Comprehensive Assessment of the Dynamics of Banana Chilling Injury by Advanced Optical Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Storage Conditions
2.2. Chlorophyll-Fluorescence Imaging
2.3. Chlorophyll-Fluorescence Analysis—Rapid Light Curves
2.4. Chlorophyll-Fluorescence Analysis—OJIP Test
2.5. UV/VIS-Spectroscopy
2.6. CIE L*a*b* Color Parameters
2.7. Statistical Analysis
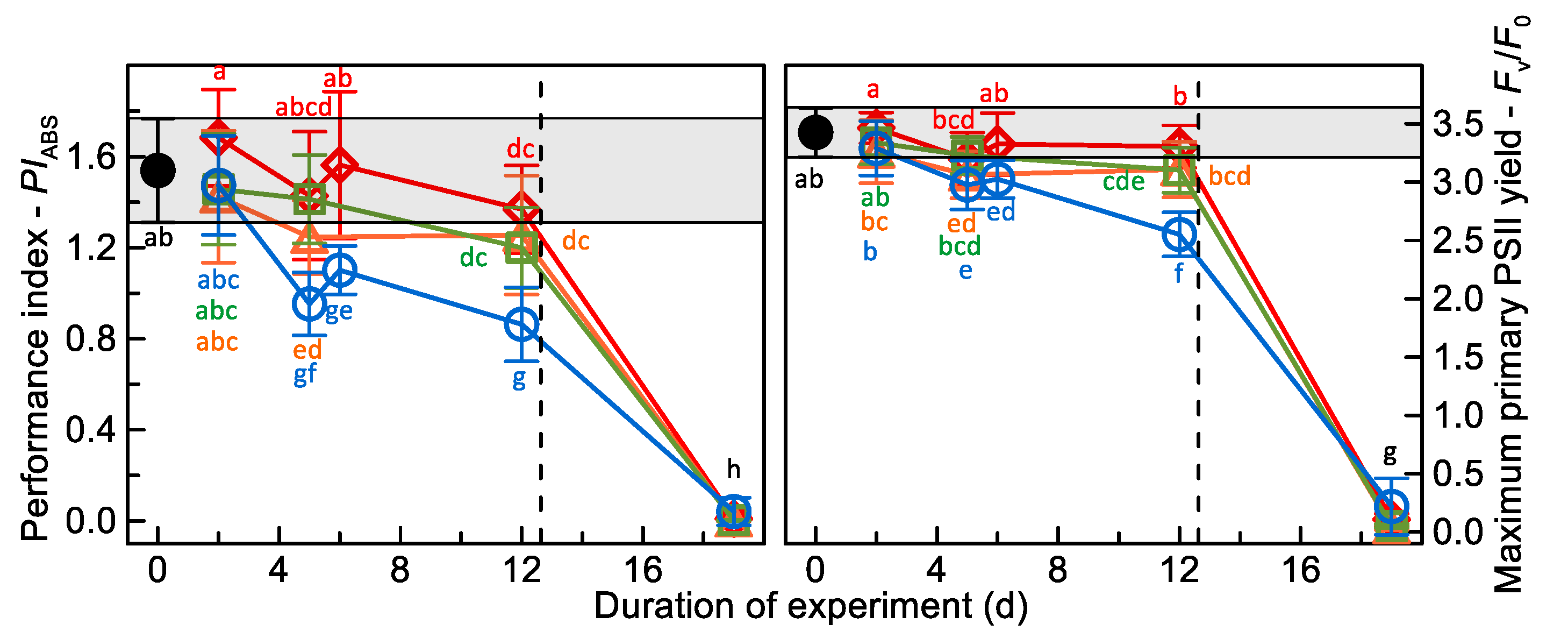
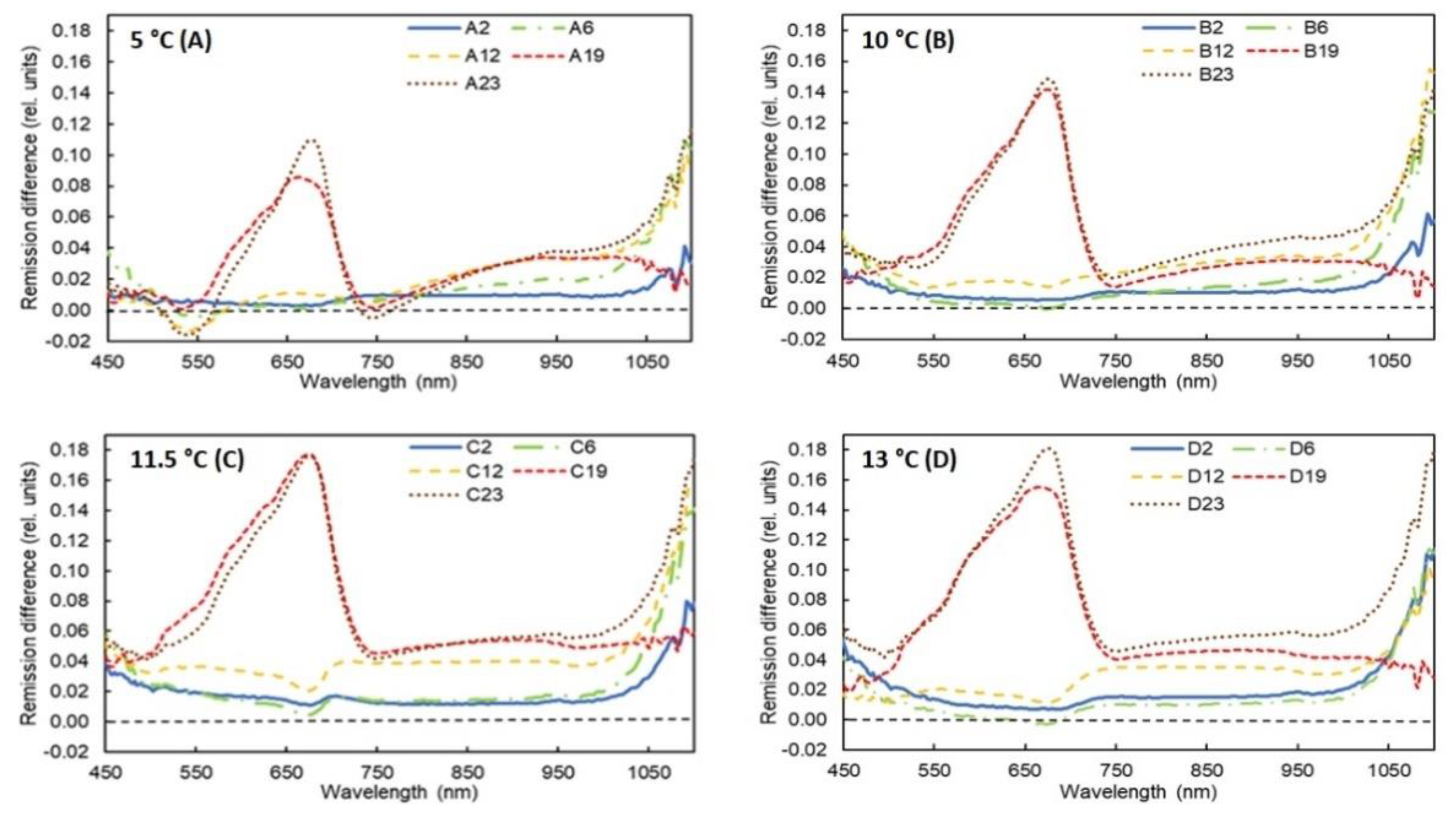
3. Results
3.1. Chlorophyll-Fluorescence Analyses
3.2. Results of Spectral Measurements
3.3. Chilling Stress Effects on Overall Appearance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Billiard, F. Fruit cold storage: Techniques and equipment. Acta Hortic. 1999, 485, 61–70. [Google Scholar] [CrossRef]
- USDA. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. In Agriculture Handbook 66; Gross, K.C., Wang, C.Y., Saltveit, M., Eds.; U.S. Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2016; p. 794. [Google Scholar]
- Parkin, K.L.; Marangoni, A.; Jackman, R.; Yada, R.; Stanley, D. Chilling injury. A review of possible mechanisms. J. Food Biochem. 1989, 13, 127–153. [Google Scholar] [CrossRef]
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573. [Google Scholar] [CrossRef]
- Lukatkin, A.; Brazaitytė, A.; Duchovskis, P. Chilling injury in chilling-sensitive plants: A review. Zemdirb. Agric. 2012, 99, 111–124. [Google Scholar]
- Murata, T. Physiological and biochemical studies of chilling injury in bananas. Physiol. Plant. 1969, 22, 401–411. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Bodbodak, S. Postharvest heat treatment for mitigation of chilling injury in fruits and vegetables. Food Bioprocess Technol. 2014, 7, 37–53. [Google Scholar] [CrossRef]
- Promyou, S.; Ketsa, S.; van Doorn, W.G. Hot water treatments delay cold-induced banana peel blackening. Postharvest Biol. Technol. 2008, 48, 132–138. [Google Scholar] [CrossRef]
- Khademia, O.; Ashtaria, M.; Razavi, F. Effects of salicylic acid and ultrasound treatments on chilling injury control and quality preservation in banana fruit during cold storage. Sci. Hortic. 2019, 249, 334–339. [Google Scholar] [CrossRef]
- Gemma, H.; Matsuyama, Y.; Wang, H.-G. Ripening characteristics and chilling injury of banana fruit. I. Effect of storage temperature on respiration, ethylene production and membrane permeability of peel and pulp tissues. Jpn. J. Trop. Agric. 1994, 38, 216–220. [Google Scholar] [CrossRef]
- Chaiprasart, P.; Gemma, H.; Iwahori, S. Changes in chlorophyll fluorescence and enzyme activity for scavenging of free radicals in banana fruits stored at low temperatures. Jpn. J. Trop. Agric. 2001, 45, 181–191. [Google Scholar] [CrossRef]
- Zsom, T.; Strohmayer, E.; Nguyen, L.G.L.; Hitka, G.; Zsom-Muha, V. Chilling injury investigation by non-destructive measuring methods during banana cold storage. Prog. Agric. Eng. Sci. 2018, 14, 147–158. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Ketsa, S.; van Doorn, W.G. Relationship between browning and the activities of polyphenol oxidase and phenylalanine ammonia lyase in banana peel during low temperature storage. Postharvest Biol. Technol. 2003, 30, 187–193. [Google Scholar] [CrossRef]
- Olorunda, A.O.; Meheriuk, M.; Looney, N.E. Some postharvest factors associated with the occurrence of chilling injury in banana. J. Sci. Food Agric. 1978, 29, 213–218. [Google Scholar] [CrossRef]
- Wang, N.-N.; Yang, Y.-C.; Sun, D.-W.; Pu, H.; Zhu, Z. Shelf-life prediction of ‘Gros Michel’ bananas with different browning levels using hyperspectral reflectance imaging. Food Anal. Methods 2015, 8, 1173–1184. [Google Scholar] [CrossRef]
- Pongprasert, N.; Sekozawa, Y.; Sugaya, S.; Gemma, H. A novel postharvest UV-C treatment to reduce chilling injury (membrane damage, browning and chlorophyll degradation) in banana peel. Sci. Hortic. 2011, 130, 73–77. [Google Scholar] [CrossRef]
- Elbagoury, M.M.; Turoop, L.; Runo, S.; Sila, D.N. Regulatory influences of methyl jasmonate and calcium chloride on chilling injury of banana fruit during cold storage and ripening. Food Sci. Nutr. 2020, 9, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, E. Banana and Plantain. In The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks. Agriculture Handbook Number 66; Gross, K., Wang, C.Y., Saltveit, M., Eds.; USDA, Agricultural Research Center, Agricultural Research Service: Beltsville, MD, USA, 2016. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-barc/beltsville-agricultural-research-center/food-quality-laboratory/docs/ah66/ (accessed on 17 March 2021).
- Luyckx, A.; Lechaudel, M.; Hubert, O.; Salmon, F.; Brat, P. Banana physiological post-harvest disorders: A review. MOJ Food Process. Technol. 2016, 3, 226–231. [Google Scholar] [CrossRef][Green Version]
- Mootoo, A.; Murr, D.P. Quality attributes of banana fruit as affected by chilling and non-chilling temperatures. In Proceedings of 21st Annual Meeting, 8–13 September 1985, Port of Spain, Trinidad and Tobago, Caribbean Food Crops Society, Puerto Rico, 8 September 1985; No 261454, p. 8. Available online: https://ageconsearch.umn.edu/record/261454/ (accessed on 17 March 2021).
- Hashim, N.; Janius, R.B.; Baranyai, L.; Rahman, R.; Osman, A.; Zude, M. Kinetic model for colour changes in bananas during the appearance of chilling injury symptoms. Food Bioprocess Technol. 2012, 5, 2952–2963. [Google Scholar] [CrossRef]
- Zhu, X.; Luo, J.; Li, Q.; Li, J.; Tonxin Liu, T.; Wang, R.; Chen, W.; Li, X. Low temperature storage reduces aroma-related volatiles production during shelf-life of banana fruit mainly by regulating key genes involved in volatile biosynthetic pathways. Postharvest Biol. Technol. 2018, 146, 68–78. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.Y.; Marangoni, A.; Parkin, K.L.; Stanley, D.W. Chilling injury. A review of qualitative aspects. J. Food Qual. 1988, 11, 253–278. [Google Scholar] [CrossRef]
- Kader, A.A. Banana. Recommendations for Maintaining Postharvest Quality; University of California (UC): Davis, CA, USA, 1996; Available online: http://postharvest.ucdavis.edu/Commodity_Resources/Fact_Sheets/Datastores/Fruit_English/?uid=9&ds=798 (accessed on 17 March 2021).
- Facundo, H.V.D.V.; Gurak, P.D.; Zerlotti Mercadante, A.; Lajolo, F.M.; Cordenunsi, B.R. Storage at low temperature differentially affects the colour and carotenoid composition of two cultivars of banana. Food Chem. 2015, 170, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.; Pflanz, M.; Regen, C.; Janius, R.B.; Rahman, R.A.; Osman, A.; Shitan, M.; Zude, M. An approach for monitoring the chilling injury appearance in bananas by means of backscattering imaging. J. Food Eng. 2013, 116, 28–36. [Google Scholar] [CrossRef]
- Seymour, G.B.; John, P.; Thompson, A.K. Inhibition of degreening in the peel of bananas ripened at tropical temperatures. II. Role of ethylene, oxygen and carbon dioxide. Ann. Appl. Biol. 1987, 110, 153–161. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Fillmore, S.; Pang, X.; Zhang, Z. Effect of high temperature on color, chlorophyll fluorescence and volatile biosynthesis in green-ripe banana fruit. Postharvest Biol. Technol. 2011, 62, 246–257. [Google Scholar] [CrossRef]
- Thomas, P.; Janave, M.T. Effect of temperature on chlorophyllase activity, chlorophyll degradation and carotenoids of Cavendish bananas during ripening. Int. J. Food Sci. Technol. 1992, 27, 57–63. [Google Scholar] [CrossRef]
- Abd El-Wahab, F.K.; Nawwar, M.A.M. Physiological and biochemical studies on chilling-injury of banana. Sci. Hortic. 1977, 7, 373–376. [Google Scholar] [CrossRef]
- Jiang, Y.; Joyce, D.C.; Jiang, W.; Lu, W. Effects of chilling temperatures on ethylene binding by banana fruit. Plant Growth Regul. 2004, 43, 109–115. [Google Scholar] [CrossRef]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- von Willert, D.J.; Matyssek, R.; Herppich, W.B. Experimentelle Pflanzenökologie, Grundlagen und Anwendungen; Georg Thieme Verlag: Stuttgart, Germany, 1995; p. 344. ISBN 9783662534632. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Matyssek, R.; Herppich, W.B. Experimentelle Pflanzenökologie, Grundlagen und Anwendungen. Springer Reference Naturwissenschaften; Springer Spektrum: Berlin/Heidelberg, Germany, 2020; p. 551. ISBN1 978-3-662-53465-6. (eBook); ISBN2 978-3-662-53463-2. (print); ISBN3 978-3-662-53464-9. (combined). [Google Scholar] [CrossRef]
- Tijskens, L.M.M.; Otma, O.; van Kooten, E.C. Photosystem II quantum yield as a measure of radical scavengers in chilling injury in cucumber fruits and bell peppers. A static, dynamic and statistical model. Planta 1994, 194, 478–486. [Google Scholar] [CrossRef]
- Purvis, A.C. Diphenylamine reduces chilling injury of green bell pepper fruit. Postharvest Biol. Technol. 2002, 25, 41–48. [Google Scholar] [CrossRef]
- Kosson, R. Chlorophyll fluorescence and chilling injury of green pepper as affected by storage conditions. Acta Hortic. 2003, 628, 379–385. [Google Scholar] [CrossRef]
- Oseko, J.; East, A.; Heyes, J. Can changes in chlorophyll fluorescence be used to determine chilling injury of cold stored feijoa? Acta Hortic. 2020, 1275, 125–132. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. Available online: https://www.walz.com/products/chl_p700/pan/overview.html (accessed on 28 August 2021).
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, C.; Schreiber, U. The polyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination: I. Saturation characteristics and partial control by the photosystem II acceptor side. Z. Naturforsch. 1987, 42c, 1246–1254. [Google Scholar] [CrossRef]
- Schreiber, U.; Neubauer, C. The polyphasic rise of chlorophyll fluorescence upon onset of strong continuous illumination: II. Partial control by the photosystem II donor side and possible ways of interpretation. Z. Naturforsch. 1987, 42c, 1255–1264. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Molec. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Govindjee. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Lazár, D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct. Plant Biol. 2006, 33, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M. PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: Protocols and applications. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 87–131. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Du, R. Nitric oxide delays chlorophyll degradation and enhances antioxidant activity in banana fruits after cold storage. Acta Physiol. Plant. 2015, 37, 74. [Google Scholar] [CrossRef]
- Zude, M. Comparison of indices and multivariate models to nondestructively predict the fruit chlorophyll by means of visible spectrometry in apple fruit. Anal. Chim. Acta 2003, 481, 119–126. [Google Scholar] [CrossRef]
- Zude, M. Non-destructive prediction of banana fruit quality using VIS/NIR spectroscopy. Fruits 2003, 58, 135–142. [Google Scholar] [CrossRef]
- Herold, B.; Truppel, I.; Zude, M.; Geyer, M. Spectral measurements on ‘Elstar’ apples during fruit development on the tree. Biosyst. Eng. 2005, 91, 173–182. [Google Scholar] [CrossRef]
- Hellebrand, H.J.; Herppich, W.B.; Beuche, H.; Dammer, K.-H.; Linke, M.; Flath, K. Investigations of plant infections by thermal vision and NIR imaging. Int. Agrophys. 2006, 20, 1–10. Available online: http://www.international-agrophysics.org/Investigations-of-plant-infections-by-thermal-vision-and-NIR-imaging,106576,0,2.html (accessed on 17 March 2021).
- Schlüter, O.; Foerster, J.; Geyer, M.; Knorr, D.; Herppich, W.B. Characterization of high hydrostatic pressure effects on fresh produce using chlorophyll fluorescence image analysis. Food Bioprocess Technol. 2009, 2, 291–299. [Google Scholar] [CrossRef]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Herppich, W.B. Chlorophyll Fluorescence Imaging for process optimisation in horticulture and fresh food production. Photosynthetica 2021, 59, 422–437. [Google Scholar] [CrossRef]
- van Heerden, P.D.R.; Tsimilli-Michael, M.; Krüger, G.H.J.; Strasser, R.J. Dark chilling effects on soybean genotypes during vegetative development: Parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiol. Plant. 2003, 117, 476–491. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Rutkowski, K.P.; Michalczuk, B.; Konopacki, P. Nondestructive determination of ‘Golden Delicious’ apple quality and harvest maturity. J. Fruit Ornam. Plant Res. 2008, 16, 39–52. Available online: http://www.inhort.pl/files/journal_pdf/journal_2008/full5%202008.pdf (accessed on 17 March 2021).
- Zsom, T.; Zsom-Muha, V.; Nguyen, L.P.L.; Nagy, D.; Hitka, G.; Polgári, P.; Baranya, L. Nondestructive detection of low temperature induced stress on postharvest quality of kápia type sweet pepper. Prog. Agric. Eng. Sci. 2020, 16, 173–186. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Sonoike, K. Various aspects of inhibition of photosynthesis under light/chilling stress: “Photoinhibition at chilling temperatures” versus “chilling damage in the light”. J. Plant Res. 1998, 111, 121–129. [Google Scholar] [CrossRef]
- Critchley, C.; Russel, W. Photoinhibition of photosynthesis in vivo: The role of protein turnover in photosystem II. Physiol. Plant. 1994, 92, 188–196. [Google Scholar] [CrossRef]
- Herppich, W.B.; Flach, M.-T.; von Willert, D.J.; Herppich, M. Field investigations in Welwitschia mirabilis during a severe drought. II. Influence of leaf age, leaf temperature and irradiance on photosynthesis and photoinhibition. Flora 1997, 192, 165–174. [Google Scholar] [CrossRef]
- Henriques, F.S. Leaf chlorophyll fluorescence: Background and fundamentals for plant biologists. Bot. Rev. 2009, 75, 249–270. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herppich, W.B.; Zsom, T. Comprehensive Assessment of the Dynamics of Banana Chilling Injury by Advanced Optical Techniques. Appl. Sci. 2021, 11, 11433. https://doi.org/10.3390/app112311433
Herppich WB, Zsom T. Comprehensive Assessment of the Dynamics of Banana Chilling Injury by Advanced Optical Techniques. Applied Sciences. 2021; 11(23):11433. https://doi.org/10.3390/app112311433
Chicago/Turabian StyleHerppich, Werner B., and Tamás Zsom. 2021. "Comprehensive Assessment of the Dynamics of Banana Chilling Injury by Advanced Optical Techniques" Applied Sciences 11, no. 23: 11433. https://doi.org/10.3390/app112311433
APA StyleHerppich, W. B., & Zsom, T. (2021). Comprehensive Assessment of the Dynamics of Banana Chilling Injury by Advanced Optical Techniques. Applied Sciences, 11(23), 11433. https://doi.org/10.3390/app112311433





