsFAPbIBr Perovskite Solar Cells
Abstract
:Featured Application
Abstract
1. Introduction
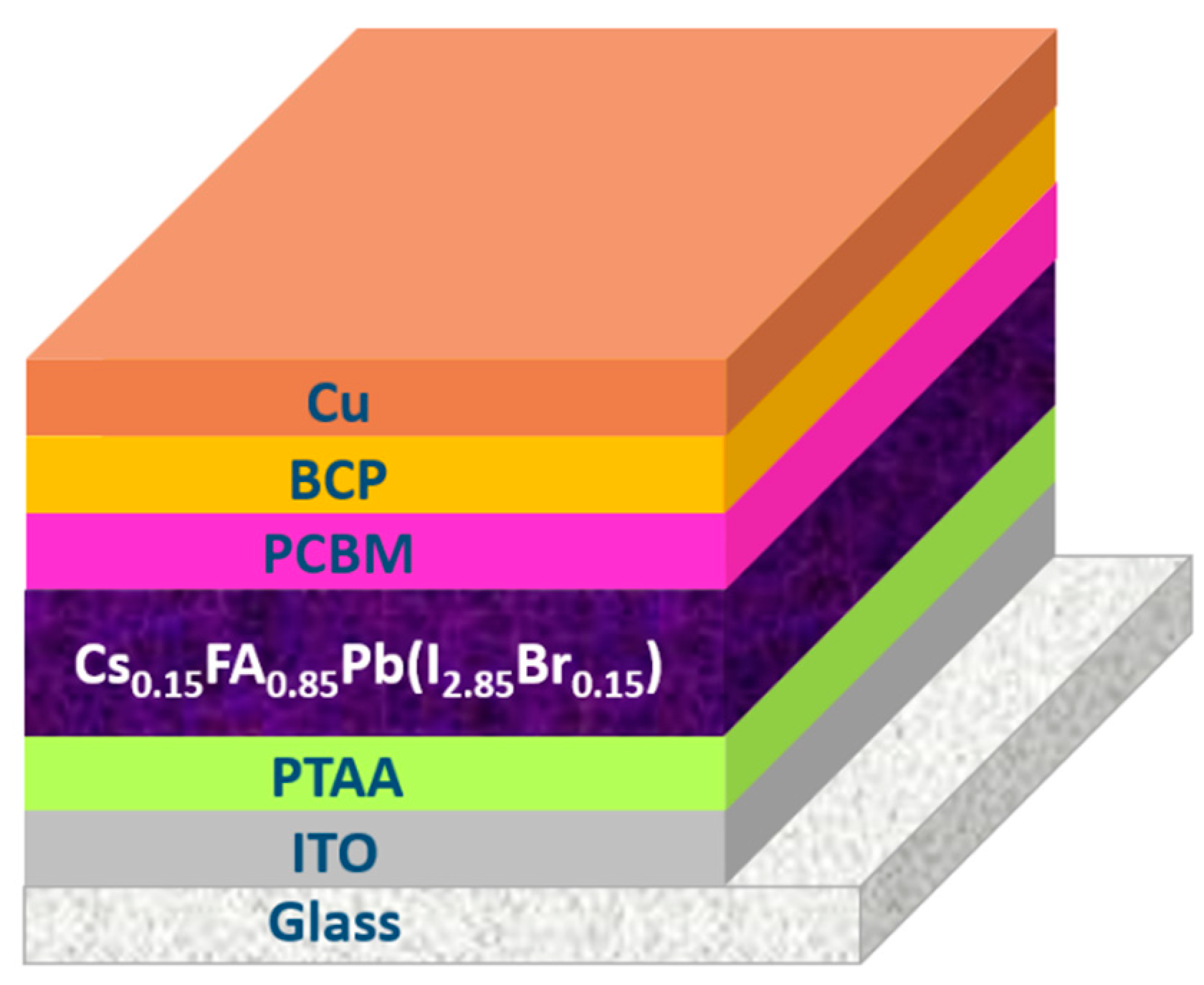
2. Materials and Methods
3. Results and Discussion
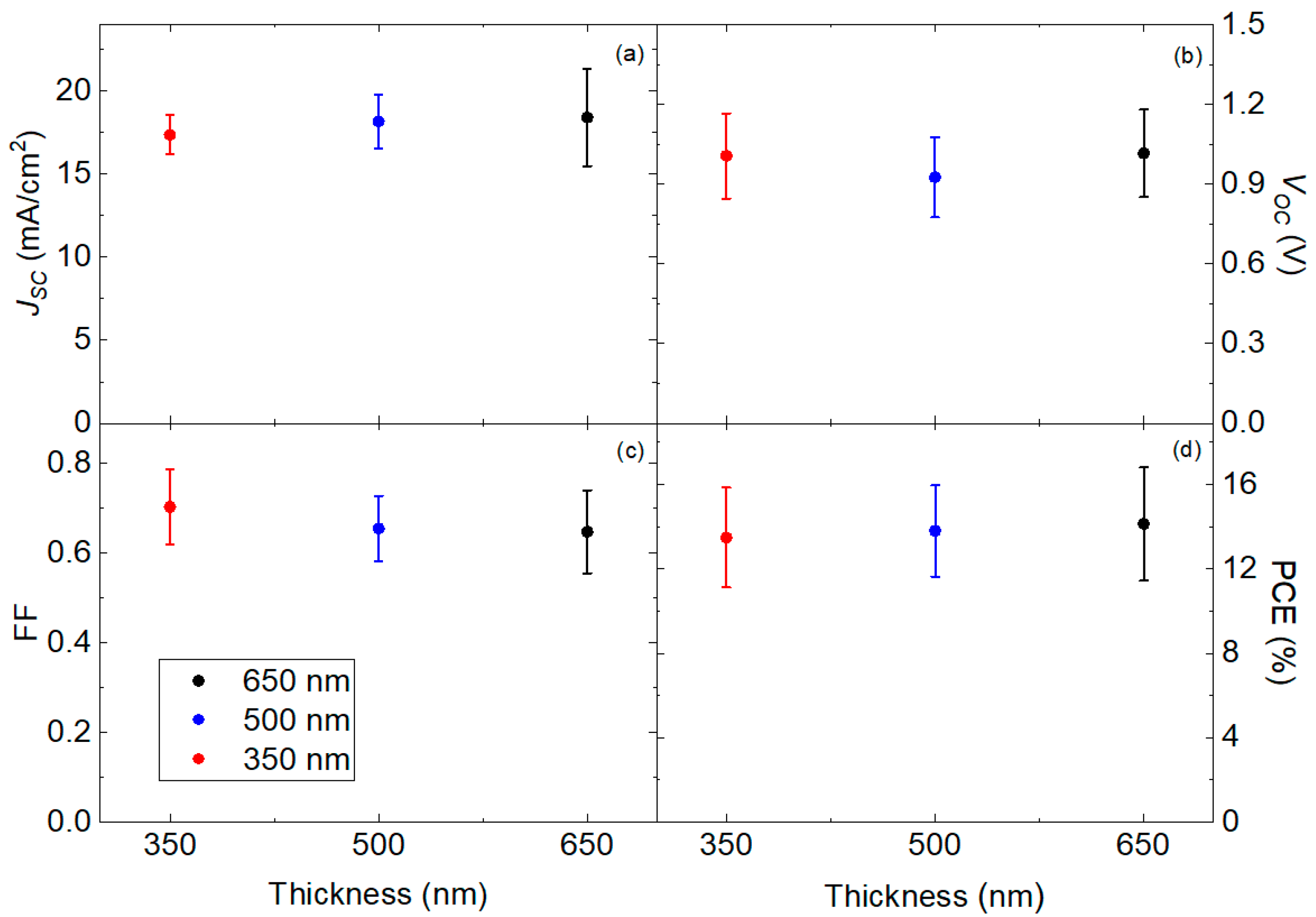
3.1. Pristine Characterization
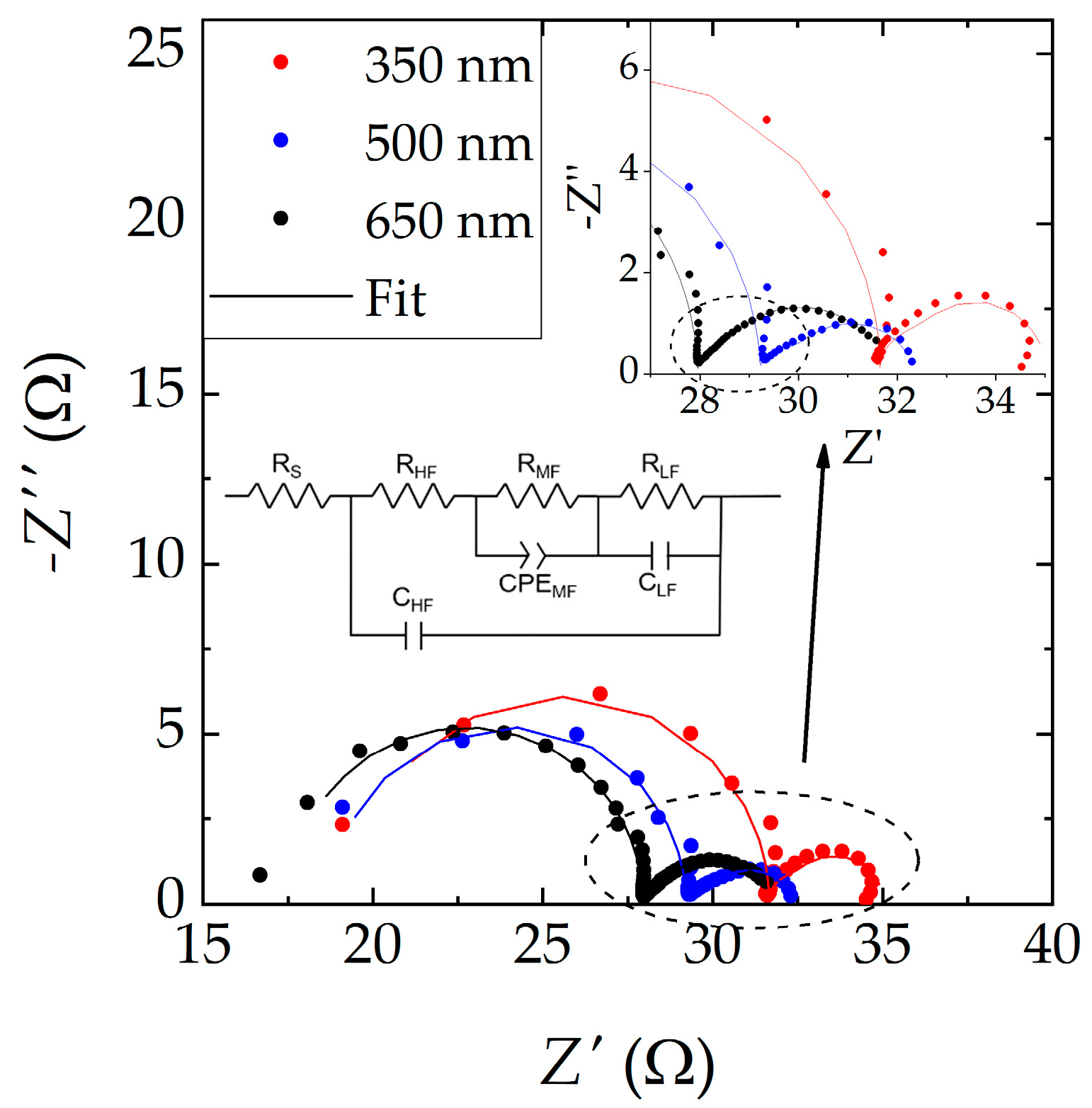
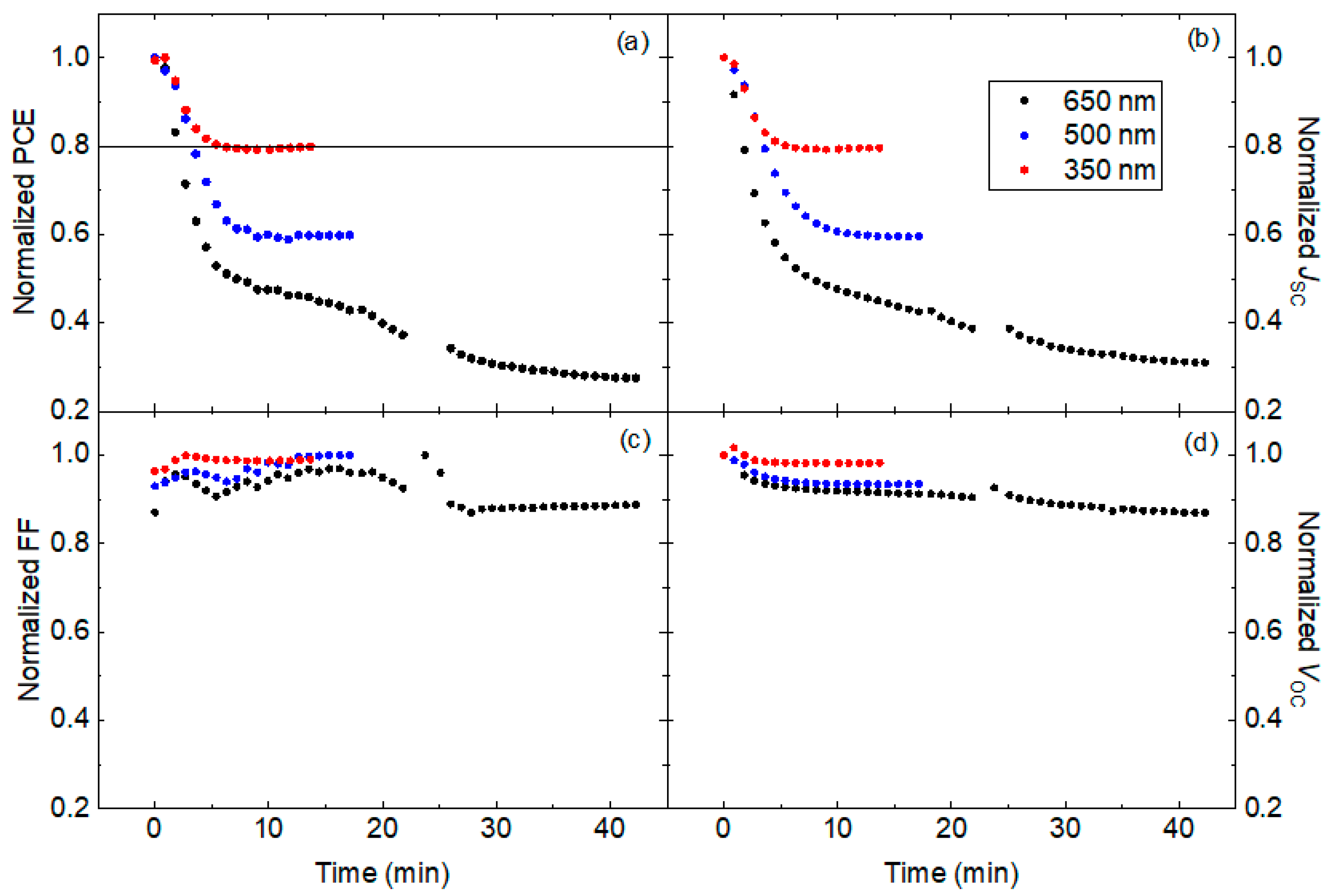
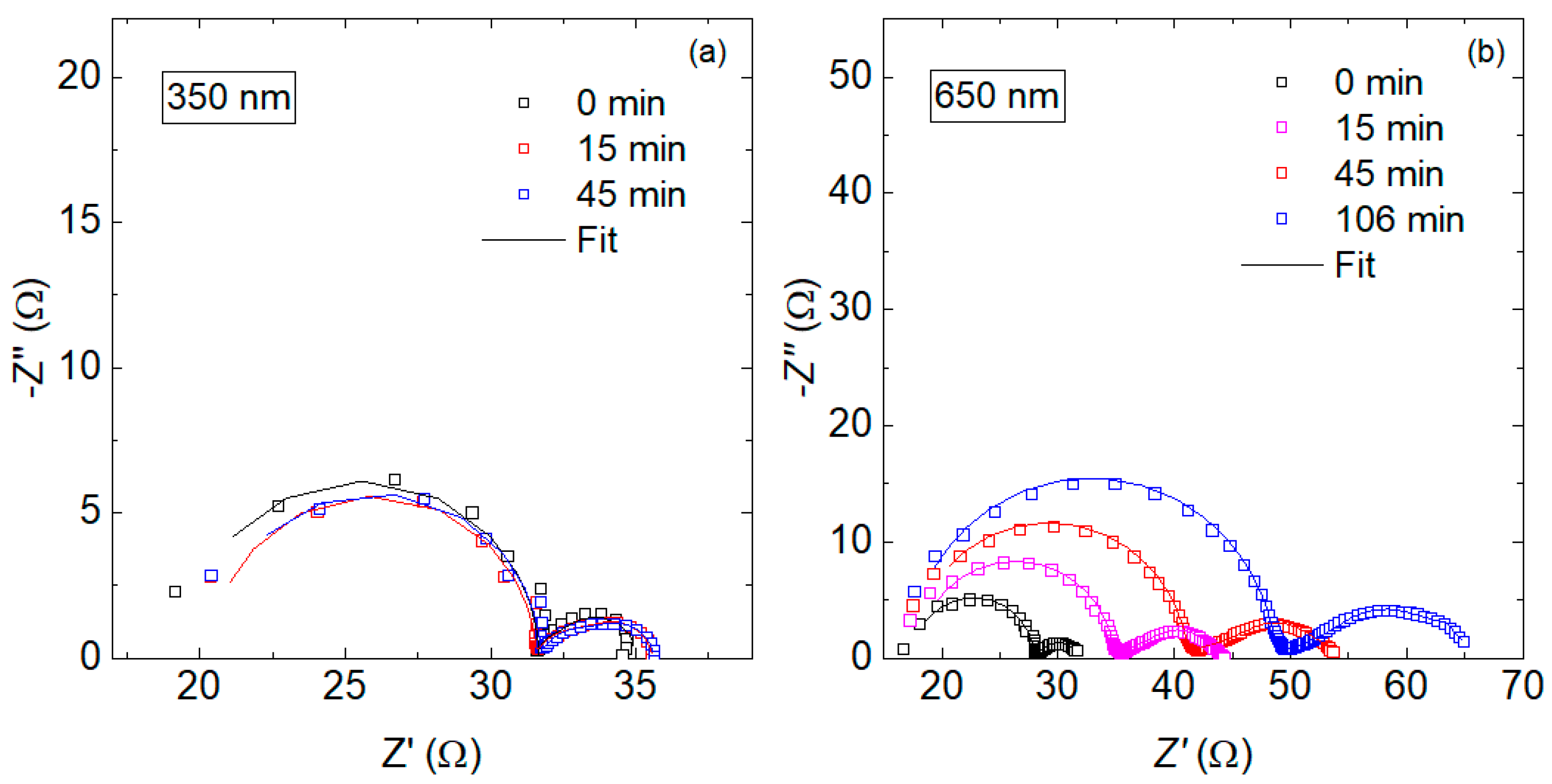
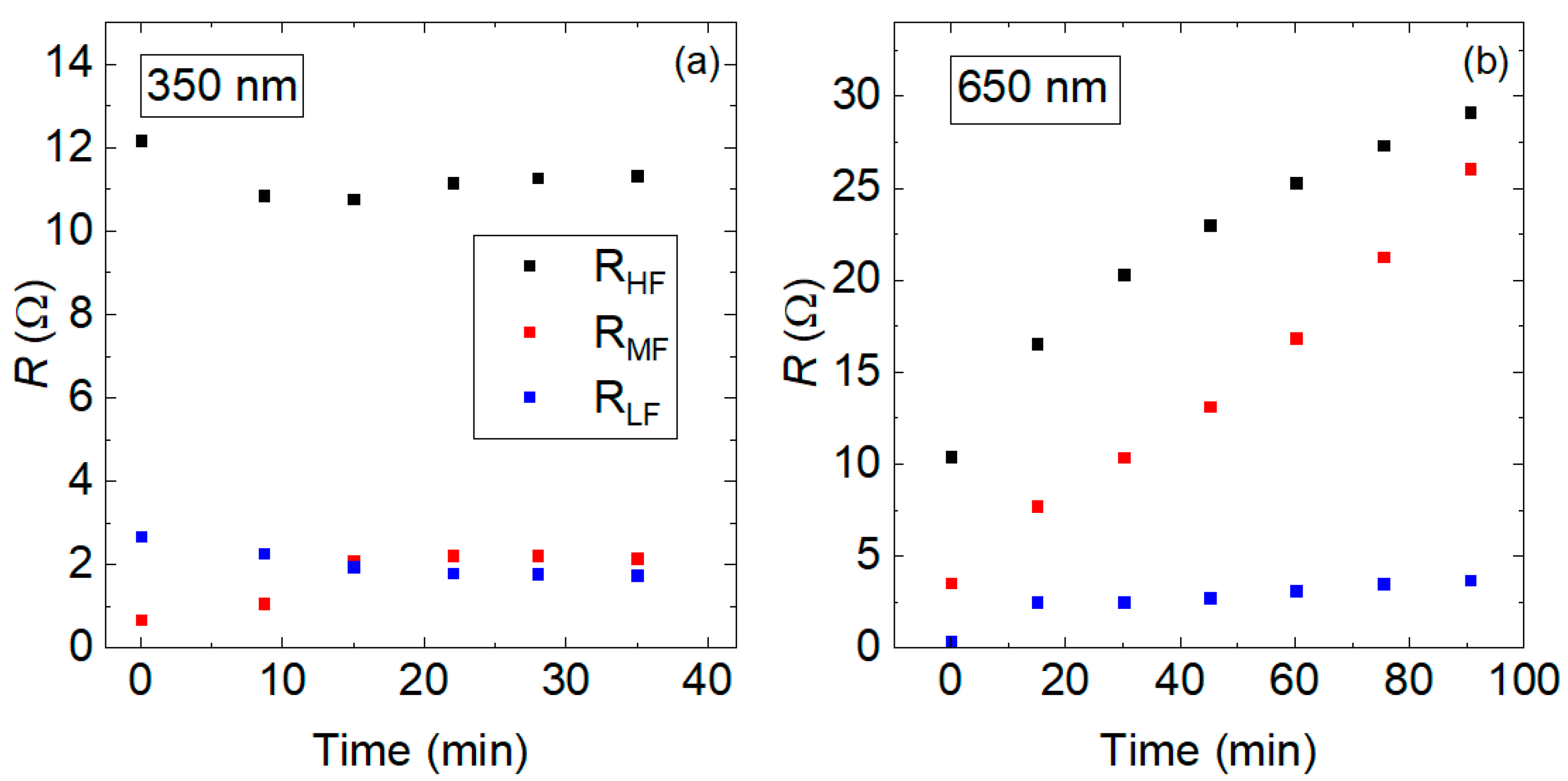
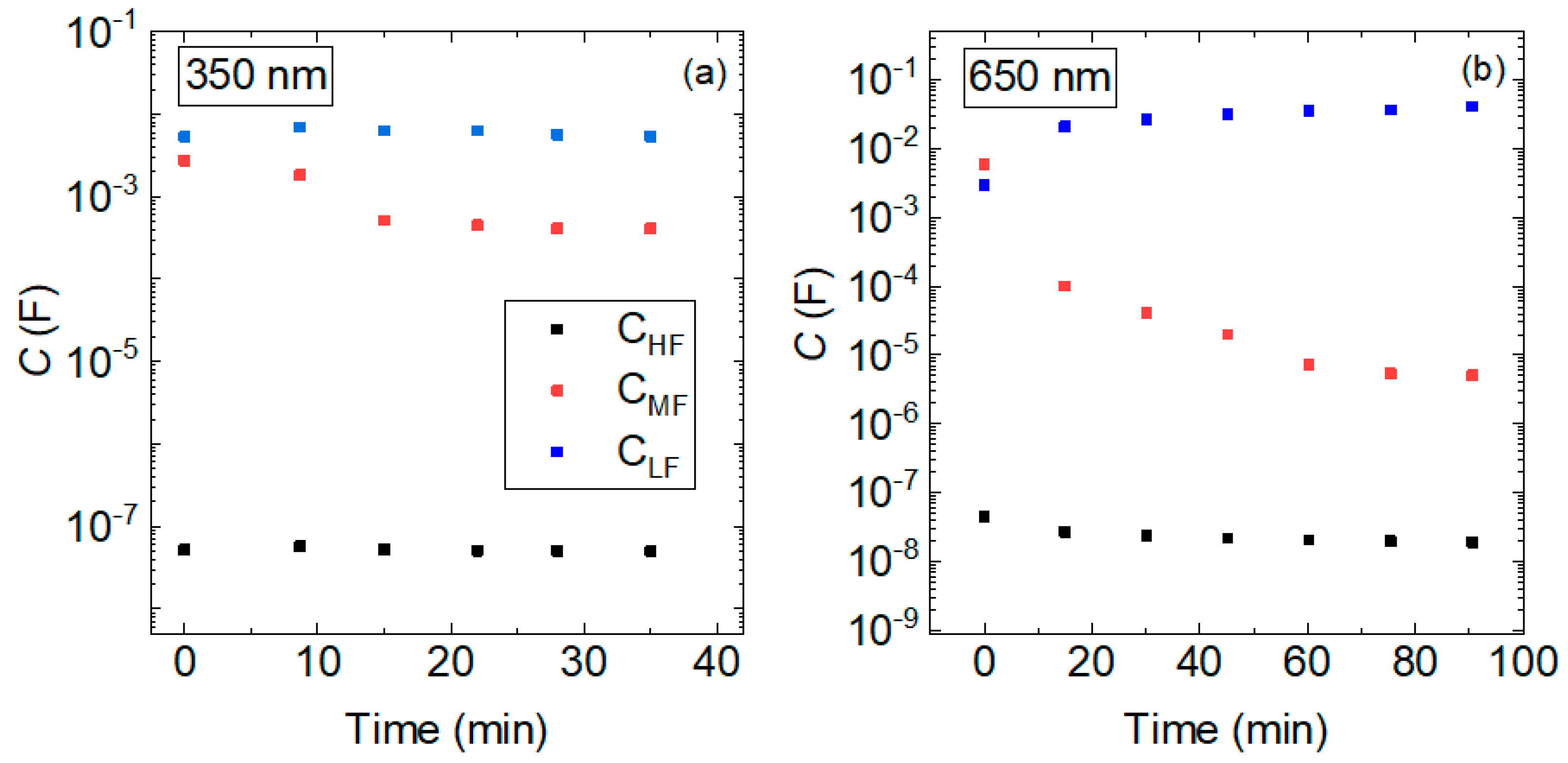
3.2. Degradation Characterization
3.3. Numerical Simulations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frost, J.M.; Butler, K.T.; Brivio, F.; Hendon, C.H.; van Schilfgaarde, M.; Walsh, A. Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 2584–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NREL Efficiency Chart. This Plot Is Courtesy of the National Renewable Energy Laboratory, Golden, CO. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies-rev211117.pdf (accessed on 24 November 2021).
- Di Giacomo, F.; Fakharuddin, A.; Jose, R.; Brown, T.M. Progress, Challenges and Perspectives in Flexible Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 3007–3035. [Google Scholar] [CrossRef] [Green Version]
- Snaith, H.J.; Abate, A.; Ball, J.M.; Eperon, G.E.; Leijtens, T.; Noel, N.K.; Stranks, S.D.; Wang, J.T.-W.; Wojciechowski, K.; Zhang, W. Anomalous Hysteresis in Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, N.K.; Uddin, A. Hysteresis in Organic-Inorganic Hybrid Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 157, 476–509. [Google Scholar] [CrossRef]
- Correa-Baena, J.-P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and Challenges of Perovskite Solar Cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-L.; Yang, C.-H.; Chuang, Y.-Y. A Comparative Study of the Effect of Fluorine Substitution on the Photovoltaic Performance of Benzothiadiazole-Based Copolymers. RSC Adv. 2016, 6, 47676–47686. [Google Scholar] [CrossRef]
- Xu, K.J.; Wang, R.T.; Xu, A.F.; Chen, J.Y.; Xu, G. Hysteresis and Instability Predicted in Moisture Degradation of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 48882–48889. [Google Scholar] [CrossRef] [PubMed]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast Oxygen Diffusion and Iodide Defects Mediate Oxygen-Induced Degradation of Perovskite Solar Cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.; Aristidou, N.; Pont, S.; Sanchez-Molina, I.; Chotchunangatchaval, T.; Wheeler, S.; Durrant, J.R.; Haque, S.A. Light and Oxygen Induced Degradation Limits the Operational Stability of Methylammonium Lead Triiodide Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 1655–1660. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-W.; Kim, S.; Bae, S.; Cho, K.; Chung, T.; Mundt, L.E.; Lee, S.; Park, S.; Park, H.; Schubert, M.C.; et al. UV Degradation and Recovery of Perovskite Solar Cells. Sci. Rep. 2016, 6, 38150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, A.; Hossain, I.M.; Moghadamzadeh, S.; Schwenzer, J.A.; Abzieher, T.; Richards, B.S.; Klampaftis, E.; Paetzold, U.W. Spectral Dependence of Degradation under Ultraviolet Light in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 21985–21990. [Google Scholar] [CrossRef] [PubMed]
- Papež, N.; Gajdoš, A.; Dallaev, R.; Sobola, D.; Sedlák, P.; Motúz, R.; Nebojsa, A.; Grmela, L. Performance Analysis of GaAs Based Solar Cells under Gamma Irradiation. Appl. Surf. Sci. 2020, 510, 145329. [Google Scholar] [CrossRef]
- Misra, R.K.; Aharon, S.; Li, B.; Mogilyansky, D.; Visoly-Fisher, I.; Etgar, L.; Katz, E.A. Temperature- and Component-Dependent Degradation of Perovskite Photovoltaic Materials under Concentrated Sunlight. J. Phys. Chem. Lett. 2015, 6, 326–330. [Google Scholar] [CrossRef]
- Abdelmageed, G.; Mackeen, C.; Hellier, K.; Jewell, L.; Seymour, L.; Tingwald, M.; Bridges, F.; Zhang, J.Z.; Carter, S. Effect of Temperature on Light Induced Degradation in Methylammonium Lead Iodide Perovskite Thin Films and Solar Cells. Sol. Energy Mater. Sol. Cells 2018, 174, 566–571. [Google Scholar] [CrossRef]
- Back, H.; Kim, G.; Kim, J.; Kong, J.; Kim, T.K.; Kang, H.; Kim, H.; Lee, J.; Lee, S.; Lee, K. Achieving Long-Term Stable Perovskite Solar Cells via Ion Neutralization. Energy Environ. Sci. 2016, 9, 1258–1263. [Google Scholar] [CrossRef]
- Li, J.; Dong, Q.; Li, N.; Wang, L. Direct Evidence of Ion Diffusion for the Silver-Electrode-Induced Thermal Degradation of Inverted Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1602922. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateen, M.; Arain, Z.; Liu, X.; Liu, C.; Yang, Y.; Ding, Y.; Ma, S.; Ren, Y.; Wu, Y.; Tao, Y.; et al. High-Performance Mixed-Cation Mixed-Halide Perovskite Solar Cells Enabled by a Facile Intermediate Engineering Technique. J. Power Sources 2020, 448, 227386. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, D.-H.; Kim, H.-S.; Seo, S.-W.; Cho, S.M.; Park, N.-G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Chai, L.; Zhong, M.; Li, X.; Wu, N.; Zhou, J. The Effect of Bromine Doping on the Perovskite Solar Cells Modified by PVP/PEG Polymer Blends. Superlattices Microstruct. 2018, 120, 279–287. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, J.; Lan, Z.; He, X.; Dong, J.; Jia, J.; Guo, P.; Lin, J.; Huang, M.; Huang, Y. Modulated CH3NH3PbI3−xBrx Film for Efficient Perovskite Solar Cells Exceeding 18%. Sci. Rep. 2017, 7, 44603. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Gangishetty, M.K.; Kelly, T.L. Effect of CH3NH3PbI3 Thickness on Device Efficiency in Planar Heterojunction Perovskite Solar Cells. J. Mater. Chem. A 2014, 2, 19873–19881. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Zhang, M.-J.; Pang, S.-P.; Huang, C.-S.; Zhou, Z.-M.; Wang, D.; Wang, N.; Cui, G.-L. Carrier Transport in CH 3 NH 3 PbI 3 Films with Different Thickness for Perovskite Solar Cells. Adv. Mater. Interfaces 2016, 3, 1600327. [Google Scholar] [CrossRef]
- Chen, J.; Zuo, L.; Zhang, Y.; Lian, X.; Fu, W.; Yan, J.; Li, J.; Wu, G.; Li, C.-Z.; Chen, H. High-Performance Thickness Insensitive Perovskite Solar Cells with Enhanced Moisture Stability. Adv. Energy Mater. 2018, 8, 1800438. [Google Scholar] [CrossRef]
- Hernández-Balaguera, E.; Romero, B.; Arredondo, B.; del Pozo, G.; Najafi, M.; Galagan, Y. The Dominant Role of Memory-Based Capacitive Hysteretic Currents in Operation of Photovoltaic Perovskites. Nano Energy 2020, 78, 105398. [Google Scholar] [CrossRef]
- Arredondo, B.; Romero, B.; Sanchez Pena, J.M.; Fernandez-Pacheco, A.; Alonso, E.; Vergaz, R.; de Dios, C. Visible Light Communication System Using an Organic Bulk Heterojunction Photodetector. Sensors 2013, 13, 12266–12276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus Statement for Stability Assessment and Reporting for Perovskite Photovoltaics Based on ISOS Procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]
- von Hauff, E. Impedance Spectroscopy for Emerging Photovoltaics. J. Phys. Chem. C 2019, 123, 11329–11346. [Google Scholar] [CrossRef] [Green Version]
- Dualeh, A.; Moehl, T.; Tétreault, N.; Teuscher, J.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Impedance Spectroscopic Analysis of Lead Iodide Perovskite-Sensitized Solid-State Solar Cells. ACS Nano 2014, 8, 362–373. [Google Scholar] [CrossRef]
- Pascoe, A.R.; Duffy, N.W.; Scully, A.D.; Huang, F.; Cheng, Y.-B. Insights into Planar CH3NH3PbI3 Perovskite Solar Cells Using Impedance Spectroscopy. J. Phys. Chem. C 2015, 119, 4444–4453. [Google Scholar] [CrossRef]
- Bou, A.; Pockett, A.; Raptis, D.; Watson, T.; Carnie, M.J.; Bisquert, J. Beyond Impedance Spectroscopy of Perovskite Solar Cells: Insights from the Spectral Correlation of the Electrooptical Frequency Techniques. J. Phys. Chem. Lett. 2020, 11, 8654–8659. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.; Ramos-Terrón, S.; Riquelme, A.; Boix, P.P.; Idígoras, J.A.; Seró, I.M.; Anta, J.A. Impedance Analysis of Perovskite Solar Cells: A Case Study. J. Mater. Chem. A 2019, 7, 12191–12200. [Google Scholar] [CrossRef]
- Todinova, A.; Contreras-Bernal, L.; Salado, M.; Ahmad, S.; Morillo, N.; Idígoras, J.; Anta, J.A. Towards a Universal Approach for the Analysis of Impedance Spectra of Perovskite Solar Cells: Equivalent Circuits and Empirical Analysis. ChemElectroChem 2017, 4, 2891–2901. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Sanchez, R.S.; Badia, L.; Garcia-Belmonte, G.; Kang, Y.S.; Mora-Sero, I.; Bisquert, J. Photoinduced Giant Dielectric Constant in Lead Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 2390–2394. [Google Scholar] [CrossRef] [PubMed]
- Almora, O.; Zarazua, I.; Mas-Marza, E.; Mora-Sero, I.; Bisquert, J.; Garcia-Belmonte, G. Capacitive Dark Currents, Hysteresis, and Electrode Polarization in Lead Halide Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Garcia-Belmonte, G.; Mora-Sero, I.; Bisquert, J.; Kang, Y.S.; Jacobsson, T.J.; Correa-Baena, J.-P.; Hagfeldt, A. Properties of Contact and Bulk Impedances in Hybrid Lead Halide Perovskite Solar Cells Including Inductive Loop Elements. J. Phys. Chem. C 2016, 120, 8023–8032. [Google Scholar] [CrossRef] [Green Version]
- Zarazua, I.; Bisquert, J.; Garcia-Belmonte, G. Light-Induced Space-Charge Accumulation Zone as Photovoltaic Mechanism in Perovskite Solar Cells. J. Phys. Chem. Lett. 2016, 7, 525–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bag, M.; Renna, L.A.; Adhikari, R.Y.; Karak, S.; Liu, F.; Lahti, P.M.; Russell, T.P.; Tuominen, M.T.; Venkataraman, D. Kinetics of Ion Transport in Perovskite Active Layers and Its Implications for Active Layer Stability. J. Am. Chem. Soc. 2015, 137, 13130–13137. [Google Scholar] [CrossRef]
- Pockett, A.; Eperon, G.E.; Sakai, N.; Snaith, H.J.; Peter, L.M.; Cameron, P.J. Microseconds, Milliseconds and Seconds: Deconvoluting the Dynamic Behaviour of Planar Perovskite Solar Cells. Phys. Chem. Chem. Phys. 2017, 19, 5959–5970. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Balaguera, E.; Arredondo, B.; del Pozo, G.; Romero, B. Exploring the Impact of Fractional-Order Capacitive Behavior on the Hysteresis Effects of Perovskite Solar Cells: A Theoretical Perspective. Commun. Nonlinear Sci. Numer. Simul. 2020, 90, 105371. [Google Scholar] [CrossRef]
- Hailegnaw, B.; Sariciftci, N.S.; Scharber, M.C. Impedance Spectroscopy of Perovskite Solar Cells: Studying the Dynamics of Charge Carriers Before and After Continuous Operation. Phys. Status Solidi A 2020, 217, 2000291. [Google Scholar] [CrossRef]
- Zarazua, I.; Han, G.; Boix, P.P.; Mhaisalkar, S.; Fabregat-Santiago, F.; Mora-Seró, I.; Bisquert, J.; Garcia-Belmonte, G. Surface Recombination and Collection Efficiency in Perovskite Solar Cells from Impedance Analysis. J. Phys. Chem. Lett. 2016, 7, 5105–5113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tress, W.; Yavari, M.; Domanski, K.; Yadav, P.; Niesen, B.; Baena, J.P.C.; Hagfeldt, A.; Graetzel, M. Interpretation and Evolution of Open-Circuit Voltage, Recombination, Ideality Factor and Subgap Defect States during Reversible Light-Soaking and Irreversible Degradation of Perovskite Solar Cells. Energy Environ. Sci. 2018, 11, 151–165. [Google Scholar] [CrossRef]
- Almora, O.; Aranda, C.; Garcia-Belmonte, G. Do Capacitance Measurements Reveal Light-Induced Bulk Dielectric Changes in Photovoltaic Perovskites? J. Phys. Chem. C 2018, 122, 13450–13454. [Google Scholar] [CrossRef]
- Arredondo, B.; Romero, B.; Beliatis, M.J.; del Pozo, G.; Martín-Martín, D.; Blakesley, J.C.; Dibb, G.; Krebs, F.C.; Gevorgyan, S.A.; Castro, F.A. Analysing Impact of Oxygen and Water Exposure on Roll-Coated Organic Solar Cell Performance Using Impedance Spectroscopy. Sol. Energy Mater. Sol. Cells 2018, 176, 397–404. [Google Scholar] [CrossRef]
- Kong, J.; Song, S.; Yoo, M.; Lee, G.Y.; Kwon, O.; Park, J.K.; Back, H.; Kim, G.; Lee, S.H.; Suh, H.; et al. Long-Term Stable Polymer Solar Cells with Significantly Reduced Burn-in Loss. Nat. Commun. 2014, 5, 5688. [Google Scholar] [CrossRef]
- Hang, P.; Xie, J.; Kan, C.; Li, B.; Zhang, Y.; Gao, P.; Yang, D.; Yu, X. Stabilizing Fullerene for Burn-in-Free and Stable Perovskite Solar Cells under Ultraviolet Preconditioning and Light Soaking. Adv. Mater. 2021, 33, 2006910. [Google Scholar] [CrossRef]
- Peters, C.H.; Sachs-Quintana, I.T.; Mateker, W.R.; Heumueller, T.; Rivnay, J.; Noriega, R.; Beiley, Z.M.; Hoke, E.T.; Salleo, A.; McGehee, M.D. The Mechanism of Burn-in Loss in a High Efficiency Polymer Solar Cell. Adv. Mater. 2012, 24, 663–668. [Google Scholar] [CrossRef]
- Bertoluzzi, L.; Boyd, C.C.; Rolston, N.; Xu, J.; Prasanna, R.; O’Regan, B.C.; McGehee, M.D. Mobile Ion Concentration Measurement and Open-Access Band Diagram Simulation Platform for Halide Perovskite Solar Cells. Joule 2020, 4, 109–127. [Google Scholar] [CrossRef]
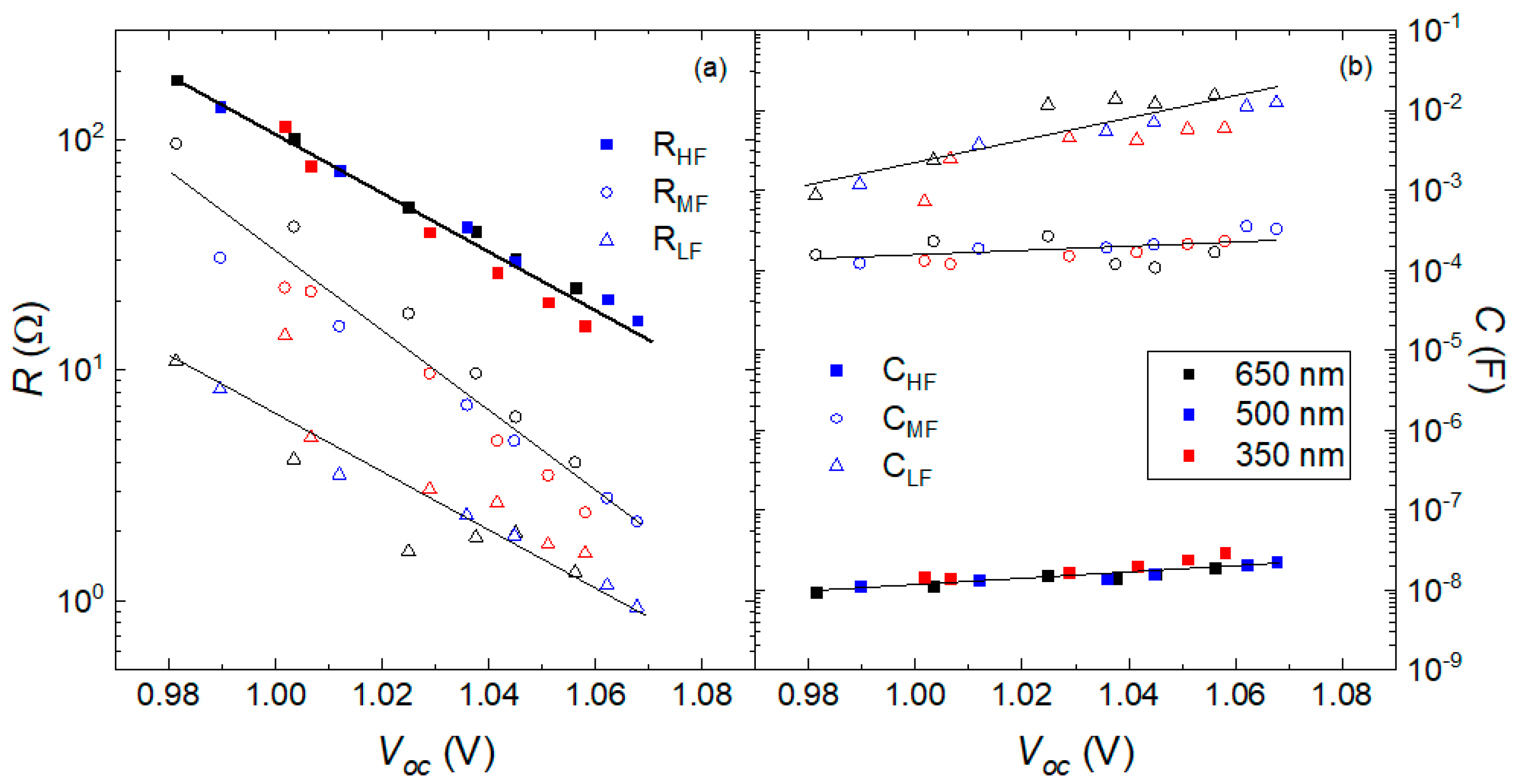
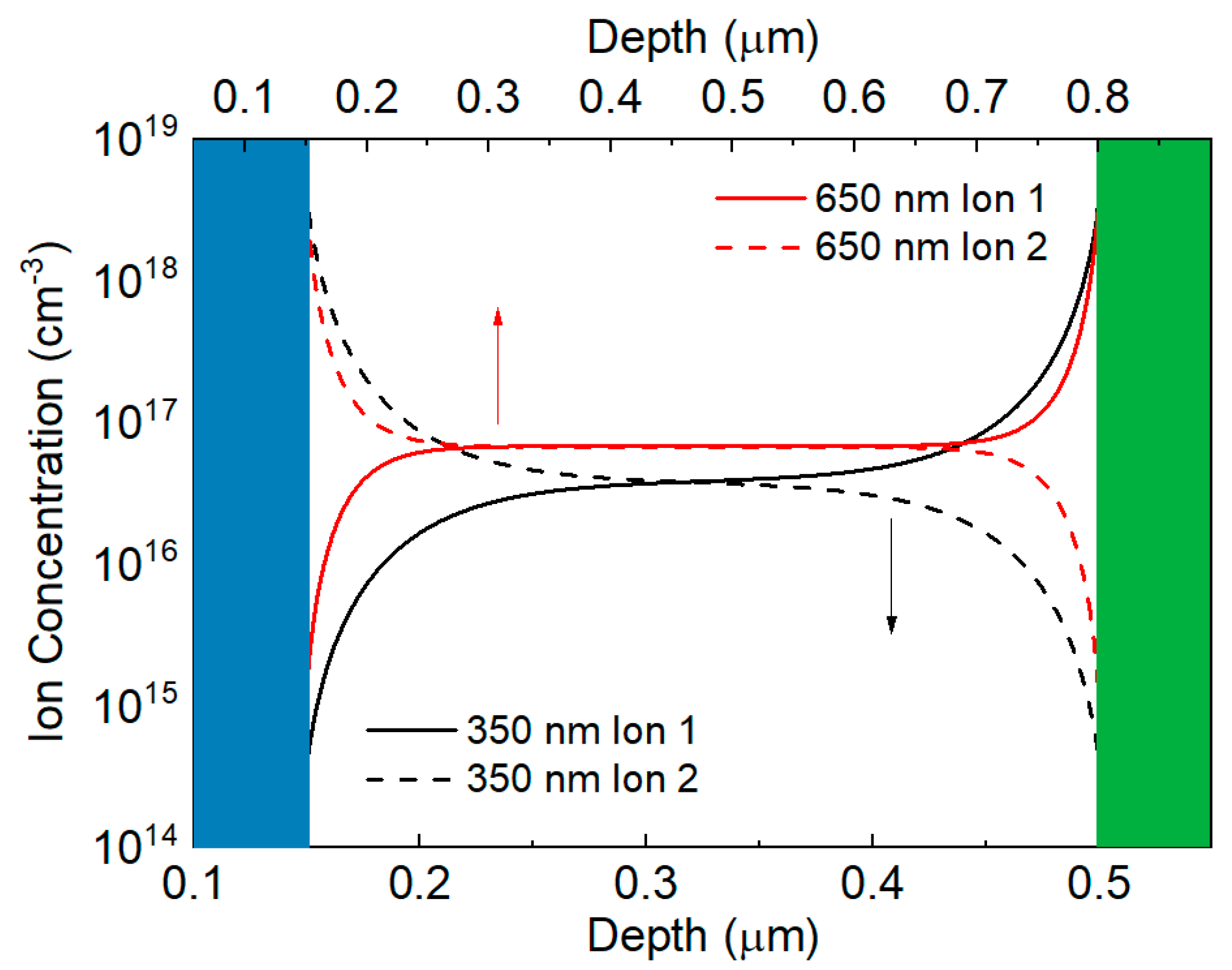
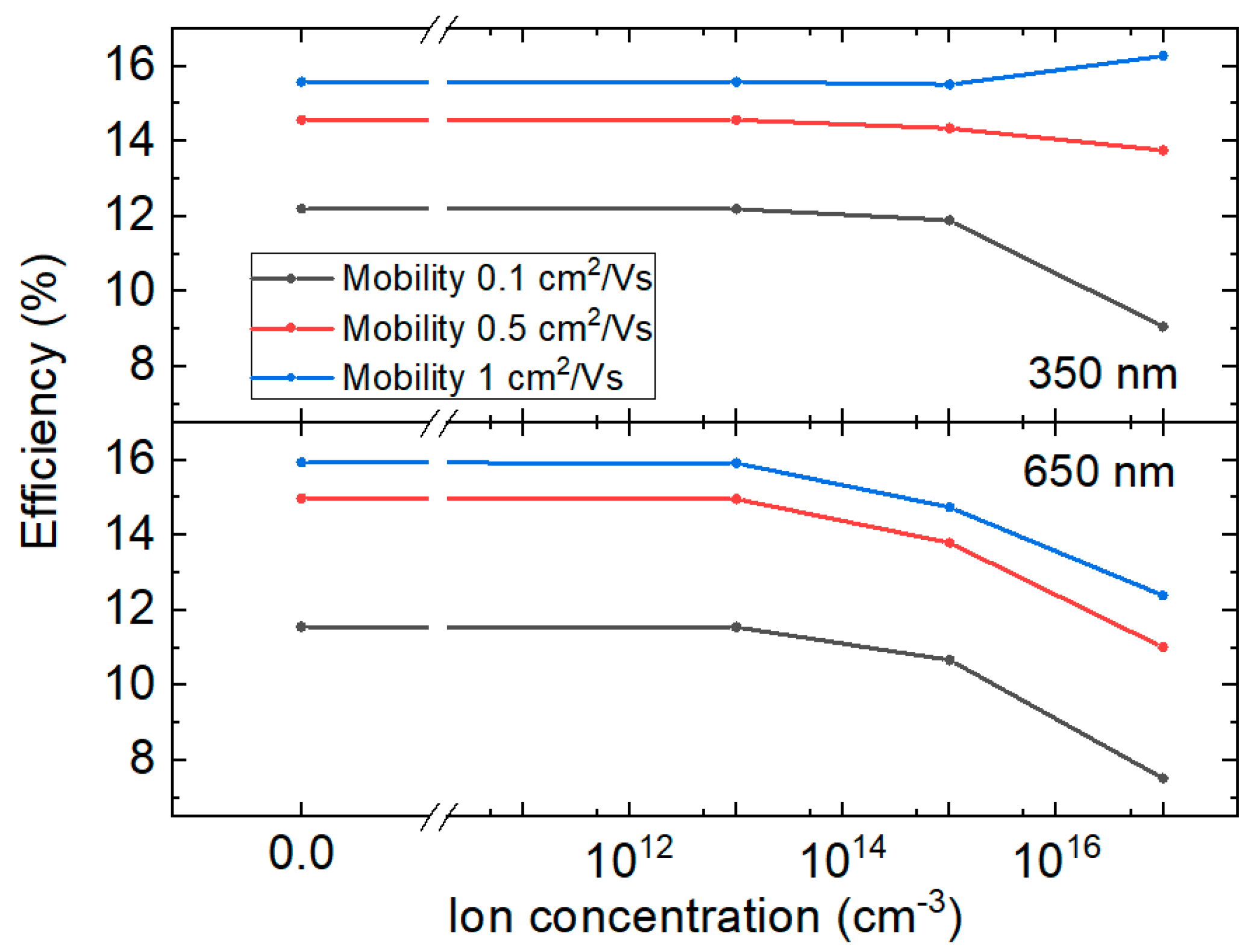
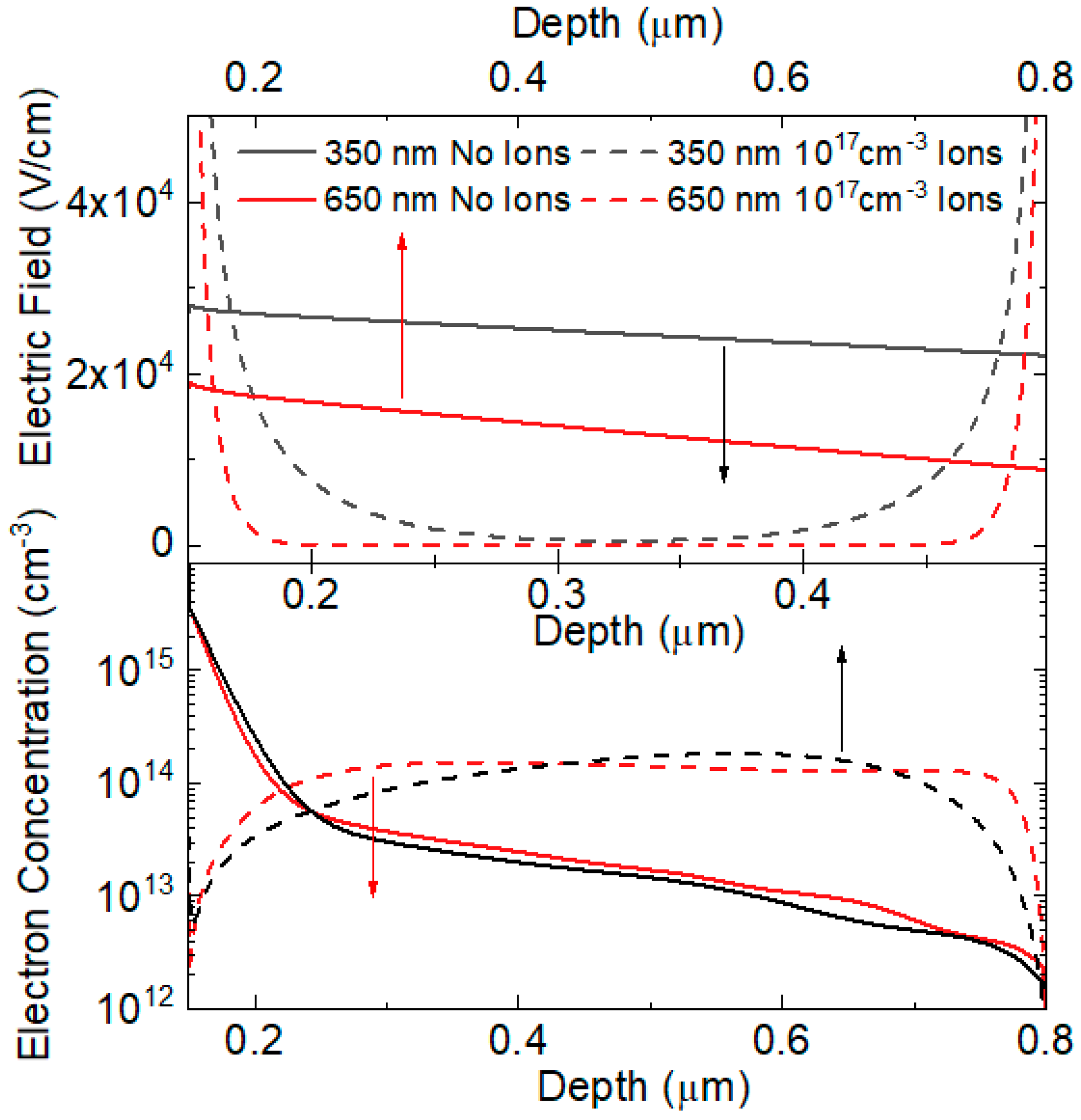
| Thickness (nm) | n from VOC | n from RHF | n from RLF | n from CLF |
|---|---|---|---|---|
| 650 | 1.2 | 1.4 | 1.5 | 1.2 |
| 500 | 1.2 | 1.4 | 1.5 | 1.4 |
| 350 | 1.3 | 1.2 | 1.2 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-González, M.C.; del Pozo, G.; Martín-Martín, D.; Muñoz-Díaz, L.; Pérez-Martínez, J.C.; Hernández-Balaguera, E.; Arredondo, B.; Galagan, Y.; Najafi, M.; Romero, B. Evaluation of Active Layer Thickness Influence in Long-Term Stability and Degradation Mechanisms in CsFAPbIBr Perovskite Solar Cells. Appl. Sci. 2021, 11, 11668. https://doi.org/10.3390/app112411668
López-González MC, del Pozo G, Martín-Martín D, Muñoz-Díaz L, Pérez-Martínez JC, Hernández-Balaguera E, Arredondo B, Galagan Y, Najafi M, Romero B. Evaluation of Active Layer Thickness Influence in Long-Term Stability and Degradation Mechanisms in CsFAPbIBr Perovskite Solar Cells. Applied Sciences. 2021; 11(24):11668. https://doi.org/10.3390/app112411668
Chicago/Turabian StyleLópez-González, Mari Carmen, Gonzalo del Pozo, Diego Martín-Martín, Laura Muñoz-Díaz, José Carlos Pérez-Martínez, Enrique Hernández-Balaguera, Belén Arredondo, Yulia Galagan, Mehrdad Najafi, and Beatriz Romero. 2021. "Evaluation of Active Layer Thickness Influence in Long-Term Stability and Degradation Mechanisms in CsFAPbIBr Perovskite Solar Cells" Applied Sciences 11, no. 24: 11668. https://doi.org/10.3390/app112411668
APA StyleLópez-González, M. C., del Pozo, G., Martín-Martín, D., Muñoz-Díaz, L., Pérez-Martínez, J. C., Hernández-Balaguera, E., Arredondo, B., Galagan, Y., Najafi, M., & Romero, B. (2021). Evaluation of Active Layer Thickness Influence in Long-Term Stability and Degradation Mechanisms in CsFAPbIBr Perovskite Solar Cells. Applied Sciences, 11(24), 11668. https://doi.org/10.3390/app112411668







