Dissolution of Biofilm Secreted by Three Different Strains of Pseudomonas aeruginosa with Bromelain, N-Acetylcysteine, and Their Combinations
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Medium
2.2. Materials
2.3. Treatments
2.4. Biofilm Formation
2.5. Treatment of Preformed Biofilms
2.6. Biofilm Assays
2.7. Statistical Analysis
3. Results
3.1. Pseudomonas aeruginosa ATCC 27853
3.1.1. As Single Agents
3.1.2. Combination of Agents
3.2. Pseudomonas aeruginosa PA01 3981
3.2.1. As Single Agents
3.2.2. Combination of Agents
3.3. Pseudomonas aeruginosa ATCC 31461
3.3.1. As Single Agents
3.3.2. Combination of Agents
3.4. Comparison of Maximum Biofilm Dissolution (BD MAX) Values of the Two Different Agents on Different Strains of Pseudomonas aeruginosa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darouiche, R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Vasilakis, V.; Yamin, F.; Reish, R.G. Surgeons’ Dilemma: Treatment of Implant-Associated Infection in the Cosmetic Breast Augmentation Patient. Aesthetic Plast. Surg. 2019, 43, 905–909. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Spitzmüller, R.; Gümbel, D.; Güthoff, C.; Zaatreh, S.; Klinder, A.; Napp, M.; Bader, R.; Mittelmeier, W.; Ekkernkamp, A.; Kramer, A.; et al. Duration of antibiotic treatment and risk of recurrence after surgical management of orthopaedic device infections: A multicenter case-control study. BMC Musculoskelet. Disord. 2019, 20, 184. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.S.; Mason, M.R.; Brooker, M.R.; O’Brien, K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J. Clin. Periodontol. 2012, 39, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Socransky, S. Microbiology of periodontal disease—present status and future considerations. J. Periodontol. 1977, 48, 497–504. [Google Scholar] [CrossRef]
- Langbach, O.; Kristoffersen, A.K.; Abesha-Belay, E.; Enersen, M.; Røkke, O.; Olsen, I. Oral, intestinal, and skin bacteria in ventral hernia mesh implants. J. Oral Microbiol. 2016, 8, 31854. [Google Scholar] [CrossRef]
- See, C.W.; Kim, T.; Zhu, D. Hernia Mesh and Hernia repair: A review. Eng. Regen. 2020, 1, 19–33. [Google Scholar]
- Falagas, M.; Kasiakou, S. Mesh-related infections after hernia repair surgery. Clin. Microbiol. Infect. 2005, 11, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrout, J.D.; Chopp, D.L.; Just, C.L.; Hentzer, M.; Givskov, M.; Parsek, M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006, 62, 1264–1277. [Google Scholar] [CrossRef]
- Davey, M.E.; Caiazza, N.C.; O’Toole, G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003, 185, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Drenkard, E.; Ausubel, F.M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002, 416, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Savage, V.J.; Chopra, I.; O’Neill, A.J. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57, 1968–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Sutherland, I. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, K.; Jularic, M.; Horsburgh, S.M.; Hirschhausen, N.; Neumann, C.; Bertling, A.; Schulte, A.; Foster, S.; Kehrel, B.E.; Peters, G.; et al. Molecular characterization of a novel Staphylococcus aureus surface protein (SasC) involved in cell aggregation and biofilm accumulation. PLoS ONE 2009, 4, e7567. [Google Scholar] [CrossRef]
- Mack, D.; Fischer, W.; Krokotsch, A.; Leopold, K.; Hartmann, R.; Egge, H.; Laufs, R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: Purification and structural analysis. J. Bacteriol. 1996, 178, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Cerca, N.; Jefferson, K.K.; Maira-Litrán, T.; Pier, D.B.; Kelly-Quintos, C.; Goldmann, D.A.; Azeredo, J.; Pier, G.B. Molecular basis for preferential protective efficacy of antibodies directed to the poorly acetylated form of staphylococcal poly-N-acetyl-β-(1-6)-glucosamine. Infect. Immun. 2007, 75, 3406–3413. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, R.; Wang, C.; Li, K.; Jiang, X.; He, H.; Hong, W. On-demand pH-sensitive surface charge-switchable polymeric micelles for targeting Pseudomonas aeruginosa biofilms development. J. Nanobiotechnol. 2021, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Nuri, R.; Shprung, T.; Shai, Y. Defensive remodeling: How bacterial surface properties and biofilm formation promote resistance to antimicrobial peptides. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 3089–3100. [Google Scholar] [CrossRef] [Green Version]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: Relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- González-Pastor, J.E.; Hobbs, E.C.; Losick, R. Cannibalism by sporulating bacteria. Science 2003, 301, 510–513. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Vuong, C.; Voyich, J.M.; Fischer, E.R.; Braughton, K.R.; Whitney, A.R.; DeLeo, F.R.; Otto, M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004, 6, 269–275. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Yang, H.; Ku, W.; Yang, S.; Liu, Z.; Lu, G. Carbon-based electrocatalysts derived from biomass for oxygen reduction reaction: A minireview. Front. Chem. 2020, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.D.; Zimmerman, M.I.; Meller, A.; Chung, M.; Swamidass, S.; Bowman, G.R. Deep learning the structural determinants of protein biochemical properties by comparing structural ensembles with DiffNets. Nat. Commun. 2021, 12, 3023. [Google Scholar] [CrossRef]
- Fleming, D.; Chahin, L.; Rumbaugh, K. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob. Agents Chemother. 2017, 61, e01998-16. [Google Scholar] [CrossRef] [Green Version]
- Taussig, S.J.; Batkin, S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J. Ethnopharmacol. 1988, 22, 191–203. [Google Scholar] [CrossRef]
- Wang, S.L.; Lin, H.T.; Liang, T.W.; Chen, Y.J.; Yen, Y.H.; Guo, S.P. Reclamation of chitinous materials by bromelain for the preparation of antitumor and antifungal materials. Bioresour. Technol. 2008, 99, 4386–4393. [Google Scholar] [CrossRef]
- Waring, W.S. Novel acetylcysteine regimens for treatment of paracetamol overdose. Ther. Adv. Drug Saf. 2012, 3, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Ehre, C.; Rushton, Z.L.; Wang, B.; Hothem, L.N.; Morrison, C.B.; Fontana, N.C.; Markovetz, M.R.; Delion, M.F.; Kato, T.; Villalon, D.; et al. An Improved Inhaled Mucolytic to Treat Airway Muco-obstructive Diseases. Am. J. Respir. Crit. Care Med. 2019, 199, 171–180. [Google Scholar] [CrossRef]
- Praveen, N.C.; Rajesh, A.; Madan, M.; Chaurasia, V.R.; Hiremath, N.V.; Sharma, A.M. In vitro Evaluation of Antibacterial Efficacy of Pineapple Extract (Bromelain) on Periodontal Pathogens. J. Int. Oral Health 2014, 6, 96–98. [Google Scholar]
- Moon, J.H.; Choi, Y.S.; Lee, H.W.; Heo, J.S.; Chang, S.W.; Lee, J.Y. Antibacterial effects of N-acetylcysteine against endodontic pathogens. J. Microbiol. 2016, 54, 322–329. [Google Scholar] [CrossRef]
- Valle, S.J.; Akhter, J.; Mekkawy, A.H.; Lodh, S.; Pillai, K.; Badar, S.; Glenn, D.; Power, M.; Liauw, W.; Morris, D.L. A novel treatment of bromelain and acetylcysteine (BromAc) in patients with peritoneal mucinous tumours: A phase I first in man study. Eur. J. Surg. Oncol. 2021, 47, 115–122. [Google Scholar] [CrossRef]
- Pérez-Vilar, J.; Mabolo, R. Gel-forming mucins. Notions from in vitro studies. Histol. Histopathol. 2007, 22, 4. [Google Scholar]
- Guillaume, O.; Pérez-Tanoira, R.; Fortelny, R.; Redl, H.; Moriarty, T.F.; Richards, R.G.; Eglin, D.; Puchner, A.P. Infections associated with mesh repairs of abdominal wall hernias: Are antimicrobial biomaterials the longed-for solution? Biomaterials 2018, 167, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Fanaei Pirlar, R.; Emaneini, M.; Beigverdi, R.; Banar, M.; van Leeuwen, W.B.; Jabalameli, F. Combinatorial effects of antibiotics and enzymes against dual-species Staphylococcus aureus and Pseudomonas aeruginosa biofilms in the wound-like medium. PLoS ONE 2020, 15, e0235093. [Google Scholar]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential role of bromelain in clinical and therapeutic applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Prescott, L.F.; Illingworth, R.N.; Critchley, J.A.; Proudfoot, A.T. Intravenous N-acetylcysteine: Still the treatment of choice for paracetamol poisoning. Br. Med. J. 1980, 280, 46–47. [Google Scholar] [CrossRef] [Green Version]
- Mekkawy, A.H.; Pillai, K.; Badar, S.; Akhter, J.; Ke, K.; Valle, S.J.; Morris, D.L. Addition of bromelain and acetylcysteine to gemcitabine potentiates tumor inhibition in vivo in human colon cancer cell line LS174T. Am. J. Cancer Res. 2021, 11, 2252–2263. [Google Scholar]
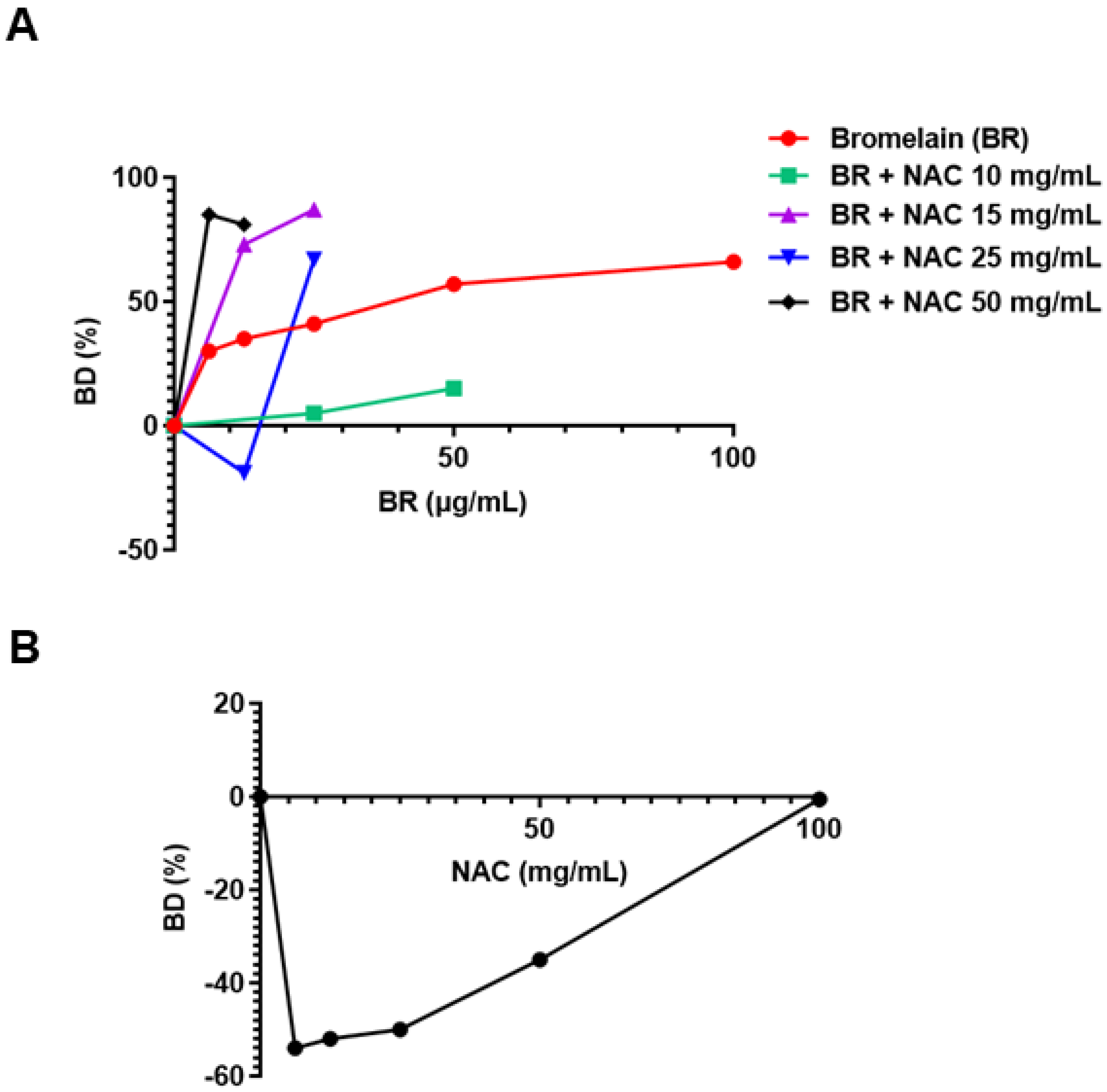
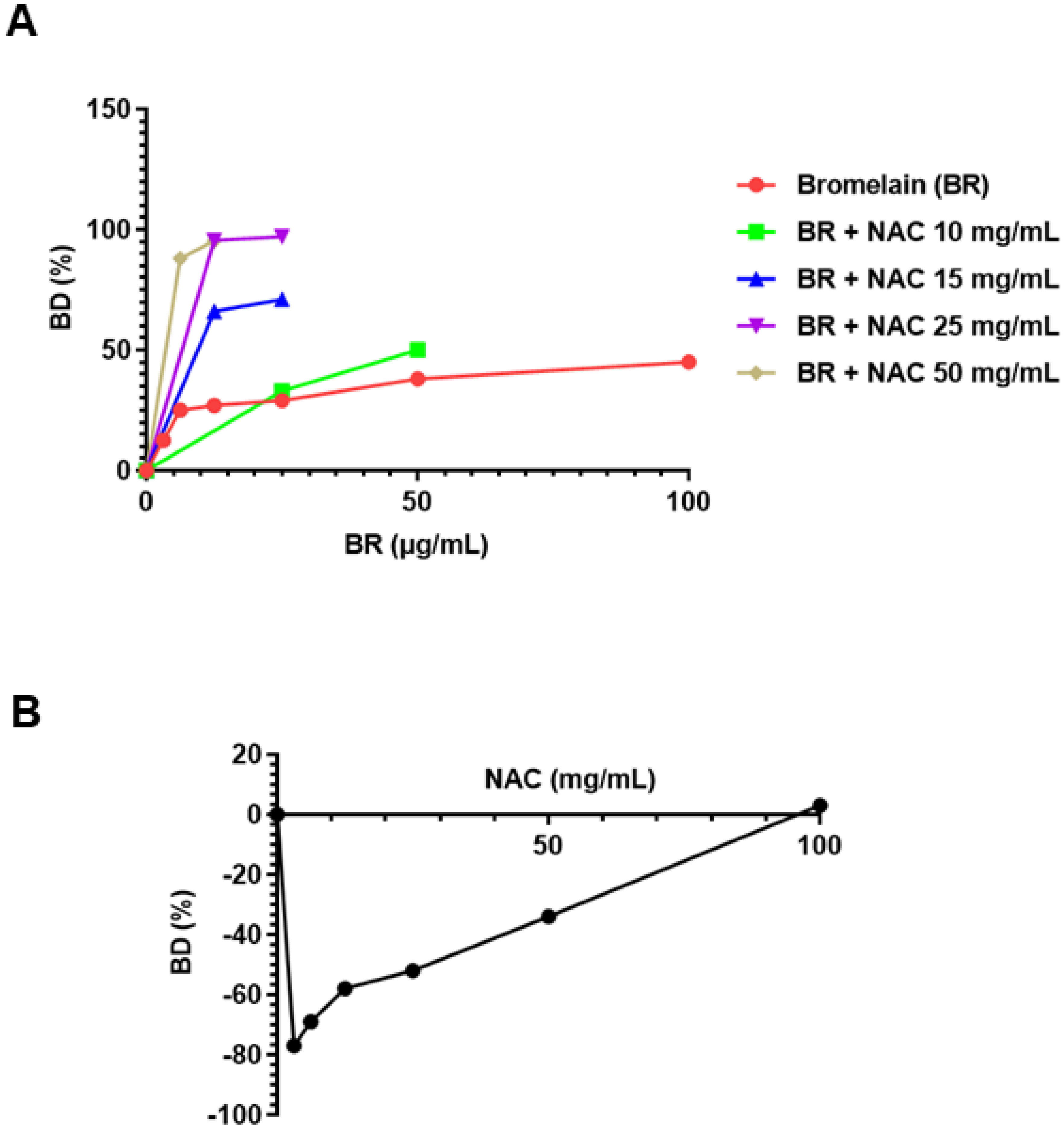

| P. aeruginosa | AGENT | Concentration | BD MAX (%) |
|---|---|---|---|
| ATCC 27853 | Bromelain | 6.25, 12.5, 25, 50, 100 µg/mL | 66 |
| N-acetylcysteine | 6.25, 12.5, 25, 50, 100 mg/mL | −54 | |
| PA01 3981 | Bromelain | 3.125, 6.25, 12.5, 25, 50, 100 µg/mL | 45 |
| N-acetylcysteine | 3.125, 6.25, 12.5, 25, 50, 100 mg/mL | 3.0 | |
| ATCC 31461 | Bromelain | 3.125, 6.25, 12.5, 25, 50, 100 µg/mL | 75 |
| N-acetylcysteine | 6.25, 12.5, 25, 50, 100 mg/mL | 57 |
| Pseudomonas aeruginosa | N-Acetylcysteine (mg/mL) | Bromelain (µg/mL) | BD MAX (%) | Combination Index (CI) |
|---|---|---|---|---|
| ATCC 27853 | 15.0 | 12.5 | 73 | 0.063 |
| 25.0 | 87 | 0.025 | ||
| 25.0 | 12.5 | −19 | antagonistic | |
| 25 | 67 | 0.209 | ||
| 50 | 6.25 | 85 | 0.008 | |
| 12.5 | 80.5 | 0.03 | ||
| PA01 3981 | 10.0 | 20 | 33 | 0.672 |
| 40 | 50 | 0.268 | ||
| 15.0 | 12.5 | 66 | 0.019 | |
| 25 | 71 | 0.022 | ||
| 25 | 12.5 | 95.5 | 0.001 | |
| 25 | 97 | 0.001 | ||
| 50 | 6.25 | 88 | 0.009 | |
| 12.5 | 95.3 | 0.003 | ||
| ATCC 31461 | 10.0 | 20,0 | 13 | 7.34 |
| 40.0 | 27 | 5.76 | ||
| 15.0 | 12.5 | 50 | 0.819 | |
| 25 | 81 | 0.403 | ||
| 25.0 | 12.5 | 80 | 0.357 | |
| 25.0 | 85 | 0.395 | ||
| 50 | 6.25 | 21 | 2.195 | |
| 12.5 | 79 | 0.581 |
| Pseudomonas aeruginosa | BR 100 µg/mL | OA | NAC 100 mg/mL | OA |
|---|---|---|---|---|
| ATCC 27853 | 66 | 2 | −54 | 3 |
| PA01 3981 | 45 | 3 | 3 | 2 |
| ATCC 31461 | 75 | 1 | 57 | 1 |
| ATCC 27853 | PA01 3981 | ATCC 31461 | |||||
|---|---|---|---|---|---|---|---|
| NAC (mg/mL) | BR (µg/mL) | BD MAX (%) | CI | BD MAX (%) | CI | BD MAX (%) | CI |
| 15 | 25 | 87 | 0.025 | - | - | 81 | 0.403 |
| 25 | 12.5 | - | - | 95.5 | 0.001 | 80 | 0.357 |
| 25 | 25 | - | - | 97 | 0.001 | 85 | 0.395 |
| 50 | 6.25 | 85 | 0.008 | 88 | 0.009 | - | - |
| 50 | 12.5 | 80.5 | 0.03 | 95.3 | 0.003 | 79 | 0.581 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, C.J.; Pillai, K.; Badar, S.; Mekkawy, A.H.; Akhter, J.; Jefferies, T.; Valle, S.J.; Morris, D.L. Dissolution of Biofilm Secreted by Three Different Strains of Pseudomonas aeruginosa with Bromelain, N-Acetylcysteine, and Their Combinations. Appl. Sci. 2021, 11, 11388. https://doi.org/10.3390/app112311388
Carter CJ, Pillai K, Badar S, Mekkawy AH, Akhter J, Jefferies T, Valle SJ, Morris DL. Dissolution of Biofilm Secreted by Three Different Strains of Pseudomonas aeruginosa with Bromelain, N-Acetylcysteine, and Their Combinations. Applied Sciences. 2021; 11(23):11388. https://doi.org/10.3390/app112311388
Chicago/Turabian StyleCarter, Carly J., Krishna Pillai, Samina Badar, Ahmed H. Mekkawy, Javed Akhter, Thomas Jefferies, Sarah J. Valle, and David L. Morris. 2021. "Dissolution of Biofilm Secreted by Three Different Strains of Pseudomonas aeruginosa with Bromelain, N-Acetylcysteine, and Their Combinations" Applied Sciences 11, no. 23: 11388. https://doi.org/10.3390/app112311388
APA StyleCarter, C. J., Pillai, K., Badar, S., Mekkawy, A. H., Akhter, J., Jefferies, T., Valle, S. J., & Morris, D. L. (2021). Dissolution of Biofilm Secreted by Three Different Strains of Pseudomonas aeruginosa with Bromelain, N-Acetylcysteine, and Their Combinations. Applied Sciences, 11(23), 11388. https://doi.org/10.3390/app112311388







