Lactic Acid Bacteria Co-Encapsulated with Lactobionic Acid: Probiotic Viability during In Vitro Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotic Microorganism, Growth Conditions and Lactic Acid Production
2.2. Co-Encapsulation of the Probiotic and the Prebiotic and Macrocapsule Characterization
2.2.1. Encapsulation
2.2.2. Bloom Test
2.3. Drying Processes
2.4. Viability over Time
2.5. Rehydration Kinetics
2.6. Simulated In Vitro Digestion of Rehydrated Freeze-Dried and Thermal-Dried Capsules
2.7. Visual Characterization and Sphericity Factor (SF)
2.8. Statistical Analysis
3. Results and Discussion
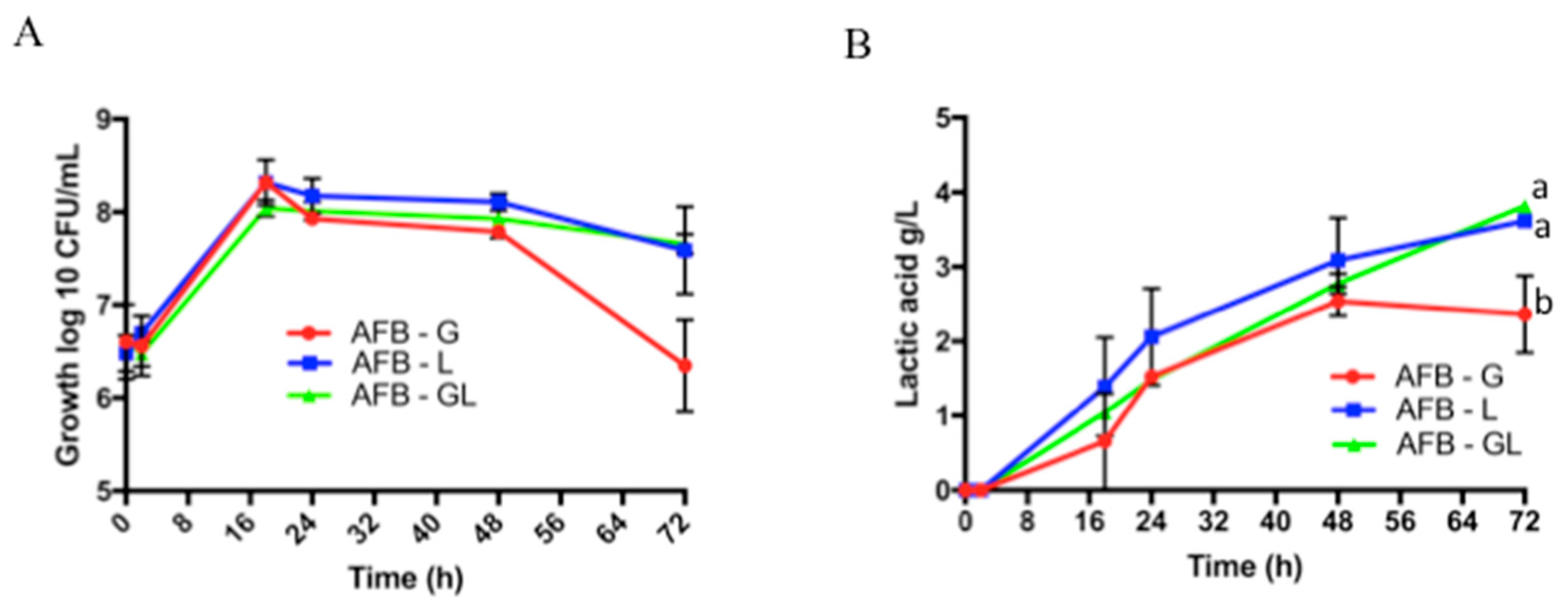
3.1. Analysis of the Growth of L. paracasei Using Lactobionic Acid as Substrate and Lactic Acid Production
3.2. Capsule Characterization
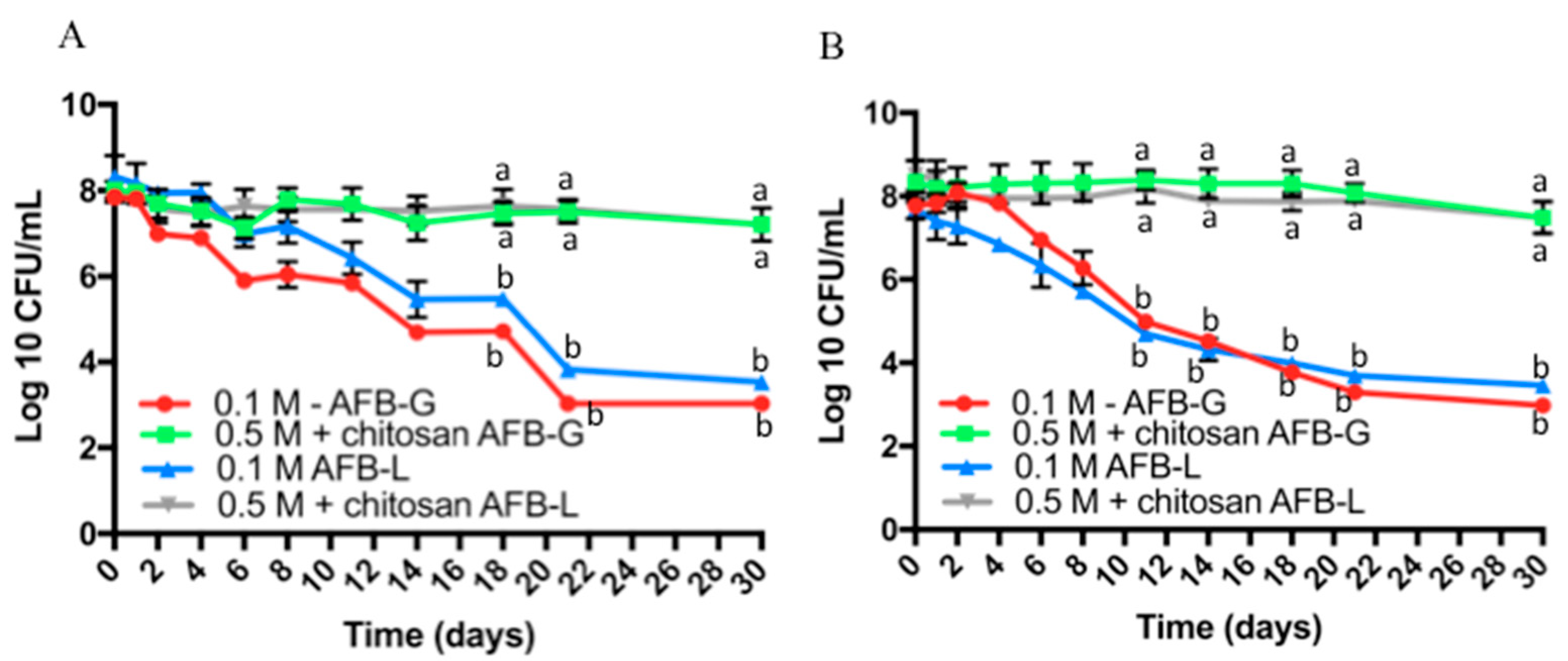
3.3. Viability over Time of the Probiotic Encapsulated in Dried Capsules
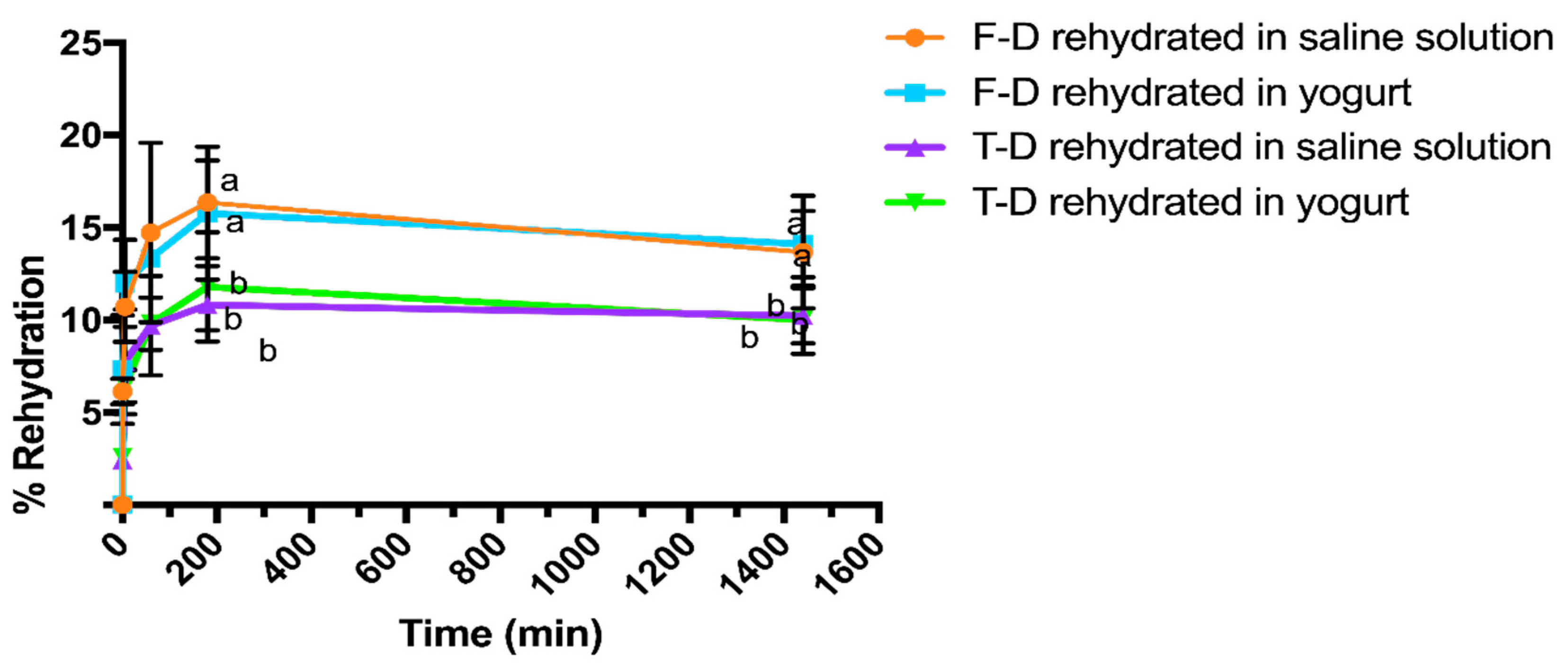
3.4. Rehydration Kinetics
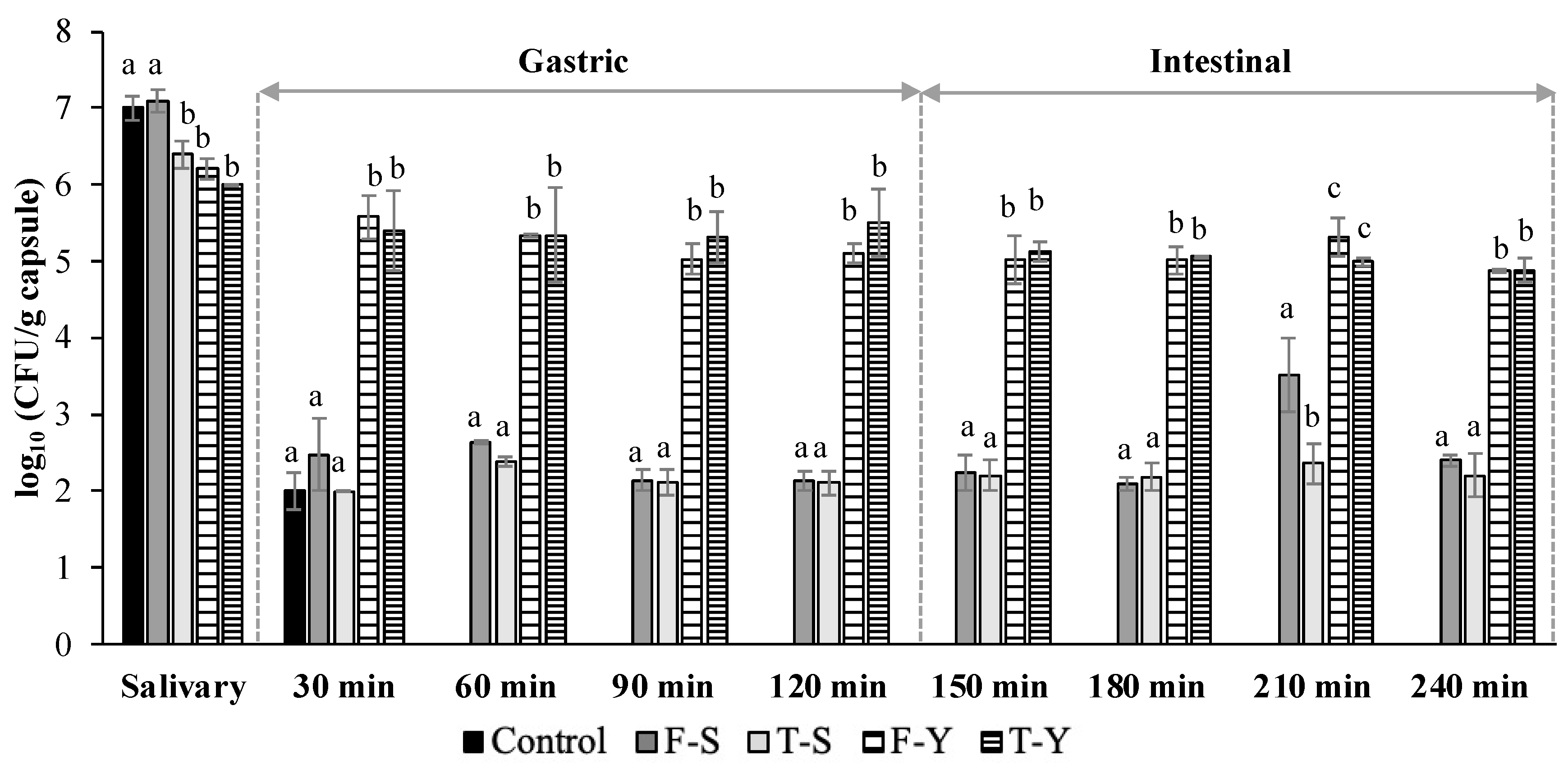
3.5. Simulated In Vitro Digestion of Rehydrated Freeze-Dried and Thermal-Dried Capsules
3.6. Visual Characterization and Sphericity Factor (SF)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Batista, A.; Silva, R.; Cappato, L.; Ferreira, M.; Nascimento, K.; Schmiele, M.; Esmerino, E.; Balthazar, C.F.; Silva, H.; Moraes, J.; et al. Developing a synbiotic fermented milk using probiotic bacteria and organic green banana flour. J. Funct. Foods 2017, 38, 242–250. [Google Scholar] [CrossRef]
- Ashwell, M. Concepts of Functional Foods. Nutr. Food Sci. 2004, 34, 47. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Puertas, C.; Marcet, I.; Rendueles, M.; Díaz, M. Bioactive synbiotic coatings with lactobionic acid and Lactobacillus plantarum CECT 9567 in the production and characterization of a new functional dairy product. J. Funct. Foods 2020, 75, 104263. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Bio-production of lactobionic acid: Current status, applications and future prospects. Biotechnol. Adv. 2013, 31, 1275–1291. [Google Scholar] [CrossRef]
- Cardoso, T.; Marques, C.; Dagostin, J.L.A.; Masson, M.L. Lactobionic Acid as a Potential Food Ingredient: Recent Studies and Applications. J. Food Sci. 2019, 84, 1672–1681. [Google Scholar] [CrossRef] [Green Version]
- Saarela, M.; Hallamaa, K.; Mattila-Sandholm, T.; Mättö, J. The effect of lactose derivatives lactulose, lactitol and lactobionic acid on the functional and technological properties of potentially probiotic Lactobacillus strains. Int. Dairy J. 2003, 13, 291–302. [Google Scholar] [CrossRef]
- Schaafsma, G. Lactose and lactose derivatives as bioactive ingredients in human nutrition. Int. Dairy J. 2008, 18, 458–465. [Google Scholar] [CrossRef]
- FDA. Code of Federal Regulations, Title 21, 21 CFR 172.720; US Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Peng, M.; Tabashsum, Z.; Anderson, M.; Truong, A.; Houser, A.K.; Padilla, J.; Akmel, A.; Bhatti, J.; Rahaman, S.O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1908–1933. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.; Batista, R.A.; Azeredo, H.M.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. Int. 2016, 90, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Nocerino, R.; Paparo, L.; Terrin, G.; Pezzella, V.; Amoroso, A.; Cosenza, L.; Cecere, G.; De Marco, G.; Micillo, M.; Albano, F.; et al. Cow’s milk and rice fermented with Lactobacillus paracasei CBA L74 prevent infectious diseases in children: A randomized controlled trial. Clin. Nutr. 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Gallo, M.; Nigro, F.; Passannanti, F.; Nanayakkara, M.; Lania, G.; Parisi, F.; Salameh, D.; Budelli, A.; Barone, M.V.; Nigro, R. Effect of pH control during rice fermentation in preventing a gliadin P31-43 entrance in epithelial cells. Int. J. Food Sci. Nutr. 2019, 70, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Labruna, G.; Nanayakkara, M.; Pagliuca, C.; Nunziato, M.; Iaffaldano, L.; D’Argenio, V.; Colicchio, R.; Budelli, A.; Nigro, R.; Salvatore, P.; et al. Celiac disease-associated Neisseria flavescens decreases mitochondrial respiration in CaCo-2 epithelial cells: Impact of Lactobacillus paracasei CBA L74 on bacterial-induced cellular imbalance. Cell. Microbiol. 2019, 21, e13035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarno, M.; Lania, G.; Cuomo, M.; Nigro, F.; Passannanti, F.; Budelli, A.; Fasano, F.; Troncone, R.; Auricchio, S.; Barone, M.V.; et al. Lactobacillus paracaseiCBA L74 interferes with gliadin peptides entrance in Caco-2 cells. Int. J. Food Sci. Nutr. 2014, 65, 953–959. [Google Scholar] [CrossRef]
- Salameh, D.; Nigro, F.; Cante, R.C.; Passannanti, F.; Gallo, M.; Budelli, A.; Marzocchella, A.; Nigro, R. Fermentation of rice flour supernatant using lactobacillus paracasei CBA L74. Chem. Eng. Trans. 2019, 75, 289–294. [Google Scholar]
- Gallo, M.; Nigro, F.; Passannanti, F.; Salameh, D.; Schiattarella, P.; Budelli, A.; Nigro, R. Lactic fermentation of cereal flour: Feasibility tests on rice, oat and wheat. Appl. Food Biotechnol. 2019, 6, 165–172. [Google Scholar]
- Cante, R.C.; Gallo, M.; Nigro, F.; Passannanti, F.; Salameh, D.; Budelli, A.; Nigro, R. Lactic fermentation of cooked navy beans by Lactobacillus paracasei CBA L74 aimed at a potential production of functional legume-based foods. Can. J. Chem. Eng. 2020, 98, 1955–1961. [Google Scholar] [CrossRef]
- Gallo, M.; Nigro, F.; Passannanti, F.; Salameh, D.; Budelli, A.; Marzocchella, A.; Nigro, R. Rice Fermentation by Lactobacillus Paracasei CBA L74. Int. J. Rice Res. 2018, 2018, 1–5. [Google Scholar]
- Gallo, M.; Passannanti, F.; Colucci Cante, R.; Nigro, F.; Salameh, D.; Schiattarella, P.; Schioppa, C.; Budelli, A.; Nigro, R. Effects of the Glucose Addition during Lactic Fermentation of Rice, Oat and Wheat Flours. Appl. Food Biotechnol. 2020, 7, 21–30. [Google Scholar]
- Gallo, M.; Passannanti, F.; Cante, R.C.; Nigro, F.; Schiattarella, P.; Zappulla, S.; Budelli, A.; Nigro, R. Lactic fermentation of cereals aqueous mixture of oat and rice flours with and without glucose addition. Heliyon 2020, 6, e04920. [Google Scholar] [CrossRef]
- Gallo, M.; Passannanti, F.; Schioppa, C.; Montella, S.; Cante, R.C.; Nigro, F.; Budelli, A.; Nigro, R. Enzymatic pretreatment and lactic fermentation of wheat flour suspension at a high solid content. J. Food Process. Preserv. 2021, 45, e15299. [Google Scholar] [CrossRef]
- Gallo, M.; Passannanti, F.; Schiattarella, P.; Esposito, A.; Colucci Cante, R.; Nigro, F.; Budelli, A.; Nigro, R. Banana Puree Lactic Fermentation: The Role of Ripeness, Heat Treatment, and Ascorbic Acid. Appl. Sci. 2021, 11, 5153. [Google Scholar] [CrossRef]
- Colucci Cante, R.; Gallo, M.; Nigro, F.; Passannanti, F.; Budelli, A.; Nigro, R. Mathematical Modeling of Lactobacillus paracasei CBA L74 Growth during Rice Flour Fermentation Performed with and without pH Control. Appl. Sci. 2021, 11, 2921. [Google Scholar] [CrossRef]
- Langa, S.; Bulck, E.V.D.; Peirotén, A.; Gaya, P.; Schols, H.; Arqués, J. Application of lactobacilli and prebiotic oligosaccharides for the development of a synbiotic semi-hard cheese. LWT 2019, 114, 108361. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Marcet, I.; Rendueles, M.; Díaz, M. Bioactive packaging based on delipidated egg yolk protein edible films with lactobionic acid and Lactobacillus plantarum CECT 9567: Characterization and use as coating in a food model. Food Hydrocoll. 2021, 119, 106849. [Google Scholar] [CrossRef]
- Khalf, M.; Dabour, N.; Kheadr, E.; Fliss, I. Viability of probiotic bacteria in maple sap products under storage and gastrointestinal conditions. Bioresour. Technol. 2010, 101, 7966–7972. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simó, G.; Fernández-Fernández, E.; Vila-Crespo, J.; Ruipérez, V.; Rodríguez-Nogales, J.M. Research progress in coating techniques of alginate gel polymer for cell encapsulation. Carbohydr. Polym. 2017, 170, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Di Natale, C.; Lagreca, E.; Panzetta, V.; Gallo, M.; Passannanti, F.; Vitale, M.; Fusco, S.; Vecchione, R.; Nigro, R.; Netti, P. Morphological and Rheological Guided Design for the Microencapsulation Process of Lactobacillus paracasei CBA L74 in Calcium Alginate Microspheres. Front. Bioeng. Biotechnol. 2021, 9, 660691. [Google Scholar] [CrossRef]
- Kaushik, V.; Roos, Y.H. Limonene encapsulation in freeze-drying of gum Arabic-sucrose-gelatin systems. LWT-Food Sci. Technol. 2007, 40, 1381–1391. [Google Scholar] [CrossRef]
- International Standard Organization ISO 9665. Adhesives-Animal Glues-Methods of Sampling and Testing; ISO: Geneva, Switzerland, 1998; Volume 1998. [Google Scholar]
- Huson, D.; Beier, S.; Flade, I.; G’orska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.-J.; Tappu, R. MEGAN Community Edition—Interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, F.; Zhang, B.; Waltzer, G.; Gidley, M.J.; Dhital, S. The interplay of α-amylase and amyloglucosidase activities on the digestion of starch in in vitro enzymic systems. Carbohydr. Polym. 2015, 117, 192–200. [Google Scholar] [CrossRef] [Green Version]
- García, C.; Ranieri, G.; Rendueles, M.; Díaz, M. Exploring encapsulation strategies as a protective mechanism to avoid amensalism in mixed populations of Pseudomonas taetrolens and Lactobacillus casei. Bioprocess Biosyst. Eng. 2019, 43, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Atencio, S.; Maestro, A.; Santamaría, E.; Gutiérrez, J.M.; González, C. Encapsulation of ginger oil in alginate-based shell materials. Food Biosci. 2020, 37, 100714. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Wang, Y.; Shi, F.; Nie, Y.; Liu, T.; Song, K. Effects of concentration variation on the physical properties of alginate-based substrates and cell behavior in culture. Int. J. Biol. Macromol. 2019, 128, 184–195. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Codex Standard for Fermented Milks, 2nd ed.; n Codex Stan., Ed.; Food Agric. Organ. United Nations: Rome, Italy, 2010; pp. 1–5. [Google Scholar]
- Mahmoud, M.; Abdallah, N.A.; El-Shafei, K.; Tawfik, N.F.; El-Sayed, H.S. Survivability of alginate-microencapsulated Lactobacillus plantarum during storage, simulated food processing and gastrointestinal conditions. Heliyon 2020, 6, e03541. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.A.; Carvalho, R.D.S.F.; Magalhães, N.S.S.; Madruga, M.S.; Athayde, A.J.A.A.; Portela, I.A. Microencapsulation of Lactobacillus acidophilus La-05 and incorporation in vegan milks: Physicochemical characteristics and survival during storage, exposure to stress conditions, and simulated gastrointestinal digestion. Food Res. Int. 2020, 135, 109295. [Google Scholar] [CrossRef] [PubMed]
- Chirife, J.; Buera, M.P. A critical review of some nonequilibrium situations and glass transitions on water activity values of foods in the microbiological growth range. J. Food Eng. 1995, 25, 531–552. [Google Scholar] [CrossRef]
- Stapelfeldt, H.; Nielsen, B.R.; Skibsted, L.H. Effect of heat treatment, water activity and storage temperature on the oxidative stability of whole milk powder. Int. Dairy J. 1997, 7, 331–339. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Lewicki, P.P. Rehydration properties of dried plant tissues. Int. J. Food Sci. Technol. 2006, 41, 1040–1046. [Google Scholar] [CrossRef]
- Melchior, S.; Marino, M.; Innocente, N.; Calligaris, S.; Nicoli, M.C. Effect of different biopolymer-based structured systems on the survival of probiotic strains during storage and in vitro digestion. J. Sci. Food Agric. 2020, 100, 3902–3909. [Google Scholar] [CrossRef]
- Bove, P.; Russo, P.; Capozzi, V.; Gallone, A.; Spano, G.; Fiocco, D. Lactobacillus plantarum passage through an oro-gastro-intestinal tract simulator: Carrier matrix effect and transcriptional analysis of genes associated to stress and probiosis. Microbiol. Res. 2013, 168, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field. LWT-Food Sci. Technol. 2013, 50, 1–16. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Camilleri, P.; Marcet, I.; Rendueles, M.; Díaz, M. Microencapsulation of Calcium Lactobionate for Protection from Microorganisms in a Solid Phase Food. Biochem. Eng. J. 2019, 150, 107281. [Google Scholar] [CrossRef]
- Bonjour, J.-P.; Benoit, V.; Payen, F.; Kraenzlin, M. Consumption of Yogurts Fortified in Vitamin D and Calcium Reduces Serum Parathyroid Hormone and Markers of Bone Resorption: A Double-Blind Randomized Controlled Trial in Institutionalized Elderly Women. J. Clin. Endocrinol. Metab. 2013, 98, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, A.L.; Deladino, L.; Martino, M. Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr. Polym. 2013, 95, 315–323. [Google Scholar] [CrossRef]
- Gorbunova, N.; Evteev, A.; Evdokimov, I.; Bannikova, A. Kinetics of ascorbic acid transport from alginate beads during in vitro digestion. J. Food Nutr. Res. 2016, 55, 148–158. [Google Scholar]
| Capsules | CaCl2 Solution | Chitosan Solution |
|---|---|---|
| A | 0.1 M + 10 min | - |
| B | 0.1 M + 30 min | - |
| C | 0.1 M + 30 min | 40 min |
| D | 0.5 M + 10 min | 40 min |
| Capsules | Carbon Source | Coating |
|---|---|---|
| I | AFB-G | 0.1M 10 min (A from Table 1) |
| II | AFB-G | 0.5M 10 min + chitosan (D from Table 1) |
| III | AFB-L | 0.1M 10 min (A from Table 1) |
| IV | AFB-L | 0.5M 10 min + chitosan (D from Table 1) |
| Capsules | A | B | C | D |
|---|---|---|---|---|
| Force (g) | 253.7 ± 7.4 a | 283.2 ± 68.3 a | 304.3 ± 52.0 a | 476.9 ± 38.7 b |
| Capsules | SF | Microscope Images | Weight (mg) |
|---|---|---|---|
| D | 0.041 ± 0.021 a |  | 71.1 ± 1.7 a |
| F-D | 0.135 ± 0.023 b | 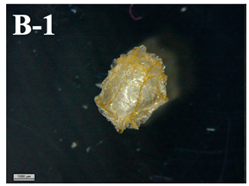 | 2.6 ± 0.2 b |
| F-D rehydrated in saline solution | 0.049 ± 0.03 a |  | 9.0 ± 0.9 c |
| F-D rehydrated in saline solution after in vitro digestion | 0.045 ± 0.01 c |  | 13.7 ± 3.1 d |
| F-D rehydrated in yogurt | 0.113 ± 0.04 b | 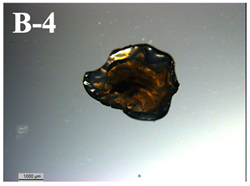 | 10.8 ± 1.3 e |
| F-D rehydrated in yogurt after in vitro digestion | 0.043 ± 0.023 a | 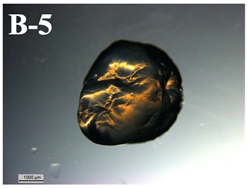 | 13.1 ± 1.1 d |
| T-D | 0.17 ± 0.082 d | 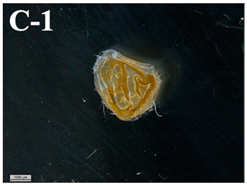 | 2.3 ± 0.4 b |
| T-D rehydrated in saline solution | 0.086 ± 0.042 d | 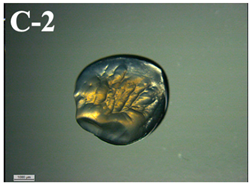 | 7.3 ± 0.4 f |
| T-D rehydrated in saline solution after in vitro digestion | 0.069 ± 0.045 e |  | 8.8 ± 0.7 c |
| T-D rehydrated in yogurt | 0.022 ± 0.013 f | 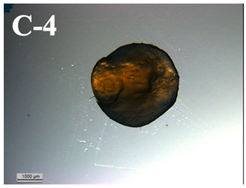 | 6.8 ± 1.2 f |
| T-D rehydrated in yogurt after in vitro digestion | 0.031 ± 0.008 f |  | 10.6 ± 1.3 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sáez-Orviz, S.; Passannanti, F.; Gallo, M.; Colucci Cante, R.; Nigro, F.; Budelli, A.L.; Rendueles, M.; Nigro, R.; Díaz, M. Lactic Acid Bacteria Co-Encapsulated with Lactobionic Acid: Probiotic Viability during In Vitro Digestion. Appl. Sci. 2021, 11, 11404. https://doi.org/10.3390/app112311404
Sáez-Orviz S, Passannanti F, Gallo M, Colucci Cante R, Nigro F, Budelli AL, Rendueles M, Nigro R, Díaz M. Lactic Acid Bacteria Co-Encapsulated with Lactobionic Acid: Probiotic Viability during In Vitro Digestion. Applied Sciences. 2021; 11(23):11404. https://doi.org/10.3390/app112311404
Chicago/Turabian StyleSáez-Orviz, Sara, Francesca Passannanti, Marianna Gallo, Rosa Colucci Cante, Federica Nigro, Andrea Luigi Budelli, Manuel Rendueles, Roberto Nigro, and Mario Díaz. 2021. "Lactic Acid Bacteria Co-Encapsulated with Lactobionic Acid: Probiotic Viability during In Vitro Digestion" Applied Sciences 11, no. 23: 11404. https://doi.org/10.3390/app112311404
APA StyleSáez-Orviz, S., Passannanti, F., Gallo, M., Colucci Cante, R., Nigro, F., Budelli, A. L., Rendueles, M., Nigro, R., & Díaz, M. (2021). Lactic Acid Bacteria Co-Encapsulated with Lactobionic Acid: Probiotic Viability during In Vitro Digestion. Applied Sciences, 11(23), 11404. https://doi.org/10.3390/app112311404









