Impact of the Middle Eocene Climatic Optimum (MECO) on Foraminiferal and Calcareous Nannofossil Assemblages in the Neo-Tethyan Baskil Section (Eastern Turkey): Paleoenvironmental and Paleoclimatic Reconstructions
Abstract
:Featured Application
Abstract
1. Introduction
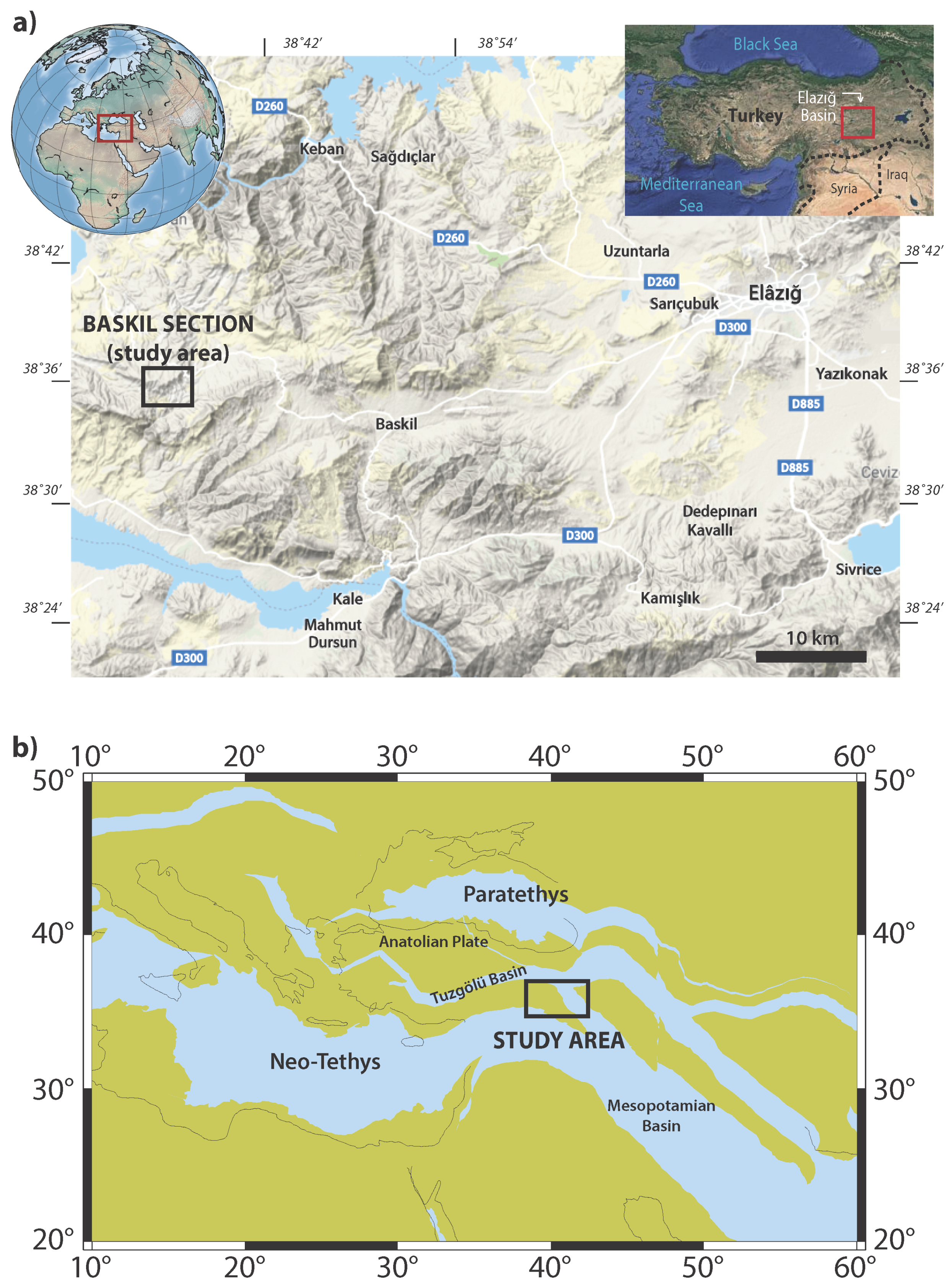
2. Geological Setting, Lithology and Bio-Magnetostratigraphic Framework
3. Materials and Methods
3.1. Field Sampling
3.2. Benthic Foraminifera and Dissolution Proxies
3.3. Planktic Foraminifera
3.4. Calcareous Nannofossils
3.5. Statistical Analyses
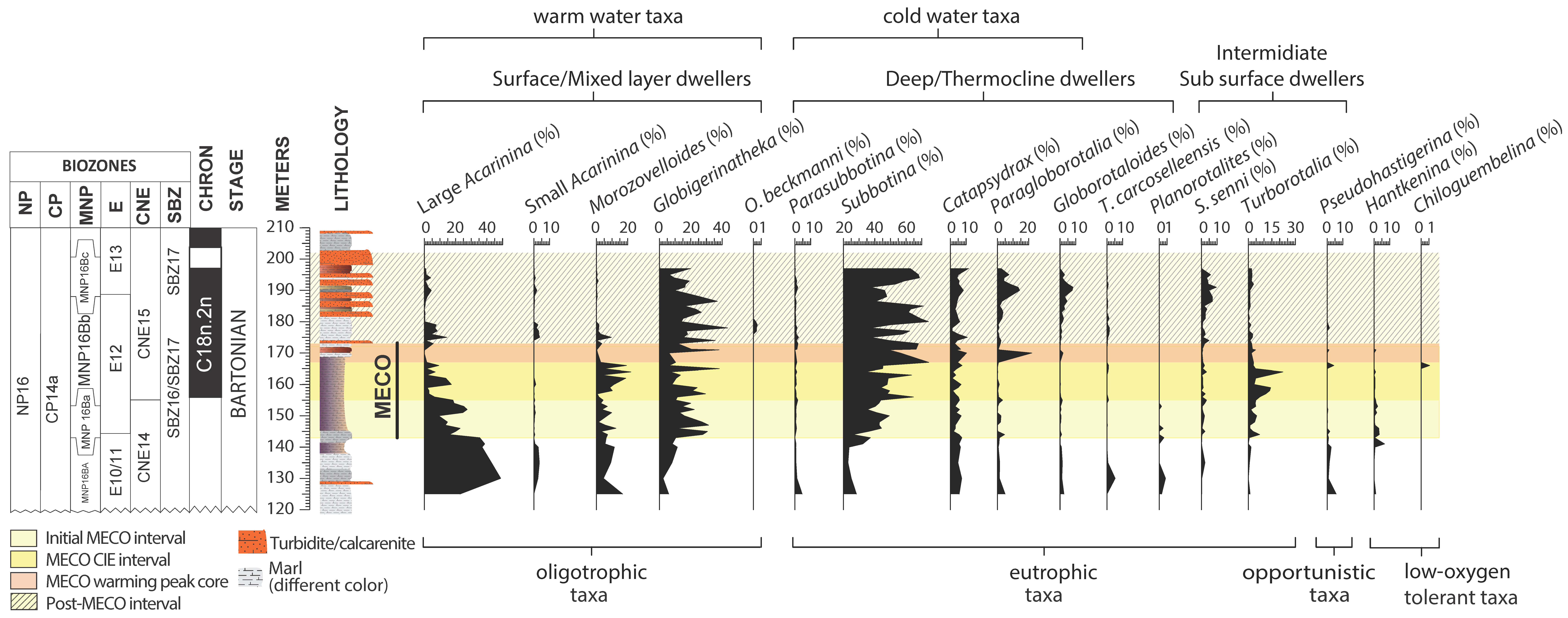
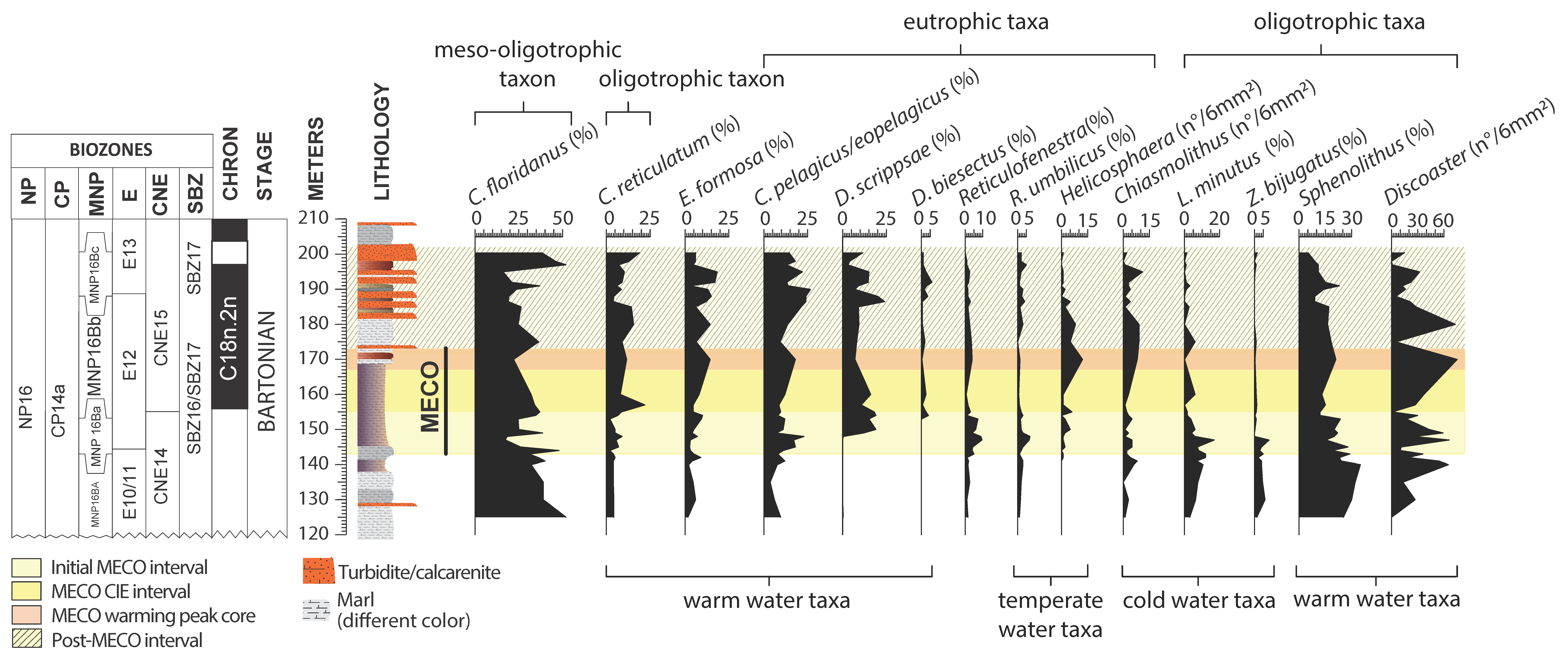
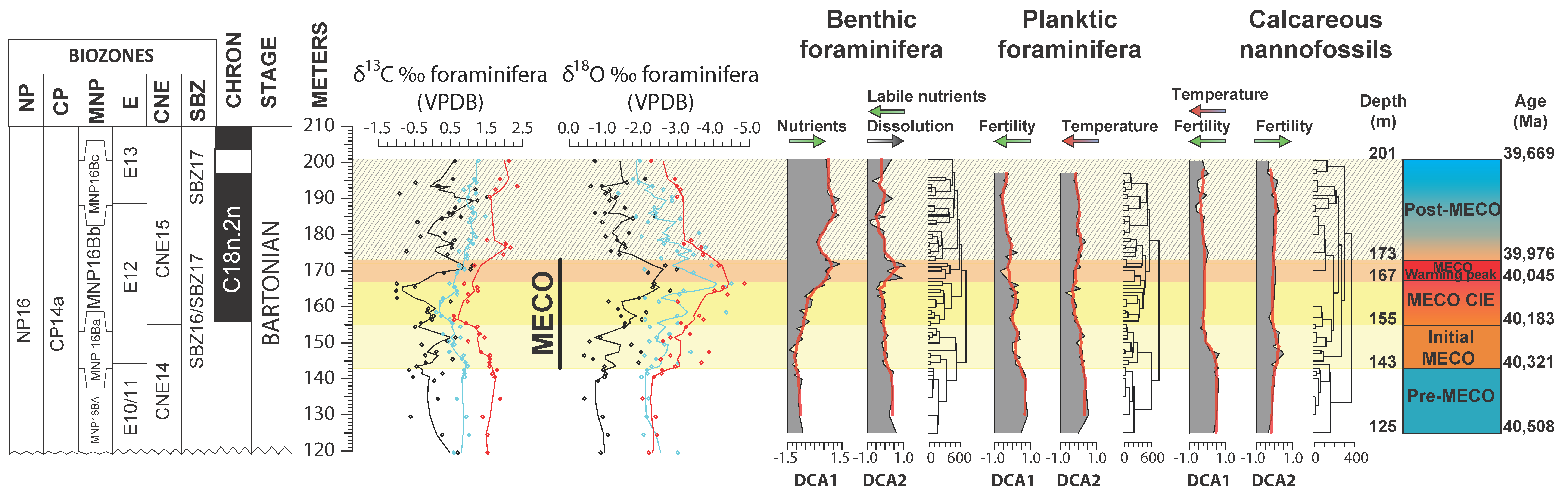
4. Results
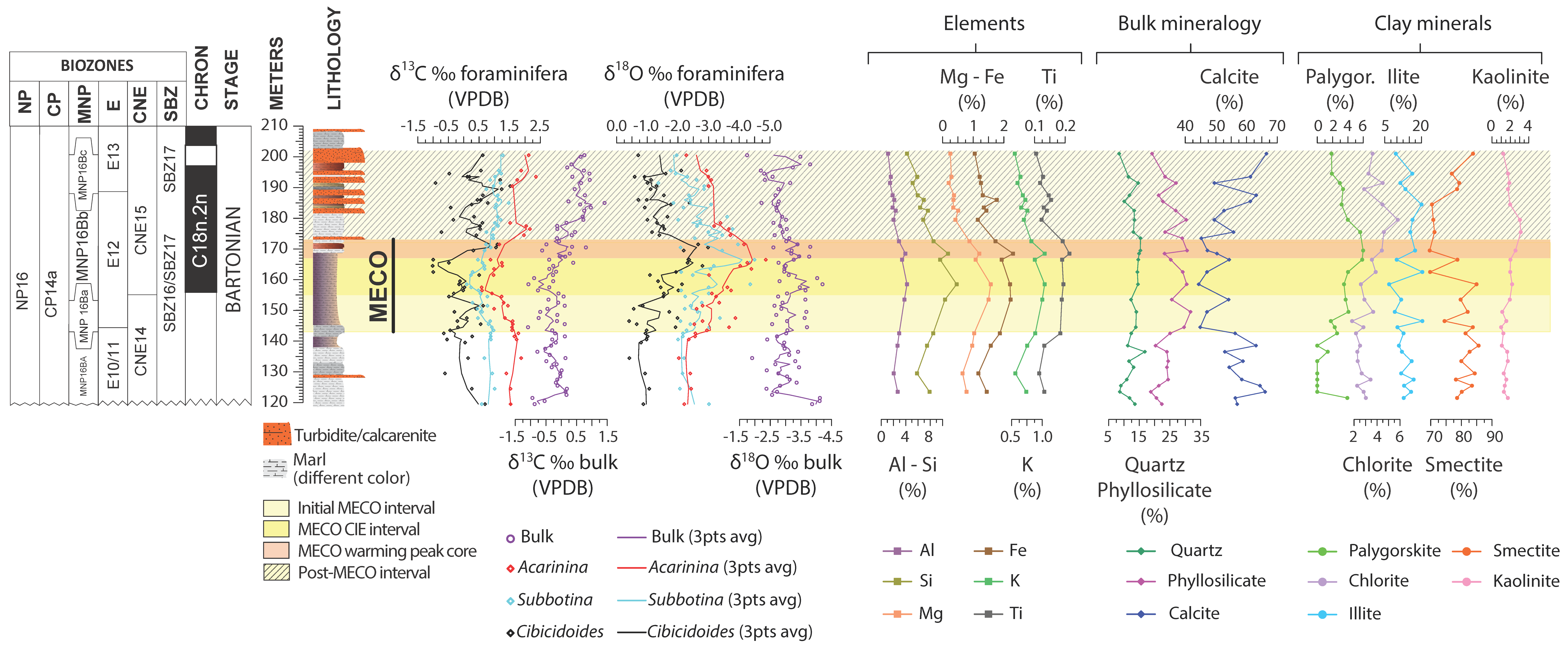
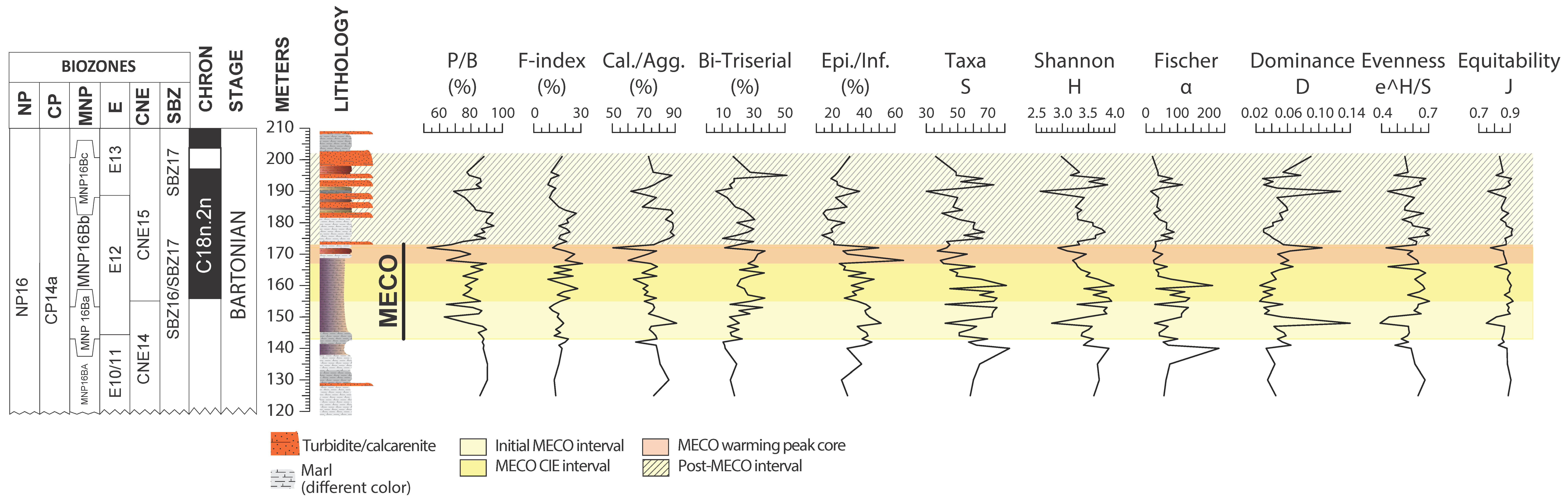
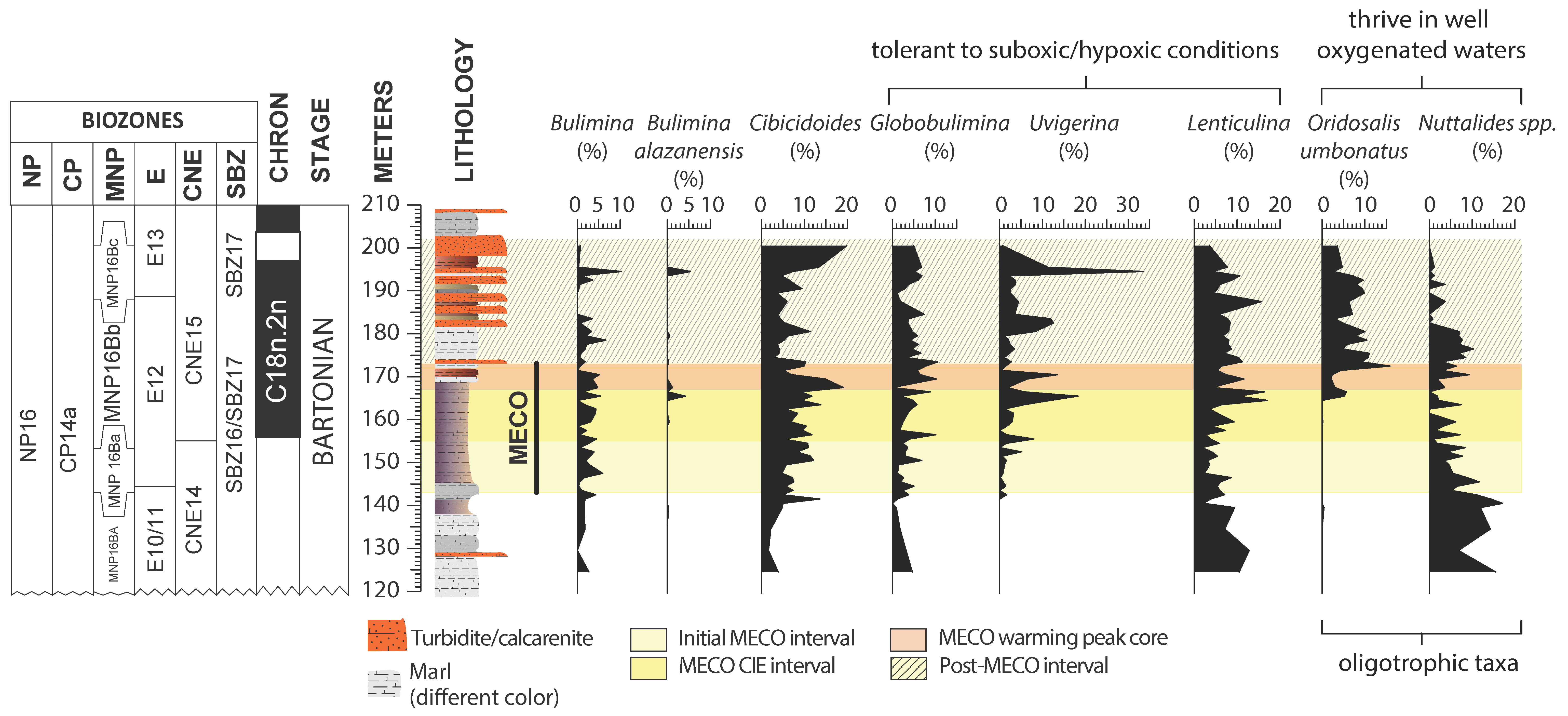
4.1. Benthic Foraminifera and Dissolution Proxies
4.2. Planktic Foraminifera
4.3. Calcareous Nannofossils
5. Discussion
5.1. Middle Eocene Benthic Foraminifera Paleoecology
5.2. Middle Eocene Planktic Foraminiferal Paleoecology
5.3. Middle Eocene Calcareous Nannofossil Paleoecology
5.4. Pre-MECO Phase (120–142 m): Stratified Water Column and Oligotrophic Conditions
5.5. The Initial MECO Phase (142–155 m): Rising of Mesotrophic Conditions
5.6. The MECO CIE Phase (155–167 m): Enhanced Eutrophic Conditions
5.7. The MECO Warming Peak Phase (167–173 m): Maximum of Surface-Water Nutrients and Dissolution
5.8. The Post-MECO Phase (173–201 m): Partial Recovery of the Pre-Event Conditions
6. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bohaty, S.M.; Zachos, J.C.; Florindo, F.; Delaney, M.L. Coupled greenhouse warming and deep-sea acidification in the middle Eocene. Paleoceanography 2009, 24, PA2207. [Google Scholar] [CrossRef]
- Westerhold, T.; Röhl, U. Orbital pacing of Eocene climate during the Middle Eocene Climate Optimum and the chron C19r event: Missing link found in the tropical western Atlantic. Geochem. Geophys. Geosyst. 2013, 14, 4811–4825. [Google Scholar] [CrossRef]
- Giorgioni, M.; Jovane, L.; Rego, E.S.; Rodelli, D.; Frontalini, F.; Coccioni, R.; Catanzariti, R.; Özcan, E. Carbon cycle instability and orbital forcing during the Middle Eocene Climatic Optimum. Sci. Rep. 2019, 9, 9357. [Google Scholar] [CrossRef] [PubMed]
- Kochhann, M.V.L.; Savian, J.F.; Tori, F.; Catanzariti, R.; Coccioni, R.; Frontalini, F.; Jovane, L.; Florindo, F.; Monechi, S. Orbital tuning for the middle Eocene to early Oligocene Monte Cagnero Section (Central Italy): Paleoenvironmental and paleoclimatic implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 577, 110563. [Google Scholar] [CrossRef]
- Bohaty, S.M.; Zachos, J.C. Significant Southern Ocean warming event in the late middle Eocene. Geology 2003, 31, 1017–1020. [Google Scholar] [CrossRef]
- Jovane, L.; Florindo, F.; Coccioni, R.; Dinarès-Turell, J.; Marsili, A.; Monechi, S.; Roberts, A.P.; Sprovieri, M. The middle Eocene climatic optimum event in the Contessa Highway section, Umbrian Apennines, Italy. Geol. Soc. Am. Bull. 2007, 119, 413–427. [Google Scholar] [CrossRef] [Green Version]
- Edgar, K.M.; Wilson, P.A.; Sexton, P.F.; Gibbs, S.J.; Roberts, A.P.; Norris, R.D. New biostratigraphy, magnetostratigraphy and isotopic insights into the Middle Eocene Climatic Optimum in low latitudes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 670–682. [Google Scholar] [CrossRef] [Green Version]
- Spofforth, D.J.A.; Agnini, C.; Pälike, H.; Rio, D.; Fornaciari, E.; Giusberti, L.; Luciani, V.; Lanci, L.; Muttoni, G. Organic carbon burial following the middle Eocene climatic optimum in the central western Tethys. Paleoceanography 2010, 25, PA3210. [Google Scholar] [CrossRef]
- Boscolo Galazzo, F.; Giusberti, L.; Luciani, V.; Thomas, E. Paleoenvironmental changes during the Middle Eocene Climatic Optimum (MECO) and its aftermath: The benthic foraminiferal record from the Alano section (NE Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 378, 22–35. [Google Scholar] [CrossRef]
- Savian, J.F.; Jovane, L.; Frontalini, F.; Trindade, R.I.F.; Coccioni, R.; Bohaty, S.M.; Wilson, P.A.; Florindo, F.; Roberts, A.P.; Catanzariti, R.; et al. Enhanced primary productivity and magnetotactic bacterial production in response to middle Eocene warming in the Neo-Tethys Ocean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 414, 32–45. [Google Scholar] [CrossRef]
- Bijl, P.K.; Houben, A.J.P.; Schouten, S.; Bohaty, S.M.; Sluijs, A.; Reichart, G.-J.; Damsté, J.S.S.; Brinkhuis, H. Transient Middle Eocene atmospheric CO2 and temperature variations. Science 2010, 330, 819–821. [Google Scholar] [CrossRef] [Green Version]
- Sluijs, A.; Zeebe, R.E.; Bijl, P.K.; Bohaty, S.M. A middle Eocene carbon cycle conundrum. Nat. Geosci. 2013, 6, 429–434. [Google Scholar] [CrossRef]
- Borelli, C.; Cramer, B.S.; Katz, M.E. Bipolar Atlantic deepwater circulation in the middle late Eocene: Effects of Southern Ocean gateway openings. Paleoceanography 2014, 29, 308–327. [Google Scholar] [CrossRef]
- George, R.; Rogers, N.; Kelley, S. Earliest magmatism in Ethiopia: Evidence for two mantle plumes in one flood basalt province. Geology 1998, 26, 923–926. [Google Scholar] [CrossRef]
- Aguirre-Diaz, G.J.; Labarthe-Hernandez, G. Fissure ignimbrites: Fissure-source origin for voluminous ignimbrites of the Sierra Madre Occidental and its relationship with Basin and Range faulting. Geology 2003, 31, 773–776. [Google Scholar] [CrossRef]
- Bowen, G.J.; Zachos, J.C. Rapid carbon sequestration at the termination of the Palaeocene-Eocene Thermal Maximum. Nat. Geosci. 2010, 3, 866–869. [Google Scholar] [CrossRef]
- Beniamovski, V.N.; Alekseev, A.S.; Ovechkina, M.N.; Oberhänsli, H. Middle to Upper Eocene disoxic–anoxic Kuma Formation (northeast Peri-Tethys): Biostratigraphy and paleoenviroments. In Causes and Consequences of Globally Warm Climates in the Early Paleogene; Wing, S.L., Ed.; Geological Society of America: Boulder, CO, USA, 2003; Volume 369, pp. 95–112. [Google Scholar]
- Luciani, V.; Giusberti, L.; Agnini, C.; Fornaciari, E.; Rio, D.; Spofforth, D.J.A.; Pälike, H. Ecological and evolutionary response of Tethyan planktonic foraminifera to the middle Eocene climatic optimum (MECO) from the Alano section (NE Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 292, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Luciani, V.; Fornaciari, E.; Papazzoni, C.A.; Dallanave, E.; Giusberti, L.; Stefani, C.; Amante, E. Integrated stratigraphy at the Bartonian–Priabonian transition: Correlation between shallow benthic and calcareous plankton zones (Varignano section, northern Italy). Geol. Soc. Am. Bull. 2020, 132, 495–520. [Google Scholar] [CrossRef]
- Edgar, K.M.; Bohaty, S.M.; Gibbs, S.J.; Sexton, P.F.; Norris, R.D.; Wilson, P.A. Symbiont ‘bleaching’ in planktic foraminifera during the Middle Eocene Climatic Optimum. Geology 2013, 41, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Toffanin, F.; Agnini, C.; Fornaciari, E.; Rio, D.; Giusberti, L.; Luciani, V.; Spofforth, D.J.A.; Pälike, H. Changes in calcareous nannofossil assemblages during the Middle Eocene Climatic Optimum: Clues from the central-western Tethys (Alano section, NE Italy). Mar. Micropaleontol. 2011, 81, 22–31. [Google Scholar] [CrossRef]
- Moebius, I.; Friedrich, O.; Edgar, K.M.; Scher, H.D.; Sexton, P. Bottom water changes in the subtropical North Atlantic and the Southern Ocean associated to the Middle Eocene Climatic Optimum. In Proceedings of the AGU Fall Meeting 2013, San Francisco, CA, USA, 9–13 December 2013. [Google Scholar]
- Moebius, I.; Friedrich, O.; Scher, H.D. Changes in Southern Ocean bottom water environments associated with the Middle Eocene Climatic Optimum (MECO). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 405, 16–27. [Google Scholar] [CrossRef]
- Witkowski, J.; Bohaty, S.M.; McCartney, K.; Harwood, D.M. Enhanced siliceous plankton productivity in response to middle Eocene warming at Southern Ocean ODP Sites 748 and 749. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 326–328, 78–94. [Google Scholar] [CrossRef]
- Witkowski, J.; Bohaty, S.M.; Edgar, K.M.; Harwood, D.M. Rapid fluctuations in mid-latitude siliceous plankton production during the Middle Eocene Climatic Optimum (ODP Site 1051, western North Atlantic). Mar. Micropaleontol. 2014, 106, 110–129. [Google Scholar] [CrossRef] [Green Version]
- Villa, G.; Fioroni, C.; Persico, D.; Roberts, A.P.; Florindo, F. Middle Eocene to Late Oligocene Antarctic glaciation/deglaciation and Southern Ocean productivity. Paleoceanography 2014, 29, 223–237. [Google Scholar] [CrossRef]
- Lyle, M.; Lyle Olivarez, A.; Backman, J.; Tripati, A. Biogenic sedimentation in the Eocene equatorial Pacific—The stuttering greenhouse and Eocene carbonate compensation depth. In Proceedings of the Ocean Drilling Program, Scientific Results; Wilson, P.A., Lyle, M., Firth, J.V., Eds.; Ocean Drilling Program: College Station, TX, USA, 2005; Volume 199, pp. 1–35. [Google Scholar] [CrossRef]
- Lyle, M.; Barron, J.; Bralower, T.J.; Huber, M.; Olivarez Lyle, A.; Ravelo, A.C.; Rea, D.K.; Wilson, P.A. Pacific Ocean and Cenozoic evolution of climate. Rev. Geophys. 2008, 46, RG2002. [Google Scholar] [CrossRef] [Green Version]
- Pea, L. Eocene-Oligocene Paleoceanography of the Subantarctic South Atlantic: Calcareous Nannofossil Reconstructions of Temperature, Nutrient, and Dissolution History. Ph.D. Thesis, Dipartimento di Scienze della Terra, Università degli Studi di Parma, Parma, Italy, 2011. [Google Scholar]
- Takata, H.; Nomura, R.; Tsujimoto, A.; Khim, B.; Chung, I. Abyssal benthic foraminifera in the eastern equatorial Pacific (IODP exp 320) during the Middle Eocene. J. Paleontol. 2013, 87, 1160–1185. [Google Scholar] [CrossRef]
- Cornaggia, F.; Bernardini, S.; Giorgioni, M.; Silva, G.L.X.; Nagy, A.I.M.; Jovane, L. Abyssal Oceanic Circulation and Acidification during the Middle Eocene Climatic Optimum (MECO). Sci. Rep. 2020, 10, 6674. [Google Scholar] [CrossRef] [Green Version]
- Rego, E.R.; Jovane, L.; Hein, J.R.; Sant’Anna, L.G.; Giorgioni, M.; Rodelli, D.; Özcan, E. Mineralogical evidence for warm and dry climatic conditions in the Neo-Tethys (eastern Turkey) during the middle Eocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 501, 45–57. [Google Scholar] [CrossRef]
- Rodelli, D.; Jovane, L.; Özcan, E.; Giorgioni, M.; Coccioni, R.; Frontalini, F.; Rego, E.S.; Brogi, A.; Catanzariti, R.; Less, G.; et al. High-resolution integrated magnetobiostratigraphy of a new middle Eocene section from the Neotethys (Elazığ Basin, eastern Turkey). Geol. Soc. Am. Bull. 2018, 130, 193–207. [Google Scholar] [CrossRef]
- Martini, E. Standard Tertiary and Quaternary calcareous nannoplankton zonation. In Proceedings of the 2nd Planktonic Conference, Rome, Italy, 1970; Farinacci, A., Ed.; Tecnoscienza: Rome, Italy, 1971; Volume 2, pp. 739–785. [Google Scholar]
- Okada, H.; Bukry, D. Supplementary modification and introduction of code numbers to the low-latitude coccolith biostratigraphic zonation (Bukry, 1973; 1975). Mar. Micropaleontol. 1980, 5, 321–325. [Google Scholar] [CrossRef]
- Fornaciari, E.; Agnini, C.; Catanzariti, R.; Rio, D.; Bolla, E.M.; Valvasoni, E. Mid–latitude calcareous nannofossil biostratigraphy and biochronology across the middle to late Eocene transition. Stratigraphy 2010, 7, 229–264. [Google Scholar]
- Agnini, C.; Fornaciari, E.; Raffi, I.; Catanzariti, R.; Pälike, H.; Backman, J.; Rio, D. Biozonation and biochronology of Paleogene calcareous nannofossils from low and middle latitudes. Newsl. Stratigr. 2014, 47, 131–181. [Google Scholar] [CrossRef]
- Wade, B.S.; Pearson, P.N.; Berggren, W.A.; Pälike, H. Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale. Earth–Sci. Rev. 2011, 104, 111–142. [Google Scholar] [CrossRef] [Green Version]
- Serra-Kiel, J.; Hottinger, L.; Caus, E.; Drobne, K.; Ferràndez, C.; Jauhri, A.K.; Less, G.; Pavlovec, R.; Pignatti, J.; Samsó, J.M.; et al. Larger foraminiferal biostratigraphy of the Tethyan Paleocene and Eocene. Bull. Société Géologique Fr. 1998, 169, 281–299. [Google Scholar]
- Less, G.; Özcan, E. Bartonian-Priabonian larger benthic foraminiferal events in the Western Tethys. Mitt. Osterr. Geol. Ges. 2012, 105, 129–140. [Google Scholar]
- Özcan, E.; Less, G.; Báldi-Beke, M.; Kollányi, K.; Kertesz, B. Biometric analysis of middle and upper Eocene Discocyclinidae and Orbitoclypeidae (Foraminifera) from Turkey and updated orthophragmine zonation in the Western Tethys. Micropaleontology 2006, 52, 485–520. [Google Scholar] [CrossRef] [Green Version]
- Özcan, E.; Less, G.; Jovane, L.; Catanzariti, R.; Frontalini, F.; Coccioni, R.; Giorgioni, M.; Rodelli, D.; Rego, E.S.; Kayğılı, S.; et al. Integrated biostratigraphy of the middle to upper Eocene Kırkgeçit Formation (Baskil Section, Elazığ, Eastern Turkey): Larger benthic foraminiferal perspective. Mediterr. Geosci. Rev. 2019, 1, 55–90. [Google Scholar] [CrossRef] [Green Version]
- Çelik, H. The effects of linear coarse-grained slope channel bodies on the orientations of fold developments: A case study from the Middle Eocene-Lower Oligocene Kırkgeçit Formation, Elazığ, eastern Turkey. Turk. J. Earth Sci. 2013, 22, 320–338. [Google Scholar] [CrossRef]
- Ogg, J.G. Geomagnetic polarity time scale. In The Geologic Time Scale 2012, 1st ed.; Gradstein, F.M., Ogg, J.G., James, G., Schmitz, M.D., Ogg, G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 85–113. [Google Scholar] [CrossRef] [Green Version]
- Lirer, F. A new technique for retrieving calcareous microfossils from lithified lime deposits. Micropaleontology 2000, 46, 365–369. [Google Scholar]
- Loeblich, A.R.; Tappan, H. Foraminiferal Genera and their Classification. Van Nostrand Reinhold Company: New York, U.S.A., 1988 [year of publication often cited erroneously as 1987; see Loeblich, A.R., Tappan, H., Publication date of foraminiferal genera and their classification. J. Paleontol. 1989, 63, 253. [Google Scholar] [CrossRef]
- Tjalsma, R.C.; Lohmann, G.P. Paleocene-Eocene Bathyal and Abyssal Benthic Foraminifera from the Atlantic Ocean. Micropaleontol. Spec. Pub. 1983, 4, 1–90. [Google Scholar]
- Van Morkhoven, F.P.C.M.; Berggren, W.A.; Edwards, A.S. (Eds.) Cenozoic Cosmopolitan Deep-Sea Benthic Foraminifera; Bulletin du Centre de Recherches Exploration-Production Elf-Aquitaine Mémoire: Pau, France, 1986; Volume 11, p. 421. [Google Scholar]
- Bolli, H.; Beckmann, J.; Saunders, J. Benthic Foraminiferal Biostratigraphy of the South. Caribbean Region, 1st ed.; Cambridge University Press: Cambridge, UK, 1994; p. 146. [Google Scholar]
- Ortiz, S.; Thomas, E. Lower-middle Eocene benthic foraminifera from the Fortuna section (Betic Cordillera, southeastern Spain). Micropaleontology 2006, 52, 97–150. [Google Scholar] [CrossRef] [Green Version]
- Boscolo Galazzo, F.; Thomas, E.; Giusberti, L. Benthic foraminiferal response to the Middle Eocene Climatic Optimum (MECO) in the South-Eastern Atlantic (ODP Site 1263). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 417, 432–444. [Google Scholar] [CrossRef]
- Holbourn, A.; Henderson, A.S.; MacLeod, N. Atlas of Benthic Foraminifera, 1st ed.; Wiley-Blackwell, Natural History Museum: London, UK, 2013; p. 656. [Google Scholar]
- Arreguín-Rodríguez, G.J.; Thomas, E.; D’haenens, S.; Speijer, R.P.; Alegret, L. Early Eocene deep-sea benthic foraminiferal faunas: Recovery from the Paleocene Eocene Thermal Maximum extinction in a greenhouse world. PLoS ONE 2018, 13, e0193167. [Google Scholar]
- Corliss, B.H. Microhabitats of benthic foraminifera within deep-sea sediments. Nature 1985, 314, 435–438. [Google Scholar] [CrossRef]
- Jones, R.W.; Charnock, M.A. ‘Morphogroups’ of agglutinating foraminifera, their life positions and feeding habits and potential applicability in paleoecological studies. Rev. Paleobiol. 1985, 4, 311–320. [Google Scholar]
- Corliss, B.H.; Chen, C. Morphotype patterns of Norwegian Sea deep-sea benthic foraminifera and ecological implications. Geology 1988, 16, 716–719. [Google Scholar] [CrossRef]
- Alegret, L.; Molina, E.; Thomas, E. Benthic foraminiferal turnover across the Cretaceous/Paleogene boundary at Agost (southeastern Spain): Paleoenvironmental inferences. Mar. Micropaleontol. 2003, 48, 251–279. [Google Scholar] [CrossRef]
- Kaminski, M.A.; Gradstein, F.M. Atlas of Paleogene Cosmopolitan Deep-Water Agglutinated Foraminifera, 1st ed.; Grzybowski Foundation Special Publication: Krakov, Poland, 2005; Volume 10, p. 547. [Google Scholar]
- Arreguín-Rodríguez, G.J.; Alegret, L.; Thomas, E. Late Paleocene-middle Eocene benthic foraminifera on a Pacific seamount (Allison Guyot, ODP Site 865): Greenhouse climate and superimposed hyperthermal events. Paleoceanography 2016, 31, 346–364. [Google Scholar] [CrossRef] [Green Version]
- Hancock, H.J.L.; Dickens, G.R. Carbonate dissolution episodes in Paleocene and Eocene sediment, Shatsky Rise, west-central Pacific. In Proceedings of the Ocean Drilling Program, Scientific Results; Bralower; Bralower, T.J., Silva, I.P., Malone, M.J., Eds.; Texas A&M University: College Station, TX, USA, 2005; Volume 198, pp. 1–24. Available online: http://www-odp.tamu.edu/publications/198_SR/116/116.htm (accessed on 26 November 2021).
- Pearson, P.N.; Olsson, R.K.; Hemblen, C.; Huber, B.T.; Berggren, W.A. Atlas of Eocene Planktonic Foraminifera, 1st ed.; Cushman Found. Special Publication for Foraminiferal Res.: Greenville, NC, USA, 2006; Volume 41, p. 513. [Google Scholar]
- Pearson, P.N.; Shackleton, N.J.; Hall, M.A. Stable isotope paleoecology of middle Eocene planktonic foraminifera and multispecies isotope stratigraphy, DSDP Site 523, South Atlantic. J. Foraminifer. Res. 1993, 23, 123–140. [Google Scholar] [CrossRef]
- Bown, P.R.; Young, J.R. Techniques. In Calcareous Nannofossil Biostratigraphy, 1st ed.; Bown, P.R., Ed.; Chapman & Hall: London, UK, 1998; pp. 16–28. [Google Scholar]
- Grimm, E.C. CONISS: A FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Francescangeli, F.; Armynot du Chatelet, E.; Billon, G.; Trentesaux, A.; Bouchet, V.M.P. Palaeo-ecological quality status based on foraminifera of Boulogne-sur-Mer harbour (Pas-de-Calais, Northeastern France) over the last 200 years. Mar. Environ. Res. 2016, 117, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Francescangeli, F.; Portela, M.; Armynot du Chatelet, E.; Billon, G.; Andersen, T.J.; Bouchet, V.M.P.; Trentesaux, A. Infilling of the Canche Estuary (eastern English Channel, France): Insight from benthic foraminifera and historical pictures. Mar. Micropaleontol. 2018, 142, 1–12. [Google Scholar] [CrossRef]
- Hill, M.O.; Gauch, H.G. Detrended correspondence analysis: An improved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- Versteegh, G.J.M.; Zonneveld, K.A.F. Determination of (palaeo-) ecological preferences of dinoflagellates by applying Detrended and Canonical Correspondence analysis to Late Pliocene dinoflagellate cyst assemblages of the south Italian Singa section. Rev. Palaeobot. Palynol. 1994, 84, 181–199. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www.r-project.org/ (accessed on 26 November 2021).
- Juggins, S. Rioja: Analysis of Quaternary Science Data. R Package Version 0.9-26. 2020. Available online: https://cran.r-project.org/package=rioja (accessed on 26 November 2021).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.3-3. 2016. Available online: https://CRAN.R-project.org/package=vegan (accessed on 26 November 2021).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Jorissen, F.J.; Fontanier, C.; Thomas, E. Paleoceanographical proxies based on deep-sea benthic foraminiferal assemblage characteristics. In Developments in Marine Geology: Proxies in Late Cenozoic Paleoceanography, 1st ed.; Hillaire-Marcel, C., de Vernal, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 1, pp. 264–325. [Google Scholar]
- Boscolo Galazzo, F.; Thomas, E.; Pagani, M.; Warren, C.; Luciani, V.; Giusberti, L. The middle Eocene climatic optimum (MECO): A multiproxy record of paleoceanographic changes in the southeast Atlantic (ODP Site 1263, Walvis Ridge). Paleoceanography 2014, 29, 1143–1161. [Google Scholar] [CrossRef]
- Moebius, I.; Friedrich, O.; Edgar, K.M.; Sexton, P.F. Episodes of intensified biological productivity in the subtropical Atlantic Ocean during the termination of the Middle Eocene Climatic Optimum (MECO). Paleoceanography 2015, 30, 1041–1058. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Cuesta, L.; Westerhold, T.; Agnini, C.; Dallanave, E.; Wilkens, R.H.; Alegret, L. Paleoenvironmental Changes at ODP Site 702 (South Atlantic): Anatomy of the Middle Eocene Climatic Optimum. Paleoceanogr. Paleoclimatol. 2019, 34, 2047–2066. [Google Scholar] [CrossRef]
- Jorissen, F.J.; De Stigter, H.C.; Widmark, J.G.V. A conceptual model explaining benthic foraminiferal microhabitats. Mar. Micropaleontol. 1995, 26, 3–15. [Google Scholar] [CrossRef]
- Jorissen, F.J. Benthic foraminiferal successions across late Quaternary Mediterranean sapropels. Mar. Geol. 1999, 153, 91–101. [Google Scholar] [CrossRef]
- Agnini, C.; Backman, J.; Boscolo-Galazzo, F.; Condon, D.J.; Fornaciari, E.; Galeotti, S.; Giusberti, L.; Grandesso, P.; Lanci, L.; Luciani, V.; et al. Proposal for the global boundary stratotype section and point (GSSP) for the Priabonian stage (Eocene) at the Alano section (Italy). Episodes 2021, 44, 151–173. [Google Scholar] [CrossRef]
- Birch, H.S.; Coxall, H.K.; Pearson, P.N. Evolutionary ecology of early Paleocene planktonic foraminifera: Size, depth habitat and symbiosis. Paleobiology 2012, 38, 374–390. [Google Scholar] [CrossRef]
- Bornemann, A.; Norris, R.D. Size-related stable isotope changes in Late Cretaceous planktic foraminifera: Implications for paleoecology and photosymbiosis. Mar. Micropaleontol. 2007, 65, 32–42. [Google Scholar] [CrossRef]
- D’Hondt, S.; Zachos, J.C.; Schultz, G. Stable isotopic signals and photosymbiosis in late Paleocene planktic foraminifera. Paleobiology 1994, 20, 391–406. [Google Scholar] [CrossRef]
- Elderfield, H.; Vautravers, M.; Cooper, M. The relationship between Mg/Ca, Sr/Ca, δ18O, and δ13C of species of planktonic foraminifera. Geochem. Geophys. Geosyst. 2002, 3, 1–13. [Google Scholar] [CrossRef]
- Norris, R.D. Symbiosis as an evolutionary innovation in the radiation of Paleocene planktic foraminifera. Paleobiology 1996, 22, 461–480. [Google Scholar] [CrossRef]
- Quillévéré, F.; Norris, R.D.; Moussa, I.; Berggren, W.A. Role of photosymbiosis and biogeography in the diversification of early Paleogene acarininids (planktonic foraminifera). Paleobiology 2001, 27, 311–326. [Google Scholar] [CrossRef]
- Takagi, H.; Moriya, K.; Ishimura, T.; Suzuki, A.; Kawahata, H.; Hirano, H. Exploring photosymbiotic ecology of planktic foraminifers from chamber-by-chamber isotopic history of individual foraminifers. Paleobiology 2015, 41, 108–121. [Google Scholar] [CrossRef]
- Wade, B.S.; Al-Sabouni, N.; Hemleben, C.; Kroon, D. Symbiont bleaching in fossil planktonic foraminifera. Evol. Ecol. 2008, 22, 253–265. [Google Scholar] [CrossRef]
- Wendler, I.; Huber, B.T.; MacLeod, K.G.; Wendler, J.E. Stable oxygen and carbon isotope systematics of exquisitely preserved Turonian foraminifera from Tanzania—Understanding isotopic signatures in fossils. Mar. Micropaleontol. 2013, 102, 1–33. [Google Scholar] [CrossRef]
- Spero, H.J.; DeNiro, M.J. The influence of photosynthesis on the δ18O and δ13C values of planktonic foraminiferal shell calcite. Symbiosis 1987, 4, 213–228. [Google Scholar]
- Bé, A.W.H.; Spero, H.J.; Anderson, O.R. Effect of symbiont elimination and reinfection on the life processes of the planktonic foraminifera Globigerinoides Sacculifer. Mar. Biol. 1982, 70, 73–86. [Google Scholar] [CrossRef]
- Wade, B.S.; Olsson, R.K. Investigation of pre-extinction dwarfing in Cenozoic planktonic foraminifera. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 284, 39–46. [Google Scholar] [CrossRef]
- Pearson, P.N.; Ditchfield, P.W.; Singano, J.; Harcourt-Brown, K.G.; Nicholas, C.J.; Olsson, R.K.; Shackleton, N.J.; Hall, M.A. Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs. Nature 2001, 413, 481–487. [Google Scholar] [CrossRef]
- Luciani, V.; D’Onofrio, R.; Dickens, G.R.; Wade, B.S. Did Photosymbiont Bleaching Lead to the Demise of Planktic Foraminifer Morozovella at the Early Eocene Climatic Optimum? Paleoceanography 2017, 32, 1115–1136. [Google Scholar] [CrossRef] [Green Version]
- Luciani, V.; D’Onofrio, R.; Filippi, G.; Moretti, S. Which was the habitat of early Eocene planktic foraminifer Chiloguembelina? Stable isotope paleobiology from the Atlantic Ocean and implication for paleoceanographic reconstructions. Glob. Planet. Chang. 2020, 191, 103216. [Google Scholar] [CrossRef]
- Boersma, A.; Premoli Silva, I.; Shackleton, N. Atlantic Eocene planktonic foraminiferal biogeography and stable isotopic paleoceanography. Paleoceanography 1987, 2, 287–331. [Google Scholar] [CrossRef]
- Shackleton, N.J.; Corfield, R.M.; Hall, M.A. Stable isotope data and the ontogeny of Paleocene planktonic foraminifera. J. Foraminifer. Res. 1985, 15, 321–336. [Google Scholar] [CrossRef]
- Wade, B.S.; Kroon, D. Middle Eocene regional climate instability: Evidence from the western North Atlantic. Geology 2002, 30, 1011–1014. [Google Scholar] [CrossRef]
- Wade, B.S. Planktonic foraminiferal biostratigraphy and mechanisms in the extinction of Morozovella in the late middle Eocene. Mar. Micropaleontol. 2004, 51, 23–38. [Google Scholar] [CrossRef]
- Sexton, P.F.; Wilson, P.A.; Pearson, P.N. Microstructural and geochemical perspectives on planktic foraminiferal preservation: ‘Glassy’ versus ‘frosty’. Geochem. Geophys. Geosyst. 2006, 7, Q12P19. [Google Scholar] [CrossRef]
- Premoli Silva, I.; Petrizzo, M.R.; Melloni, D. Data report: Planktonic foraminiferal biostratigraphy across the Cretaceous/Paleocene boundary at Shatsky Rise (ODP Leg 198, northwest Pacific). In Proceedings of the Ocean Drilling Program: Scientific Results; Bralower, T.J., Premoli Silva, I., Malone, M.J., Eds.; Ocean Drilling Program: College Station, TX, USA, 2005; Volume 198, pp. 1–16. [Google Scholar] [CrossRef]
- Burgess, C.E.; Pearson, P.N.; Lear, C.H.; Morgans, H.E.G.; Handley, L.; Pancost, R.D.; Schouten, S. Middle Eocene climate cyclicity in the southern Pacific: Implications for global ice volume. Geology 2008, 36, 651–654. [Google Scholar] [CrossRef]
- Pearson, P.N.; Palmer, M.R. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 2000, 406, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Coxall, H.K.; Wilson, P.A.; Pearson, P.N.; Sexton, P.F. Iterative evolution of digitate planktonic foraminifera. Paleobiology 2007, 33, 495–516. [Google Scholar] [CrossRef] [Green Version]
- Resig, J.M.; Kroopnick, P.M. Isotopic and distributional evidence of a planktonic habit for the foraminiferal genus Streptochilus Brönnimann and Resig, 1971. Mar. Micropaleontol. 1983, 8, 235–248. [Google Scholar] [CrossRef]
- Boersma, A.; Premoli Silva, I. Atlantic paleogene biserial heterohelicid foraminifera and oxygen minima. Paleoceanography 1989, 4, 271–286. [Google Scholar] [CrossRef]
- Hallock, P.; Premoli Silva, I.; Boersma, A. Similarities between planktonic and larger foraminiferal evolutionary trends through Paleogene paleoceanographic changes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 83, 43–64. [Google Scholar] [CrossRef]
- Luciani, V.; Giusberti, L.; Agnini, C.; Backman, J.; Fornaciari, E.; Rio, D. The Paleocene–Eocene Thermal Maximum as recorded by Tethyan planktonic foraminifera in the Forada section (northern Italy). Mar. Micropaleontol. 2007, 64, 189–214. [Google Scholar] [CrossRef]
- Wei, W.; Wise, S.W., Jr. Biogeographic gradients of middle Eocene-Oligocene calcareous nannoplankton in the South Atlantic Ocean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 79, 29–61. [Google Scholar] [CrossRef]
- Persico, D.; Villa, G. Eocene–Oligocene calcareous nannofossils from Maud Rise and Kerguelen Plateau/Antarctica): Paleoecological and paleoceanographic implications. Mar. Micropaleontol. 2004, 52, 153–179. [Google Scholar] [CrossRef]
- Villa, G.; Fioroni, C.; Pea, L.; Bohaty, S.M.; Persico, D. Middle Eocene–late Oligocene climate variability: Calcareous nannofossil response at Kerguelen Plateau, Site 748. Mar. Micropaleontol. 2008, 69, 173–192. [Google Scholar] [CrossRef]
- Tori, F. Variabilità Climatica e Ciclicità Nell’intervallo Eocene Oligocene: Dati dai Nannofossili Calcarei. Ph.D. Thesis, Department of Earth Sciences, University of Florence, Florence, Italy, 2008; p. 222. [Google Scholar]
- Bukry, D. Cenozoic coccolith and silicoflagellate stratigraphy, offshore northwest Africa, DSDP Leg 41. In Deep Sea Drilling Project Initial Reports; Lancelot, Y., Seibold, E., Dean, W.E., Jansa, L.F., Eremeev, V., Gardner, J., Cepek, P., Krasheninnikov, V.A., Pflaumann, U., Johnson, D., et al., Eds.; Deep Sea Drilling Project; U.S. Govt. Printing Office: Washington, DC, USA, 1997; Volume 41, pp. 689–719. [Google Scholar]
- Aubry, M.-P. Late Paleogene calcareous nannoplankton evolution: A tale of climatic deterioration. In Eocene-Oligocene Climatic and Biotic Evolution; Prothero, D.R., Berggren, W.A., Eds.; Princeton University Press: Princeton, NJ, USA, 1992; pp. 272–309. [Google Scholar] [CrossRef]
- Aubry, M.-P. Early Paleogene calcareous nannoplankton evolution: A tale of climatic amelioration. In Late Paleocene–Early Eocene Biotic and Climatic Events in the Marine and Terrestrial Records; Aubry, M.-P., Lucas, S.G., Berggren, W.A., Eds.; Columbia University Press: New York, NY, USA, 1998; pp. 158–201. [Google Scholar]
- Bralower, T.J. Evidence of surface water oligotrophy during the Paleocene-Eocene thermal maximum: Nannofossil assemblage data from Ocean Drilling Program Site 690, Maud Rise, Weddell Sea. Paleoceanography 2002, 17, 13-1–13-12. [Google Scholar] [CrossRef]
- Gibbs, S.J.; Bralower, T.J.; Bown, P.R.; Zachos, J.C.; Bybell, L.M. Shelf and open ocean calcareous phytoplankton assemblages across the Paleocene–Eocene thermal Maximum: Implications for global productivity gradients. Geology 2006, 34, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Agnini, C.; Fornaciari, E.; Rio, D.; Tateo, F.; Backman, J.; Giusberti, L. Responses of calcareous nannofossil assemblages, mineralogy and geochemistry to the environmental perturbations across the Paleocene/Eocene boundary in the Venetian Pre-Alps. Mar. Micropaleontol. 2007, 63, 19–38. [Google Scholar] [CrossRef]
- Schneider, L.J.; Bralower, T.J.; Kump, L.R. Response of nannoplankton to early Eocene ocean destratification. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 310, 152–162. [Google Scholar] [CrossRef]
- Kalb, A.L.; Bralower, T.J. Nannoplankton origination events and environmental changes in the late Paleocene and early Eocene. Mar. Micropaleontol. 2012, 92–93, 1–15. [Google Scholar] [CrossRef]
- Kleijne, A. Holooccoliphorids from the Indian Ocean, Red Sea, Mediterranean and North Atlantic Ocean. Mar. Micropaleontol. 1991, 17, 1–76. [Google Scholar] [CrossRef]
- Gibbs, S.; Shackleton, N.; Young, J. Orbitally forced climate signals in mid-Pliocene nannofossil assemblages. Mar. Micropaleontol. 2004, 51, 39–56. [Google Scholar] [CrossRef]
- Bukry, D. Low-latitude coccolith biostratigraphic zonation. In Proceedings of the Ocean Drilling Program, Initial Reports; Edgar, N.T., Saunders, J.B., Eds.; Ocean Drilling Program: College Station, TX, USA, 1973; Volume 15, pp. 685–703. [Google Scholar] [CrossRef]
- Angori, E.; Bernaola, G.; Monechi, S. Calcareous nannofossil assemblages and their response to the Paleocene-Eocene Thermal Maximum event at different latitudes: ODP Site 690 and Tethyan sections. In Large Ecosystem Perturbations: Causes and Consequences; Monechi, S., Coccioni, R., Rampino, M., Eds.; Special Paper of the Geological Society of America: Boulder, CO, USA, 2007; Volume 424, pp. 69–85. [Google Scholar] [CrossRef]
- Arrhenius, S. On the reaction rate of the inversion of non-refined sugar upon souring. Phys. Chem. 1889, 4, 226–248. [Google Scholar]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 1771–1789. [Google Scholar] [CrossRef]
- Olivarez Lyle, A.; Lyle, M.W. Missing organic carbon in Eocene marine sediments: Is metabolism the biological feedback that maintains end-member climates? Paleoceanography 2006, 21, PA2007. [Google Scholar] [CrossRef]
- John, E.H.; Pearson, P.N.; Coxall, H.K.; Birch, H.; Wade, B.S.; Foster, G.L. Warm ocean processes and carbon cycling in the Eocene. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2013, 371, 20130099. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gray, E.; Thomas, E.; Brandon, M.; Zachos, J.; Paytan, A. Carbon sequestration during the Palaeocene–Eocene Thermal Maximum by an efficient biological pump. Nat. Geosci. 2014, 7, 382–388. [Google Scholar] [CrossRef]
- Wei, W.; Wise, S.W., Jr. Paleogene calcareous nannofossil magnetobiostratigraphy: Results from South Atlantic DSDP 516. Mar. Micropaleontol. 1989, 14, 119–152. [Google Scholar] [CrossRef]
- Petrizzo, M.R.; Leoni, G.; Speijer, R.P.; De Bernardi, B.; Felletti, F. Dissolution susceptibility of some Paleogene planktonic foraminifera from ODP Site 1209 (Shatsky Rise, Pacific Ocean). J. Foraminifer. Res. 2008, 38, 357–371. [Google Scholar] [CrossRef] [Green Version]
- Blaj, T.; Backman, J.; Raffi, I. Late Eocene to Oligocene preservation history and biochronology of calcareous nannofossils from paleo-equatorial Pacific Ocean sediments. Riv. Ital. Paleontol. Stratigr. 2009, 115, 67–84. [Google Scholar]
- Hallock, P. Fluctuations in the trophic resource continuum: A factor in global diversity cycles? Paleoceanography 1987, 2, 457–471. [Google Scholar] [CrossRef]
- Çemen, I.; Göncüolu, M.C.; Dirik, K. Structural Evolution of the Tuz Gölü Basin in Central Anatolia, Turkey. J. Geol. 1999, 107, 693–706. [Google Scholar] [CrossRef]
| Most Abundant Genera | Most Abundant Species | Average (%) | Maximum (%) |
|---|---|---|---|
| Anomalinoides | Anomalinoides alazanensis Anomalinoides spissiformis | 1.5 2.0 | 16.5 6.9 |
| Asterigerina fimbriata | 2.6 | 25.4 | |
| Bathysiphon | Bathysiphon sp. | 3.7 | 34.3 |
| Bulimina | |||
| Cibicidoides | Cibicidoides hadjibulakensis | 5.0 | 17.2 |
| Eponides plummerae | 3.2 | 13.2 | |
| Gaudryina sp. | 2.8 | 10.7 | |
| Glomospira charoides | 1.8 | 10 | |
| Gyroidinoides | Gyroidinoides complanatus | 1.8 | 3.8 |
| Hanzawaia ammophila | 2.7 | 12.4 | |
| Lagena | Lagena sp. | 1.5 | 7.7 |
| Laevidentalina gracilis | 1.9 | 6.6 | |
| Lenticulina | Lenticulina cultrate Lenticulina turbinata | 2.8 1.6 | 10.7 6.0 |
| Nodosaria | Nodosaria longiscata | 2.1 | 17.3 |
| Nuttallides | Nuttallides truempyi | 5.0 | 17.1 |
| Oridorsalis | Oridorsalis umbonatus | 3.5 | 15.8 |
| Osangularia | Osangularia plummearae | 2.1 | 22.7 |
| Uvigerina | Uvigerina rippensis | 3.1 | 18.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Onofrio, R.; Zaky, A.S.; Frontalini, F.; Luciani, V.; Catanzariti, R.; Francescangeli, F.; Giorgioni, M.; Coccioni, R.; Özcan, E.; Jovane, L. Impact of the Middle Eocene Climatic Optimum (MECO) on Foraminiferal and Calcareous Nannofossil Assemblages in the Neo-Tethyan Baskil Section (Eastern Turkey): Paleoenvironmental and Paleoclimatic Reconstructions. Appl. Sci. 2021, 11, 11339. https://doi.org/10.3390/app112311339
D’Onofrio R, Zaky AS, Frontalini F, Luciani V, Catanzariti R, Francescangeli F, Giorgioni M, Coccioni R, Özcan E, Jovane L. Impact of the Middle Eocene Climatic Optimum (MECO) on Foraminiferal and Calcareous Nannofossil Assemblages in the Neo-Tethyan Baskil Section (Eastern Turkey): Paleoenvironmental and Paleoclimatic Reconstructions. Applied Sciences. 2021; 11(23):11339. https://doi.org/10.3390/app112311339
Chicago/Turabian StyleD’Onofrio, Roberta, Amr S. Zaky, Fabrizio Frontalini, Valeria Luciani, Rita Catanzariti, Fabio Francescangeli, Martino Giorgioni, Rodolfo Coccioni, Ercan Özcan, and Luigi Jovane. 2021. "Impact of the Middle Eocene Climatic Optimum (MECO) on Foraminiferal and Calcareous Nannofossil Assemblages in the Neo-Tethyan Baskil Section (Eastern Turkey): Paleoenvironmental and Paleoclimatic Reconstructions" Applied Sciences 11, no. 23: 11339. https://doi.org/10.3390/app112311339
APA StyleD’Onofrio, R., Zaky, A. S., Frontalini, F., Luciani, V., Catanzariti, R., Francescangeli, F., Giorgioni, M., Coccioni, R., Özcan, E., & Jovane, L. (2021). Impact of the Middle Eocene Climatic Optimum (MECO) on Foraminiferal and Calcareous Nannofossil Assemblages in the Neo-Tethyan Baskil Section (Eastern Turkey): Paleoenvironmental and Paleoclimatic Reconstructions. Applied Sciences, 11(23), 11339. https://doi.org/10.3390/app112311339











