The Metabolic Stability of Antimicrobial Peptides IK8 in Plasma and Liver S9
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.2.1. HPLC Sample Preparation
2.2.2. Q-TOF Sample Preparation
2.3. Equipment and Chromatographic Conditions
3. Results
3.1. Plasma Metabolic Stability
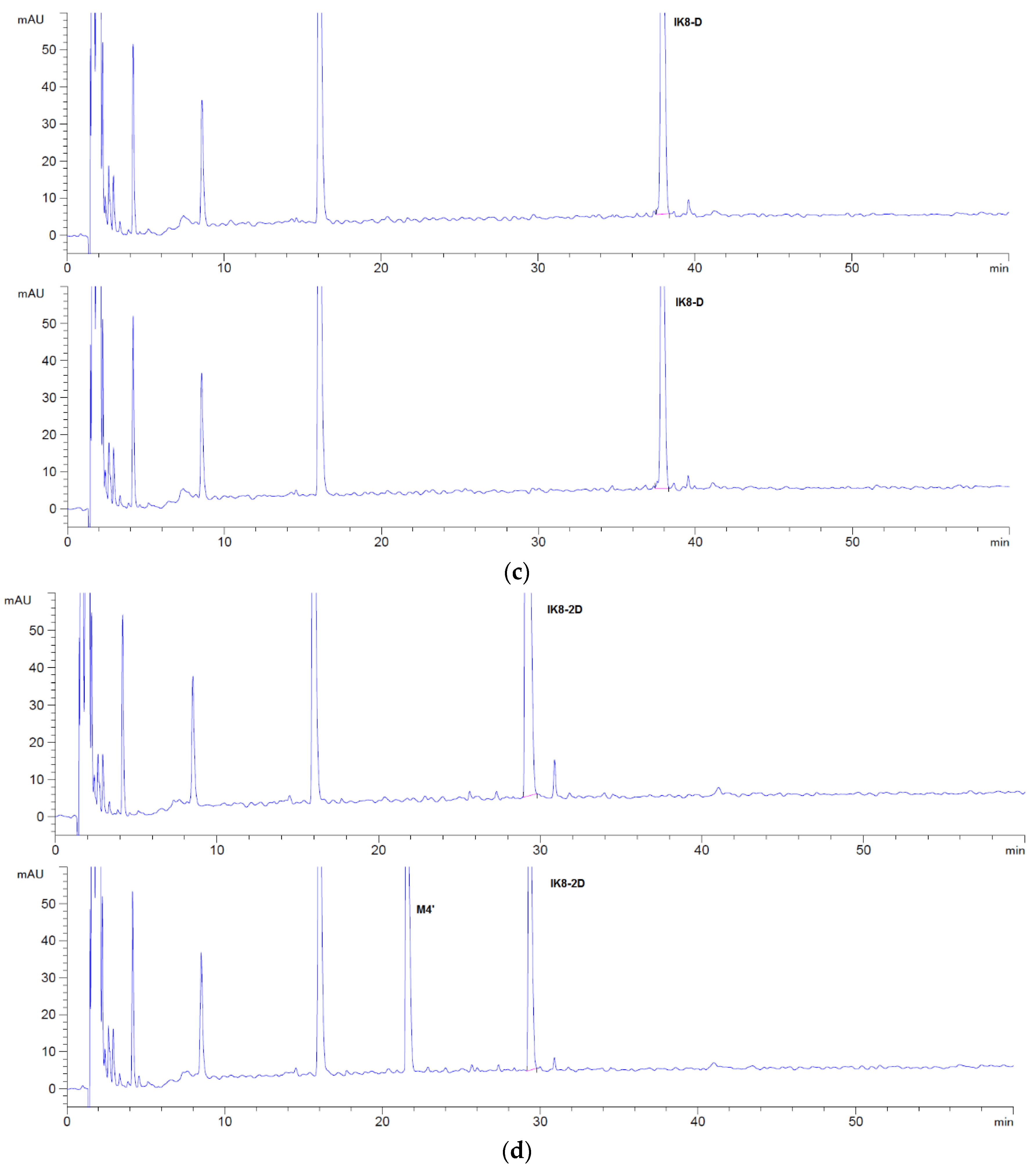
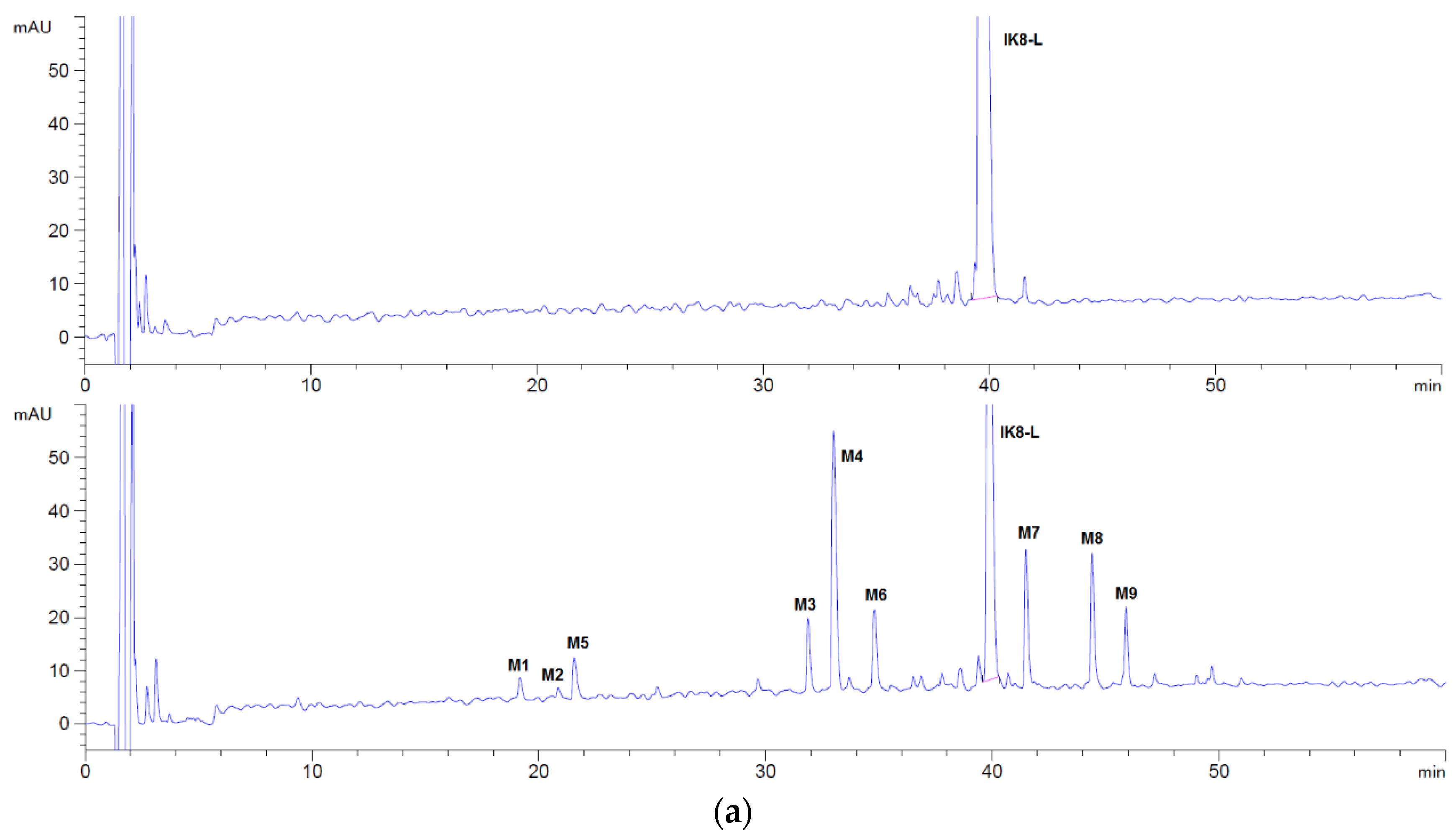
3.1.1. Incubation Chromatograms between Three Configurations IK8 and Plasma of Different Species (Using Rat Plasma Incubation as an Example)
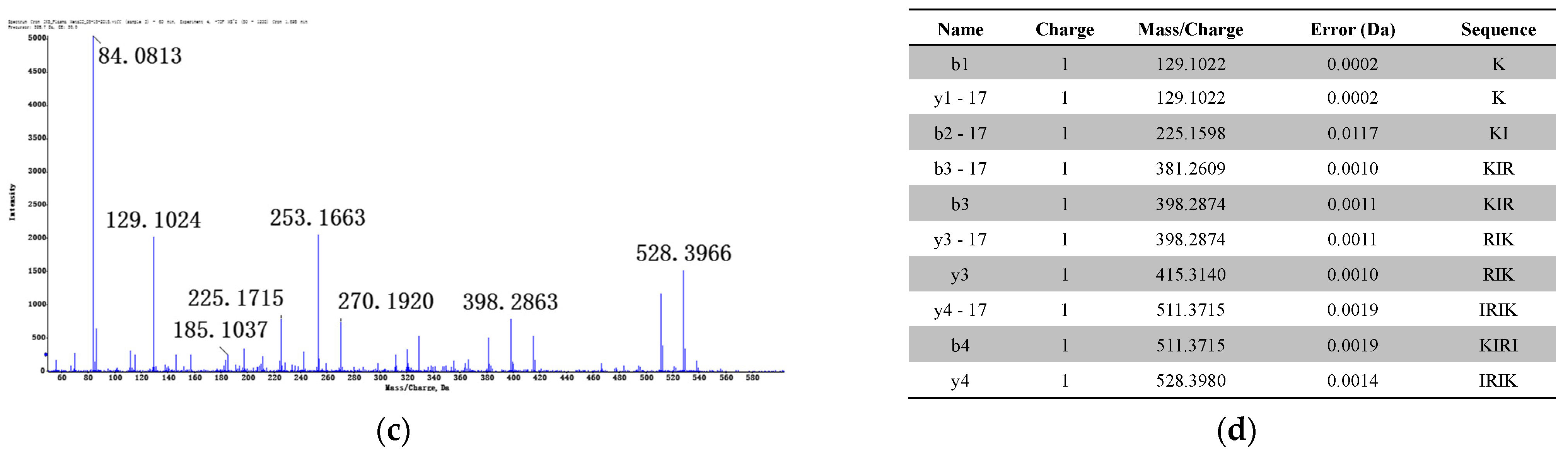
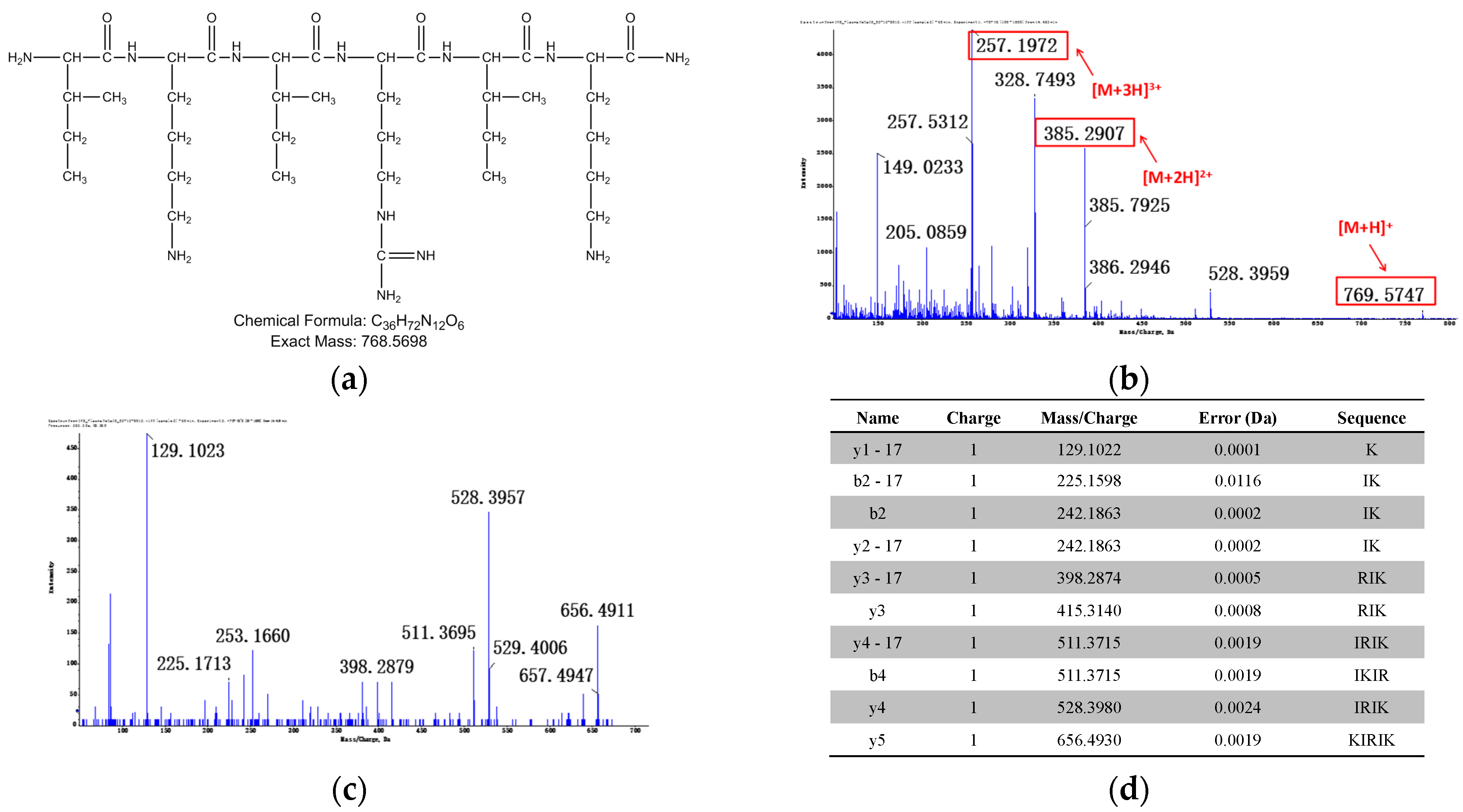
3.1.2. Identification of Metabolites after Incubation between Three Configurations IK8 and Plasma of Different Species (Using Rat Plasma Incubation as an Example)
3.2. Liver S9 Metabolic Stability
3.2.1. Incubation Chromatograms between Three Configurations IK8 and Liver S9 of Different Species (Using Rat Liver S9 Incubation as an Example)
3.2.2. Identification of Metabolites after Incubation between Three Configurations IK8 and Liver S9 of Different Species (Using Rat Liver S9 Incubation as an Example)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2016, 22, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Essig, A.; Hofmann, D.; Münch, D.; Gayathri, S.; Künzler, M.; Kallio, P.T.; Sahl, H.G.; Wider, G.; Schneider, T.; Aebi, M. Copsin, a novel peptide-based fungal antibiotic interfering with the peptidoglycan synthesis. J. Biol. Chem. 2014, 289, 34953–34964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Patočka, J.; Kuča, K. Insect antimicrobial peptides, a mini review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, Q.; Lu, Z.; Gao, Y.; Trembleau, L.; Ebel, R.; Andersen, J.H.; Philips, C.; Law, S.; Deng, H. Discovery and biosynthetic investigation of a new antibacterial dehydrated non-ribosomal tripeptide. Angew. Chem. 2021, 60, 3229–3237. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet. Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Erdem Büyükkiraz, M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Chin, W.; Dong, H.; Xu, L.; Zhong, G.; Huang, Y.; Li, L.; Xu, K.; Wu, M.; Hedrick, J.L.; et al. Biodegradable antimicrobial polycarbonates with in vivo efficacy against multidrug-resistant MRSA systemic infection. Adv. Healthc. Mater. 2015, 4, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Boparai, J.K.; Sharma, P.K. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Florin, T.; Maracci, C.; Graf, M.; Karki, P.; Klepacki, D.; Berninghausen, O.; Beckmann, R.; Vázquez-Laslop, N.; Wilson, D.N.; Rodnina, M.V.; et al. An antimicrobial peptide that inhibits translation by trapping release factors on the ribosome. Nat. Struct. Mol. Biol. 2017, 24, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.; Lövdahl, T.; Simonovic, D.; Hansen, K.; Andersen, A.J.C.; Devold, H.; Richard, C.S.M.; Andersen, J.H.; Strøm, M.B.; Haug, T. Antimicrobial activity of small synthetic peptides based on the marine peptide turgencin A: Prediction of antimicrobial peptide sequences in a natural peptide and strategy for optimization of potency. Int. J. Mol. Sci. 2020, 21, 5460. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. Development of a novel hybrid antimicrobial peptide for targeted killing of Pseudomonas aeruginosa. Eur. J. Med. Chem. 2020, 185, 111814. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B. Review: Lessons learned from clinical trials using antimicrobial peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Chin, W.; Zhong, G.; Pu, Q.; Yang, C.; Lou, W.; De Sessions, P.F.; Periaswamy, B.; Lee, A.; Liang, Z.C.; Ding, X.; et al. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nat. Commun. 2018, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Falciani, C.; Lozzi, L.; Pollini, S.; Luca, V.; Carnicelli, V.; Brunetti, J.; Lelli, B.; Bindi, S.; Scali, S.; Di Giulio, A.; et al. Isomerization of an antimicrobial peptide broadens antimicrobial spectrum to gram-positive bacterial pathogens. PLoS ONE 2012, 7, e46259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, Z.Y.; Cheng, J.; Huang, Y.; Xu, K.; Ji, Z.; Fan, W.; Yang, Y.Y. Effect of stereochemistry, chain length and sequence pattern on antimicrobial properties of short synthetic β-sheet forming peptide amphiphiles. Biomaterials 2014, 35, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Cheng, J.; Liang, Z.C.; Xu, L.; Lou, W.; Bao, C.; Ong, Z.Y.; Dong, H.; Yang, Y.Y.; Fan, W. Short Synthetic β-Sheet Antimicrobial Peptides for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Burn Wound Infections. Adv. Healthc. Mater. 2017, 6, 1601134. [Google Scholar] [CrossRef] [PubMed]









| M1 | M2 | M3 | M4 | |
|---|---|---|---|---|
| Human | 2.3 | 19.1 | 11.8 | 66.8 |
| Monkey | 6.7 | 15.4 | 9.8 | 68.0 |
| Dog | ND | ND | ND | 100 |
| Rat | 2.4 | 22.2 | 6.7 | 68.7 |
| Mouse | ND | 2.5 | ND | 97.5 |
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | |
|---|---|---|---|---|---|---|---|---|---|
| Human | 4.0 | 3.9 | 5.3 | 62.0 | 1.8 | 7.5 | 4.8 | 4.5 | 6.3 |
| Monkey | 2.5 | 0.0 | 2.2 | 44.1 | 4.6 | 18.9 | 9.0 | 6.5 | 12.2 |
| Dog | 9.2 | 0.0 | 4.9 | 17.5 | 23.2 | 11.2 | 14.0 | 12.0 | 8.0 |
| Rat | 2.1 | 1.0 | 7.7 | 35.2 | 5.8 | 9.5 | 14.7 | 14.2 | 9.9 |
| Mouse | 4.5 | 0.0 | 3.7 | 67.6 | 3.7 | 4.8 | 7.4 | 5.5 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Zhou, S.; Xu, M.; Zeng, S.; Fan, W.; Yu, L.; Lin, N. The Metabolic Stability of Antimicrobial Peptides IK8 in Plasma and Liver S9. Appl. Sci. 2021, 11, 11661. https://doi.org/10.3390/app112411661
Mao Y, Zhou S, Xu M, Zeng S, Fan W, Yu L, Lin N. The Metabolic Stability of Antimicrobial Peptides IK8 in Plasma and Liver S9. Applied Sciences. 2021; 11(24):11661. https://doi.org/10.3390/app112411661
Chicago/Turabian StyleMao, Yingying, Shaojun Zhou, Mingcheng Xu, Su Zeng, Weimin Fan, Lushan Yu, and Nengming Lin. 2021. "The Metabolic Stability of Antimicrobial Peptides IK8 in Plasma and Liver S9" Applied Sciences 11, no. 24: 11661. https://doi.org/10.3390/app112411661
APA StyleMao, Y., Zhou, S., Xu, M., Zeng, S., Fan, W., Yu, L., & Lin, N. (2021). The Metabolic Stability of Antimicrobial Peptides IK8 in Plasma and Liver S9. Applied Sciences, 11(24), 11661. https://doi.org/10.3390/app112411661








