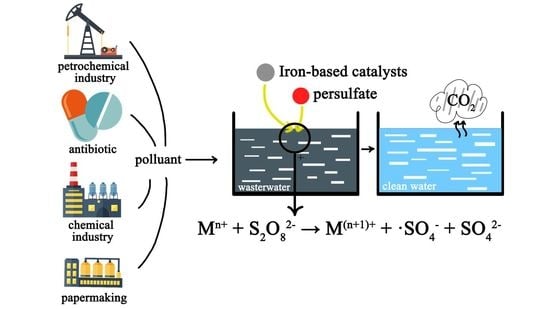
A Review of Activation Persulfate by Iron-Based Catalysts for Degrading Wastewater
Abstract
:1. Introduction
2. Activation Persulfate by Various Iron-Based Catalysts
2.1. MeFe2O4 (Me = Cu, Co, Zn, etc.)
2.2. MeFe2O4 Combined with the Carrier
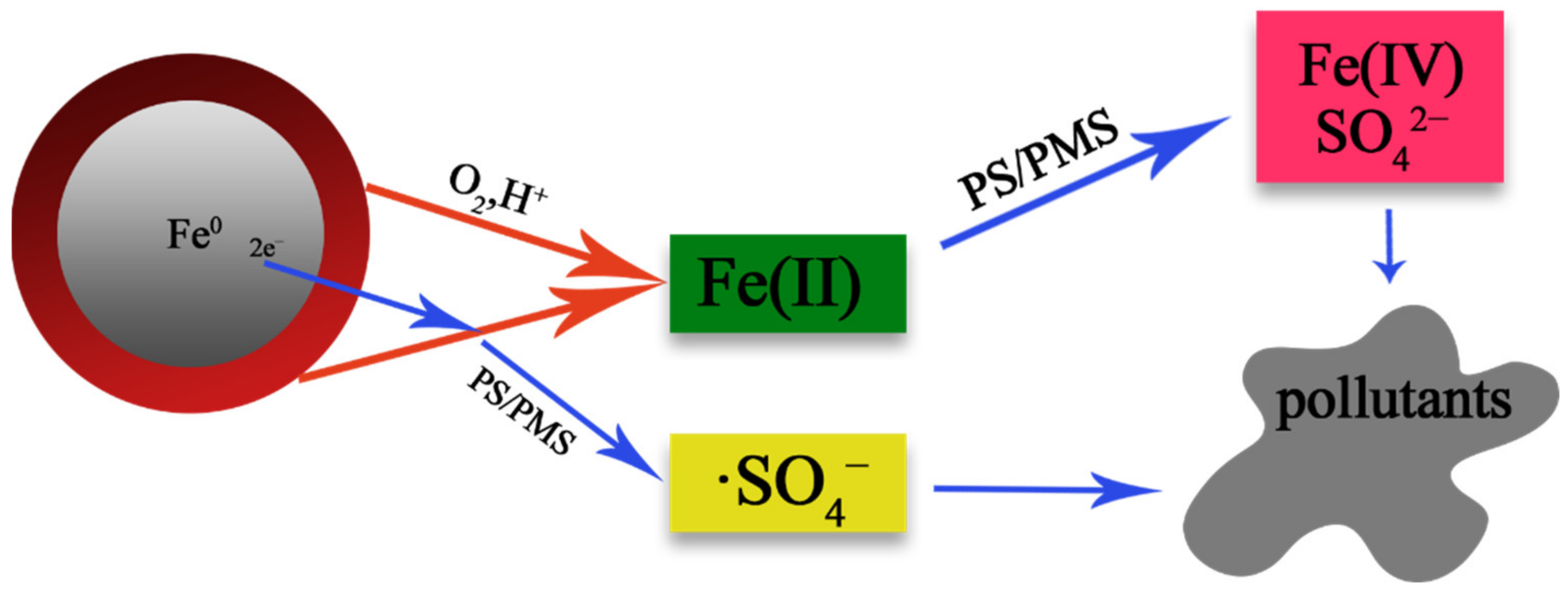
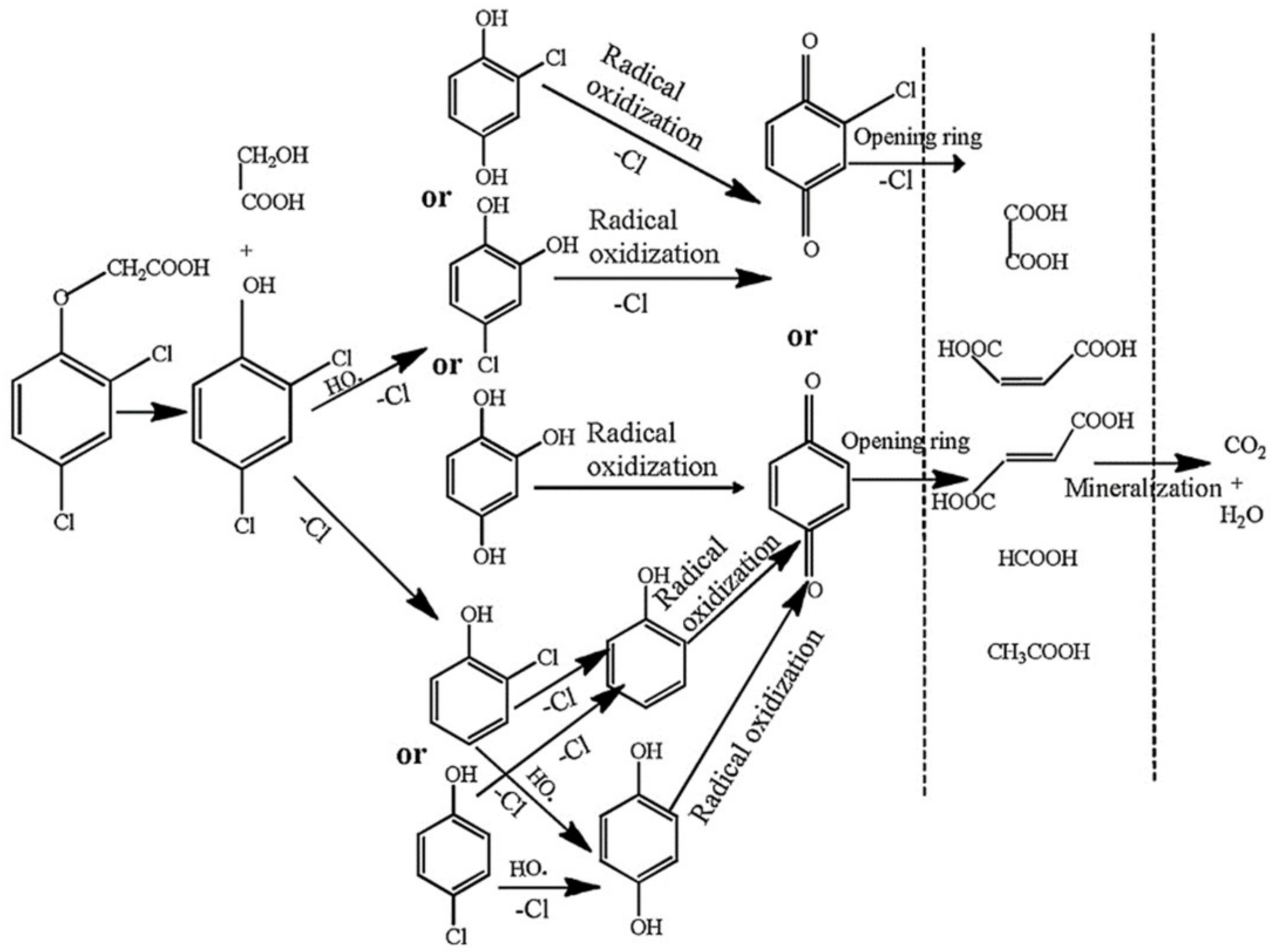
2.3. Activation Persulfate by Fe0
2.4. Fe3O4
3. Comparison of the Performance of Different Iron-Based Catalysts
4. Coupling Activation of Iron-Based Catalysts under Auxiliary Action
4.1. Photocatalytic Activation
4.2. Piezoelectric Catalytic Activation
4.3. Summary
5. Conclusions and Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Bi, W.; Zhang, Q.; Dong, X.; Zhang, X. Hydrothermal carbonation carbon-based photocatalysis under visible light: Modification for enhanced removal of organic pollutant and novel insight into the photocatalytic mechanism. J. Hazard. Mater. 2021, 127821. [Google Scholar] [CrossRef]
- Van Gijn, K.; Chen, Y.L.; van Oudheusden, B.; Gong, S.; de Wilt, H.A.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Optimizing biological effluent organic matter removal for subsequent micropollutant removal. J. Environ. Chem. Eng. 2021, 9, 106247. [Google Scholar] [CrossRef]
- Zeng, H.; Lan, H.; An, X.; Repo, E.; Park, Y.; Pastushok, O.; Liu, H.; Qu, J. Insight into electroreductive activation process of peroxydisulfate for eliminating organic pollution: Essential role of atomic hydrogen. Chem. Eng. J. 2021, 426, 128355. [Google Scholar] [CrossRef]
- Giannakis, S.; Samoili, S.; Rodríguez-Chueca, J. A meta-analysis of the scientific literature on (photo)Fenton and persulfate advanced oxidation processes: Where do we stand and where are we heading to? Curr. Opin. Green Sustain. Chem. 2021, 29, 100456. [Google Scholar] [CrossRef]
- Tian, Y.; Jia, N.; Zhou, L.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. Photo-Fenton-like degradation of antibiotics by inverse opal WO3 co-catalytic Fe2+/PMS, Fe2+/H2O2 and Fe2+/PDS processes: A comparative study. Chemosphere 2021, 132627. [Google Scholar] [CrossRef]
- Sathe, S.M.; Chakraborty, I.; Dubey, B.K.; Ghangrekar, M.M. Microbial fuel cell coupled Fenton oxidation for the cathodic degradation of emerging contaminants from wastewater: Applications and challenges. Environ. Res. 2022, 204, 112135. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Vakili, M.; Farraji, H.; Aziz, S.Q. Combined ozone oxidation process and adsorption methods for the removal of acetaminophen and amoxicillin from aqueous solution; kinetic and optimisation. Environ. Technol. Innov. 2019, 15, 100404. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, G.; Chen, S.; Liu, F.; Wang, Y.; Zhao, C. Multifunctional Cl-S double-doped carbon nitride nanotube unit in catalytic ozone oxidation synergistic photocatalytic system: Generation of ROS-rich region and effective treatment of organic wastewater. Chem. Eng. J. 2022, 430, 132843. [Google Scholar] [CrossRef]
- Tozar, T.; Boni, M.; Staicu, A.; Pascu, M.L. Optical Characterization of Ciprofloxacin Photolytic Degradation by UV-Pulsed Laser Radiation. Molecules 2021, 26, 2324. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, P.; Smallridge, A.; Irtassam, A.; Yeager, T. Scalable production of hydroxyl radicals (.OH) via homogeneous photolysis of hydrogen peroxide using a continuous-flow photoreactor. Chem. Eng. J. 2022, 427, 131762. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Lai, W.W.-P.; Lin, A.Y.-C. Sunlight photolysis mitigates the formation of N-nitrosodimethylamine (NDMA) during the chloramination of methadone. Chem. Eng. J. 2020, 384, 123307. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. A potential natural solar light active photocatalyst using magnetic ZnFe2O4 @ TiO2/Cu nanocomposite as a high performance and recyclable platform for degradation of naproxen from aqueous solution. J. Clean. Prod. 2020, 268, 122023. [Google Scholar] [CrossRef]
- Yin, R.; Chen, Y.; Hu, J.; Jin, S.; Guo, W.; Zhu, M. Peroxydisulfate bridged photocatalysis of covalent triazine framework for carbamazepine degradation. Chem. Eng. J. 2022, 427, 131613. [Google Scholar] [CrossRef]
- Tang, P.; Liu, B.; Xie, W.; Wang, P.; He, Q.; Bao, J.; Zhang, Y.; Zhang, Z.; Li, J.; Ma, J. Synergistic mechanism of combined ferrate and ultrafiltration process for shale gas wastewater treatment. J. Membr. Sci. 2022, 641, 119921. [Google Scholar] [CrossRef]
- Manoli, K.; Li, R.; Kim, J.; Feng, M.; Huang, C.-H.; Sharma, V.K. Ferrate(VI)-peracetic acid oxidation process: Rapid degradation of pharmaceuticals in water. Chem. Eng. J. 2022, 429, 132384. [Google Scholar] [CrossRef]
- Pan, B.; Feng, M.; Qin, J.; Dar, A.A.; Wang, C.; Ma, X.; Sharma, V.K. Iron(V)/Iron(IV) species in graphitic carbon nitride-ferrate(VI)-visible light system: Enhanced oxidation of micropollutants. Chem. Eng. J. 2022, 428, 132610. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean. Prod. 2020, 250, 119553. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Liu, F.; Fu, W.; Lin, L.; Li, B. Comparison of chemical and biological degradation of sulfonamides: Solving the mystery of sulfonamide transformation. J. Hazard. Mater. 2021, 127661. [Google Scholar] [CrossRef]
- Shimizu, A.; Takada, H.; Koike, T.; Takeshita, A.; Saha, M.; Rinawati; Nakada, N.; Murata, A.; Suzuki, T.; Suzuki, T.; et al. Ubiquitous occurrence of sulfonamides in tropical Asian waters. Sci. Total Environ. 2013, 452–453, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Guo, Q.; Wang, X.; Zhang, L.; Gao, X.; Luo, Y. Highly efficient degradation of sulfamethoxazole (SMX) by activating peroxymonosulfate (PMS) with CoFe2O4 in a wide pH range. Sep. Purif. Technol. 2021, 276, 119403. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Synergistic effect of PMS activation by Fe0@Fe3O4 anchored on N, S, O co-doped carbon composite for degradation of sulfamethoxazole. Chem. Eng. J. 2022, 427, 131960. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Zhong, S.; Zhang, L. AOPs-based remediation of petroleum hydrocarbons-contaminated soils: Efficiency, influencing factors and environmental impacts. Chemosphere 2019, 246, 125726. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.; Liu, Y.; Wang, L.; Li, D.; Liu, C.; Gong, M.; Zhang, Z.; Yang, T.; Ma, J. Remarkable enhancement of a photochemical Fenton-like system (UV-A/Fe(II)/PMS) at near-neutral pH and low Fe(II)/peroxymonosulfate ratio by three alpha hydroxy acids: Mechanisms and influencing factors. Sep. Purif. Technol. 2019, 224, 142–151. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Xue, J.; Ma, Y.; Xie, Z.; Wu, L.; Huang, M.; Zhang, Z. Persulfate-based degradation of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in aqueous solution: Review on influences, mechanisms and prospective. J. Hazard. Mater. 2020, 393, 122405. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.-R.; Li, W.-Q.; He, C.-S.; Wang, Y.-R.; Liu, X.-C.; Zhou, G.-N.; Mu, Y. Oxygen vacancy on hollow sphere CuFe2O4 as an efficient Fenton-like catalysis for organic pollutant degradation over a wide pH range. Appl. Catal. B Environ. 2021, 291, 120069. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Wang, S.; Gong, T.; Hua, M.; Qian, J.; Pan, B. Metal-free biomass with abundant carbonyl groups as efficient catalyst for the activation of peroxymonosulfate and degradation of sulfamethoxazole. Chem. Eng. J. 2022, 430, 132767. [Google Scholar] [CrossRef]
- Zhang, W.; Qian, L.; Han, L.; Yang, L.; Ouyang, D.; Long, Y.; Wei, Z.; Dong, X.; Liang, C.; Li, J.; et al. Synergistic roles of Fe(II) on simultaneous removal of hexavalent chromium and trichloroethylene by attapulgite-supported nanoscale zero-valent iron/persulfate system. Chem. Eng. J. 2022, 430, 132841. [Google Scholar] [CrossRef]
- Keerthana, S.P.; Yuvakkumar, R.; Ravi, G.; Pavithra, S.; Thambidurai, M.; Dang, C.; Velauthapillai, D. Pure and Ce-doped spinel CuFe2O4 photocatalysts for efficient rhodamine B degradation. Environ. Res. 2021, 200, 111528. [Google Scholar] [CrossRef]
- Wu, R.; Qu, J.; He, H.; Yu, Y. Removal of azo-dye Acid Red B (ARB) by adsorption and catalytic combustion using magnetic CuFe2O4 powder. Appl. Catal. B Environ. 2004, 48, 49–56. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, L.; Wang, N.; Tang, H. Sulfate radicals induced degradation of tetrabromobisphenol A with nanoscaled magnetic CuFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Appl. Catal. B Environ. 2013, 129, 153–162. [Google Scholar] [CrossRef]
- Salami, R.; Amini, M.; Bagherzadeh, M.; Chae, K.H. Vanadium oxide-supported copper ferrite nanoparticles: A reusable and highly efficient catalyst for rhodamine B degradation via activation of peroxymonosulfate. Appl. Organomet. Chem. 2021, 35. [Google Scholar] [CrossRef]
- Yi, X.-H.; Ji, H.; Wang, C.-C.; Li, Y.; Li, Y.-H.; Zhao, C.; Wang, A.; Fu, H.; Wang, P.; Zhao, X.; et al. Photocatalysis-activated SR-AOP over PDINH/MIL-88A(Fe) composites for boosted chloroquine phosphate degradation: Performance, mechanism, pathway and DFT calculations. Appl. Catal. B Environ. 2021, 293, 120229. [Google Scholar] [CrossRef]
- Duan, P.; Liu, X.; Liu, B.; Akram, M.; Li, Y.; Pan, J.; Yue, Q.; Gao, B.; Xu, X. Effect of phosphate on peroxymonosulfate activation: Accelerating generation of sulfate radical and underlying mechanism. Appl. Catal. B Environ. 2021, 298, 120532. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zhang, D.; Guo, Y.; Gao, S.; Li, E.; Zheng, H. Effects of acid, acid-ZVI/PMS, Fe(II)/PMS and ZVI/PMS conditioning on the wastewater activated sludge (WAS) dewaterability and extracellular polymeric substances (EPS). J. Environ. Sci. 2020, 91, 73–84. [Google Scholar] [CrossRef]
- Wei, W.; Zhou, D.; Feng, L.; Li, X.; Hu, L.; Zheng, H.; Wang, Y. The graceful art, significant function and wide application behavior of ultrasound research and understanding in carbamazepine (CBZ) enhanced removal and degradation by Fe0/PDS/US. Chemosphere 2021, 278, 130368. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Xu, S.; Fu, H.; Li, Z.; Li, K.; Sheng, K.; Du, J.; Lu, X.; Li, X.; et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4. Chem. Eng. J. 2020, 387, 124094. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A.; Ma, J. Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites. J. Hazard. Mater. 2017, 336, 81–92. [Google Scholar] [CrossRef]
- Huang, Y.; Han, C.; Liu, Y.; Nadagouda, M.N.; Machala, L.; O’Shea, K.E.; Sharma, V.K.; Dionysiou, D.D. Degradation of atrazine by ZnxCu1−xFe2O4 nanomaterial-catalyzed sulfite under UV–vis light irradiation: Green strategy to generate SO4−. Appl. Catal. B Environ. 2018, 221, 380–392. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Song, Y.; Yang, Y.; Mo, S.; Guo, D.; Liu, L. Fast construction of (Fe2O3)x@Ni-MOF heterostructure nanosheets as highly active catalyst for water oxidation. J. Alloys Compd. 2022, 892, 162149. [Google Scholar] [CrossRef]
- Lyu, L.; Won Kim, C.; Seong, K.-D.; Kang, J.; Liu, S.; Yamauchi, Y.; Piao, Y. Defect engineering induced heterostructure of Zn-birnessite@spinel ZnMn2O4 nanocrystal for flexible asymmetric supercapacitor. Chem. Eng. J. 2021, 133115. [Google Scholar] [CrossRef]
- Xie, X.; Wang, B.; Wang, Y.; Ni, C.; Sun, X.; Du, W. Spinel structured MFe2O4 (M = Fe, Co, Ni, Mn, Zn) and their composites for microwave absorption: A review. Chem. Eng. J. 2022, 428, 131160. [Google Scholar] [CrossRef]
- Xian, G.; Kong, S.; Li, Q.; Zhang, G.; Zhou, N.; Du, H.; Niu, L. Synthesis of Spinel Ferrite MFe2O4 (M = Co, Cu, Mn, and Zn) for Persulfate Activation to Remove Aqueous Organics: Effects of M-Site Metal and Synthetic Method. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Zhang, X.; Cui, X.; He, S.; Liang, H.; Ding, A. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate. Chem. Eng. J. 2020, 384, 123319. [Google Scholar] [CrossRef]
- Mostafa, S.; Rosario-Ortiz, F.L. Singlet oxygen formation from wastewater organic matter. Environ. Sci. Technol. 2013, 47, 8179–8186. [Google Scholar] [CrossRef]
- Yi, Q.; Ji, J.; Shen, B.; Dong, C.; Liu, J.; Zhang, J.; Xing, M. Singlet Oxygen Triggered by Superoxide Radicals in a Molybdenum Cocatalytic Fenton Reaction with Enhanced REDOX Activity in the Environment. Environ. Sci. Technol. 2019, 53, 9725–9733. [Google Scholar] [CrossRef]
- Mikrut, M.; Mazuryk, O.; Macyk, W.; van Eldik, R.; Stochel, G. Generation and photogeneration of hydroxyl radicals and singlet oxygen by particulate matter and its inorganic components. J. Environ. Chem. Eng. 2021, 9, 106478. [Google Scholar] [CrossRef]
- Liu, F.; Li, W.; Wu, D.; Tian, T.; Wu, J.-F.; Dong, Z.-M.; Zhao, G.-C. New insight into the mechanism of peroxymonosulfate activation by nanoscaled lead-based spinel for organic matters degradation: A singlet oxygen-dominated oxidation process. J. Colloid Interface Sci. 2020, 572, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Junaid, M.; Lin, F.; Zhang, S.; Xu, N. Degradation of sulphachloropyridazine sodium in column reactor packed with CoFe2O4−loaded quartz sand via peroxymonosulfate activation: Insights into the amorphous phase, efficiency, and mechanism. Chem. Eng. J. 2020, 390, 124549. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; Yao, G.; Lai, B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024. [Google Scholar] [CrossRef]
- Ratanaphain, C.; Viboonratanasri, D.; Prompinit, P.; Krajangpan, S.; Khan, E.; Punyapalakul, P. Reactivity characterization of SiO2-coated nano zero-valent iron for iodoacetamide degradation: The effects of SiO2 thickness, and the roles of dehalogenation, hydrolysis and adsorption. Chemosphere 2022, 286, 131816. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wan, J.; Wang, Y.; Yan, Z.; Ma, Y. Activation of persulfate by molecularly imprinted Fe-MOF-74@SiO2 for the targeted degradation of dimethyl phthalate: Effects of operating parameters and chlorine. Chem. Eng. J. 2021, 422, 130406. [Google Scholar] [CrossRef]
- Wang, L.; Guan, R.; Qi, Y.; Zhang, F.; Li, P.; Wang, J.; Qu, P.; Zhou, G.; Shi, W. Constructing Zn-P charge transfer bridge over ZnFe2O4-black phosphorus 3D microcavity structure: Efficient photocatalyst design in visible-near-infrared region. J. Colloid Interface Sci. 2021, 600, 463–472. [Google Scholar] [CrossRef]
- Shao, S.; Deng, J.; Lv, X.; Ji, H.; Xiao, Y.; Zhu, X.; Feng, K.; Xu, H.; Zhong, J. Black phosphorus nanoflakes decorated hematite photoanode with functional phosphate bridges for enhanced water oxidation. Chem. Eng. J. 2021, 425, 131500. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Beitollahi, H.; Tajik, S.; Eshghi, A.; Azizi, S. Electro-oxidation of hydrazine on NiFe2O4-rGO as a high-performance nano-electrocatalyst in alkaline media. Mater. Chem. Phys. 2022, 275, 125313. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D.; Ma, C.; Yang, Z.; Yue, H.; Zhang, D.; Sun, Z. Conductive Fe2N/N-rGO composite boosts electrochemical redox reactions in wide temperature accommodating lithium-sulfur batteries. Chem. Eng. J. 2022, 427, 131622. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Ramachandran, K.; Sheet, S.; Yoo, D.J.; Lee, Y.S.; Satish kumar, Y.; Kim, A.R.; Gnana kumar, G. Pigeon-Excreta-Mediated Synthesis of Reduced Graphene Oxide (rGO)/CuFe2O4 Nanocomposite and Its Catalytic Activity toward Sensitive and Selective Hydrogen Peroxide Detection. ACS Sustain. Chem. Eng. 2017, 5, 4897–4905. [Google Scholar] [CrossRef]
- Othman, I.; Abu Haija, M.; Ismail, I.; Zain, J.H.; Banat, F. Preparation and catalytic performance of CuFe2O4 nanoparticles supported on reduced graphene oxide (CuFe2O4/rGO) for phenol degradation. Mater. Chem. Phys. 2019, 238. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.C.; Xia, D.; Wong, P.K.; Li, Y. Graphene and g-C3N4 nanosheets cowrapped elemental alpha-sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light. Environ. Sci. Technol. 2013, 47, 8724–8732. [Google Scholar] [CrossRef]
- Sun, B.; Ma, W.; Wang, N.; Xu, P.; Zhang, L.; Wang, B.; Zhao, H.; Lin, K.A.; Du, Y. Retraction of “Polyaniline: A New Metal-Free Catalyst for Peroxymonosulfate Activation with Highly Efficient and Durable Removal of Organic Pollutants”. Environ. Sci. Technol. 2021, 55, 3451. [Google Scholar] [CrossRef]
- Hao, P.; Hu, M.; Xing, R.; Zhou, W. Synergistic degradation of methylparaben on CuFe2O4-rGO composite by persulfate activation. J. Alloys Compd. 2020, 823, 153757. [Google Scholar] [CrossRef]
- Li, R.; Cai, M.; Xie, Z.; Zhang, Q.; Zeng, Y.; Liu, H.; Liu, G.; Lv, W. Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B Environ. 2019, 244, 974–982. [Google Scholar] [CrossRef]
- Gan, L.; Zhong, Q.; Geng, A.; Wang, L.; Song, C.; Han, S.; Cui, J.; Xu, L. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Sci. Total Environ. 2019, 694, 133705. [Google Scholar] [CrossRef]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, H.; Ma, J.; Kong, Y.; Komarneni, S. Efficient degradation of rhodamine B by magnetically separable ZnS–ZnFe2O4 composite with the synergistic effect from persulfate. Chemosphere 2019, 237, 124547. [Google Scholar] [CrossRef] [PubMed]
- Kohantorabi, M.; Hosseinifard, M.; Kazemzadeh, A. Catalytic activity of a magnetic Fe2O3@CoFe2O4 nanocomposite in peroxymonosulfate activation for norfloxacin removal. N. J. Chem. 2020, 44, 4185–4198. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, M.; Wang, L.; Qu, R.; Wang, Z. Catalytic degradation of 2-phenylbenzimidazole-5-sulfonic acid by peroxymonosulfate activated with nitrogen and sulfur co-doped CNTs-COOH loaded CuFe2O4. Chem. Eng. J. 2017, 307, 95–104. [Google Scholar] [CrossRef]
- Cuervo Lumbaque, E.; Lopes Tiburtius, E.R.; Barreto-Rodrigues, M.; Sirtori, C. Current trends in the use of zero-valent iron (Fe0) for degradation of pharmaceuticals present in different water matrices. Trends Environ. Anal. Chem. 2019, 24, e00069. [Google Scholar] [CrossRef]
- Hussain, I.; Zhang, Y.; Huang, S.; Gao, Q. Degradation of p-chloroaniline by FeO3−xH3−2x/Fe0 in the presence of persulfate in aqueous solution. RSC Adv. 2015, 5, 41079–41087. [Google Scholar] [CrossRef]
- Rodriguez, S.; Vasquez, L.; Romero, A.; Santos, A. Dye Oxidation in Aqueous Phase by Using Zero-Valent Iron as Persulfate Activator: Kinetic Model and Effect of Particle Size. Ind. Eng. Chem. Res. 2014, 53, 12288–12294. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Sun, Y.; Liang, L.; Pan, B.; Zhang, W.; Guan, X. Advances in Sulfidation of Zerovalent Iron for Water Decontamination. Environ. Sci. Technol. 2017, 51, 13533–13544. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Pang, S.-Y.; Zhou, Y.; Gao, Y.; Guan, C.; Jiang, J. Further understanding the involvement of Fe(IV) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment. Chem. Eng. J. 2019, 371, 842–847. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H. Mn-based catalysts for sulfate radical-based advanced oxidation processes: A review. Environ. Int. 2019, 133, 105141. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Weng, C.H.; Ding, F.; Lin, Y.T.; Liu, N. Effective decolorization of polyazo direct dye Sirius Red F3B using persulfate activated with Fe-0 aggregate. Sep. Purif. Technol. 2015, 147, 147–155. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Wang, Y.; Huang, M. Influence of particle size of zero-valent iron and dissolved silica on the reactivity of activated persulfate for degradation of acid orange 7. Chem. Eng. J. 2014, 237, 487–496. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Pang, S.; Gao, Y.; Zhou, Y.; Cao, Y.; Jiang, J. Relative contribution of ferryl ion species (Fe(IV)) and sulfate radical formed in nanoscale zero valent iron activated peroxydisulfate and peroxymonosulfate processes. Water Res. 2020, 172, 115504. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, M.; Pan, Y. Enhanced degradation of 2,4-dichlorophenoxyacetic acid by pre-magnetization Fe-C activated persulfate: Influential factors, mechanism and degradation pathway. J. Hazard. Mater. 2018, 353, 454–465. [Google Scholar] [CrossRef]
- Ye, C.; Liu, P.; Ma, Z.; Xue, C.; Zhang, C.; Zhang, Y.; Liu, J.; Liu, C.; Sun, X.; Mu, Y. High H2O2 Concentrations Observed during Haze Periods during the Winter in Beijing: Importance of H2O2 Oxidation in Sulfate Formation. Environ. Sci. Technol. Lett. 2018, 5, 757–763. [Google Scholar] [CrossRef]
- Peng, X.; Xi, B.; Zhao, Y.; Shi, Q.; Meng, X.; Mao, X.; Jiang, Y.; Ma, Z.; Tan, W.; Liu, H.; et al. Effect of Arsenic on the Formation and Adsorption Property of Ferric Hydroxide Precipitates in ZVI Treatment. Environ. Sci. Technol. 2017, 51, 10100–10108. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cagnetta, G.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep. Purif. Technol. 2020, 239, 116534. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Gao, J.; Li, X.; Yu, H.; Wang, N.; Du, P.; Yu, R.; Li, H.; Fan, X.; et al. Synergistic degradation of chloramphenicol by ultrasound-enhanced nanoscale zero-valent iron/persulfate treatment. Sep. Purif. Technol. 2020, 240, 116575. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Y.; Zhou, M.; Xing, K.; Lv, W.; Wang, W.; Chen, H.; Yao, Y. Nitrogen-doped porous carbon encapsulating iron nanoparticles for enhanced sulfathiazole removal via peroxymonosulfate activation. Chemosphere 2020, 250, 126300. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, J.; Liu, Q.; Liu, X.; Gao, B. A novel stabilized carbon-coated nZVI as heterogeneous persulfate catalyst for enhanced degradation of 4-chlorophenol. Environ. Int. 2020, 138, 105639. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Wang, Y. Synthesis of novel core–shell Fe0@Fe3O4 as heterogeneous activator of persulfate for oxidation of dibutyl phthalate under neutral conditions. Chem. Eng. J. 2016, 301, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhong, J.; Zhang, L.; Fan, Y.; Yang, Z.; Shih, K.; Li, H.; Wu, D.; Yan, B. Activation of peroxymonosulfate by Fe0@Fe3O4 core-shell nanowires for sulfate radical generation: Electron transfer and transformation products. Sep. Purif. Technol. 2020, 247, 116942. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Wang, J.; Li, W. Fe@C carbonized resin for peroxymonosulfate activation and bisphenol S degradation. Environ. Pollut. 2019, 252, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Wang, K.; Lu, J.; Chen, J.; Dong, X.; Xia, D.; Zhang, A.; Wang, Q. Activation of peroxymonosulfate by magnetic carbon supported Prussian blue nanocomposite for the degradation of organic contaminants with singlet oxygen and superoxide radicals. Chemosphere 2019, 218, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Zhang, X.; Wang, S.; Xia, Z.; Zeng, G.; Ding, J.; Ren, N. Construction of Fe3O4@β-CD/g-C3N4 nanocomposite catalyst for degradation of PCBs in wastewater through photodegradation and heterogeneous Fenton oxidation. Chem. Eng. J. 2022, 429, 132445. [Google Scholar] [CrossRef]
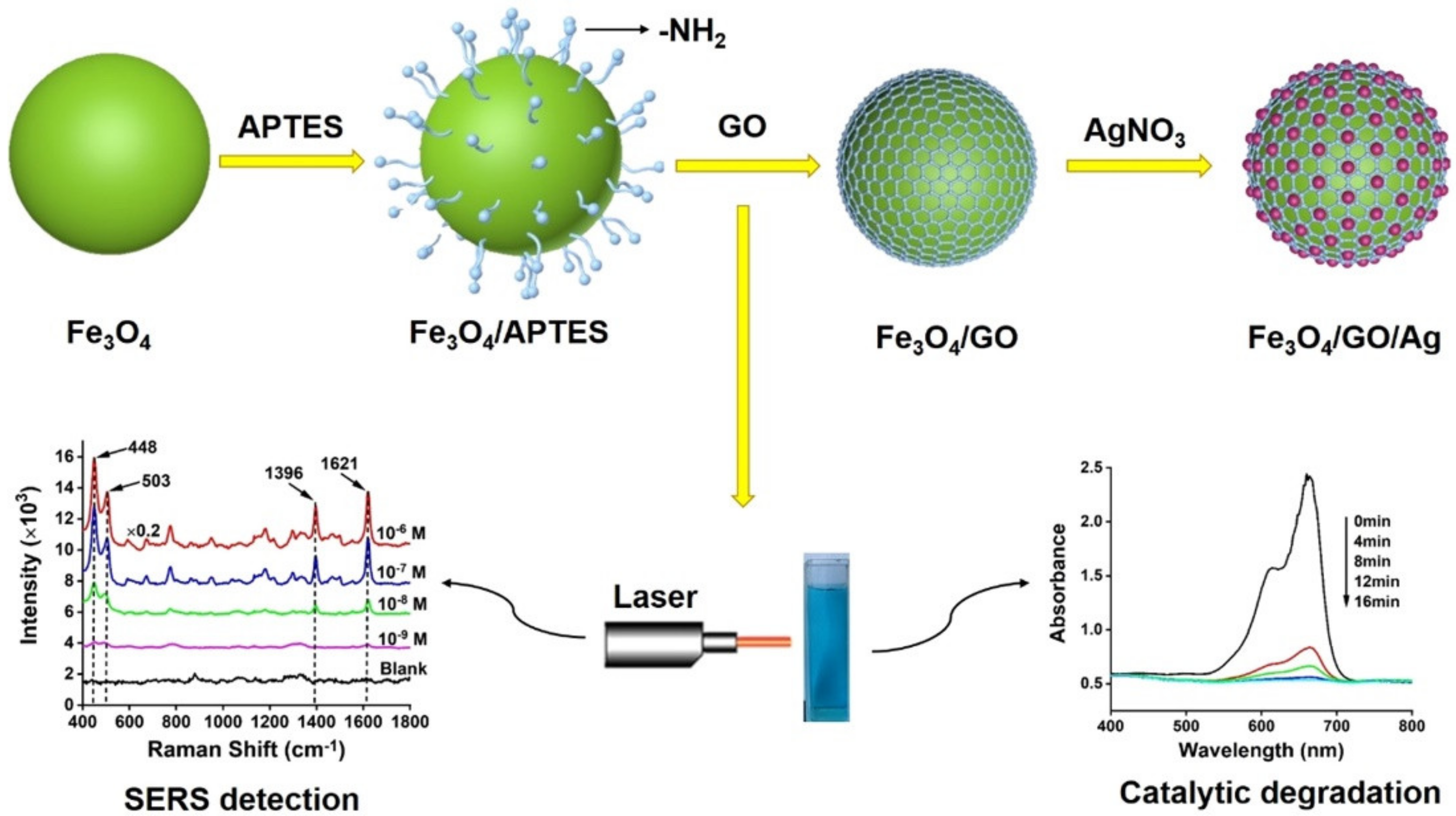
- He, J.; Song, G.; Wang, X.; Zhou, L.; Li, J. Multifunctional magnetic Fe3O4/GO/Ag composite microspheres for SERS detection and catalytic degradation of methylene blue and ciprofloxacin. J. Alloys Compd. 2022, 893, 162226. [Google Scholar] [CrossRef]
- Zhen, J.; Zhang, S.; Zhuang, X.; Ahmad, S.; Lee, T.; Si, H.; Cao, C.; Ni, S.-Q. Sulfate radicals based heterogeneous peroxymonosulfate system catalyzed by CuO-Fe3O4-Biochar nanocomposite for bisphenol A degradation. J. Water Process Eng. 2021, 41, 102078. [Google Scholar] [CrossRef]
- Yin, F.; Wang, C.; Lin, K.-Y.A.; Tong, S. Persulfate activation for efficient degradation of norfloxacin by a rGO-Fe3O4 composite. J. Taiwan Inst. Chem. Eng. 2019, 102, 163–169. [Google Scholar] [CrossRef]
- Yan, J.; Lei, M.; Zhu, L.; Anjum, M.N.; Zou, J.; Tang, H. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate. J. Hazard. Mater. 2011, 186, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Xiong, Z.; Ao, Z.; Pu, S.; Yao, G.; Lai, B. Core-shell magnetic Fe3O4@Zn/Co-ZIFs to activate peroxymonosulfate for highly efficient degradation of carbamazepine. Appl. Catal. B Environ. 2020, 277, 119136. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Chen, X.; Liu, L.; Wang, Y.; Zhou, S.; Long, X.; Yu, J.; Jiao, F. Iron-based catalysts for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Chem. Eng. J. 2021, 421, 127845. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Lu, J.-D. The effect of two ferromagnetic metal stripes on valley polarization of electrons in a graphene. Phys. Lett. A 2020, 384, 126402. [Google Scholar] [CrossRef]
- Brovini, E.M.; de Deus, B.C.T.; Vilas-Boas, J.A.; Quadra, G.R.; Carvalho, L.; Mendonça, R.F.; Pereira, R.D.O.; Cardoso, S.J. Three-bestseller pesticides in Brazil: Freshwater concentrations and potential environmental risks. Sci. Total Environ. 2021, 771, 144754. [Google Scholar] [CrossRef]
- Wang, F.; Gao, J.; Zhai, W.; Cui, J.; Liu, D.; Zhou, Z.; Wang, P. Effects of antibiotic norfloxacin on the degradation and enantioselectivity of the herbicides in aquatic environment. Ecotoxicol. Environ. Saf. 2021, 208, 111717. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Harnett, K.G.; Chin, A.; Schuh, S.M. BPA and BPA alternatives BPS, BPAF, and TMBPF, induce cytotoxicity and apoptosis in rat and human stem cells. Ecotoxicol. Environ. Saf. 2021, 216, 112210. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Yi, K.; Hou, L.; Li, X.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Khan, J.A.; He, X.; Khan, H.M.; Shah, N.S.; Dionysiou, D.D. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82-/Fe2+ and UV/HSO5−/Fe2+ processes: A comparative study. Chem. Eng. J. 2013, 218, 376–383. [Google Scholar] [CrossRef]
- Devi, L.G.; Munikrishnappa, C.; Nagaraj, B.; Rajashekhar, K.E. Effect of chloride and sulfate ions on the advanced photo Fenton and modified photo Fenton degradation process of Alizarin Red S. J. Mol. Catal. A Chem. 2013, 374–375, 125–131. [Google Scholar] [CrossRef]
- Benkelberg, H.-J.; Warneck, P. Photodecomposition of Iron(III) Hydroxo and Sulfato Complexes in Aqueous Solution: Wavelength Dependence of OH and SO4− Quantum Yields. J. Phys. Chem. 1995, 99, 5214–5221. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, H.M.; Boccelli, D.; Dionysiou, D.D. Efficient degradation of lindane in aqueous solution by iron (II) and/or UV activated peroxymonosulfate. J. Photochem. Photobiol. A Chem. 2016, 316, 37–43. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Catalytic degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) by nano-Fe2O3 activated peroxymonosulfate: Influential factors and mechanism determination. Chemosphere 2017, 169, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.; Chiron, S. Solar photo-Fenton like using persulphate for carbamazepine removal from domestic wastewater. Water Res. 2014, 48, 229–236. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Brienza, M.; Goetz, V.; Chiron, S. Solar photo-Fenton using peroxymonosulfate for organic micropollutants removal from domestic wastewater: Comparison with heterogeneous TiO2 photocatalysis. Chemosphere 2014, 117, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wang, J.; Wang, Y.; Jin, C.; Ganeshraja, A.S. Visible-light-induced photocatalysis and peroxymonosulfate activation over ZnFe2O4 fine nanoparticles for degradation of Orange II. Catal. Sci. Technol. 2016, 6, 2296–2304. [Google Scholar] [CrossRef]
- Cai, C.; Liu, J.; Zhang, Z.; Zheng, Y.; Zhang, H. Visible light enhanced heterogeneous photo-degradation of Orange II by zinc ferrite (ZnFe2O4) catalyst with the assistance of persulfate. Sep. Purif. Technol. 2016, 165, 42–52. [Google Scholar] [CrossRef]
- Villa, S.M.; Maturi, M.; Santaniello, T.; Migliorini, L.; Locatelli, E.; Franchini, M.C.; Milani, P. Quantitative Spectral Electromechanical Characterization of Soft Piezoelectric Nanocomposites. Sens. Actuators A Phys. 2021, 113196. [Google Scholar] [CrossRef]
- Tan, D.; Jiang, C.; Sun, N.; Huang, J.; Zhang, Z.; Zhang, Q.; Bu, J.; Bi, S.; Guo, Q.; Song, J. Piezoelectricity in monolayer MXene for nanogenerators and piezotronics. Nano Energy 2021, 90, 106528. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Fan, S.; Chen, X.; Qin, M.; Long, D.; Tadé, M.O.; Liu, S. Impact of oxygen vacancy occupancy on piezo-catalytic activity of BaTiO3 nanobelt. Appl. Catal. B Environ. 2020, 279, 119340. [Google Scholar] [CrossRef]
- Yang, X.; Li, P.; Wu, B.; Li, H.; Zhou, G. A flexible piezoelectric-triboelectric hybrid nanogenerator in one structure with dual doping enhancement effects. Curr. Appl. Phys. 2021, 32, 50–58. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, H.; Leng, H.; You, H.; Jia, Y.; Wang, S. Ultrasonic role to activate persulfate/chlorite with foamed zero-valent-iron: Sonochemical applications and induced mechanisms. Ultrason. Sonochem. 2021, 78, 105750. [Google Scholar] [CrossRef]
- Nie, G.; Yao, Y.; Duan, X.; Xiao, L.; Wang, S. Advances of piezoelectric nanomaterials for applications in advanced oxidation technologies. Curr. Opin. Chem. Eng. 2021, 33. [Google Scholar] [CrossRef]
- Li, Y.-T.; Zhang, J.-J.; Li, Y.-H.; Chen, J.-L.; Du, W.-Y. Treatment of soil contaminated with petroleum hydrocarbons using activated persulfate oxidation, ultrasound, and heat: A kinetic and thermodynamic study. Chem. Eng. J. 2022, 428, 131336. [Google Scholar] [CrossRef]
- Xu, Q.; Li, Z.; You, H.; Li, H.; Yu, Y. Foamed-Fe0 via phase interface polishing by ultrasound to activate persulfate for treating triphenylmethane derivative. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Zhou, T.; Zou, X.; Mao, J.; Wu, X. Decomposition of sulfadiazine in a sonochemical Fe0-catalyzed persulfate system: Parameters optimizing and interferences of wastewater matrix. Appl. Catal. B Environ. 2016, 185, 31–41. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Zhou, M.; Cai, J.; Tian, Y. Enhanced removal of antibiotics from secondary wastewater effluents by novel UV/pre-magnetized Fe0/H2O2 process. Water Res. 2019, 153, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Chakma, S.; Praneeth, S.; Moholkar, V.S. Mechanistic investigations in sono-hybrid (ultrasound/Fe2+/UVC) techniques of persulfate activation for degradation of Azorubine. Ultrason. Sonochem. 2017, 38, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, J.; Ding, Z.; Zhao, Z.; Xu, X.; Fang, Z. Ultrasound irritation enhanced heterogeneous activation of peroxymonosulfate with Fe3O4 for degradation of azo dye. Ultrason. Sonochem. 2017, 34, 953–959. [Google Scholar] [CrossRef]
- Sajjadi, S.; Khataee, A.; Bagheri, N.; Kobya, M.; Senocak, A.; Demirbas, E.; Karaoglu, A.G. Degradation of diazinon pesticide using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite under ultrasound irradiation. J. Ind. Eng. Chem. 2019, 77, 280–290. [Google Scholar] [CrossRef]
- Qiu, P.; Xue, N.; Cheng, Z.; Kai, X.; Zeng, Y.; Xu, M.; Zhang, S.; Xu, C.; Liu, F.; Guo, Z. The cooperation of photothermal conversion, photocatalysis and sulfate radical-based advanced oxidation process on few-layered graphite modified graphitic carbon nitride. Chem. Eng. J. 2021, 417, 127993. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Hasija, V.; Sharma, V.; Sharma, S.; Raizada, P.; Singh, M.; Saini, A.K.; Hosseini-Bandegharaei, A.; Thakur, V.K. Systematic review on applicability of magnetic iron oxides–integrated photocatalysts for degradation of organic pollutants in water. Mater. Today Chem. 2019, 14, 100186. [Google Scholar] [CrossRef]

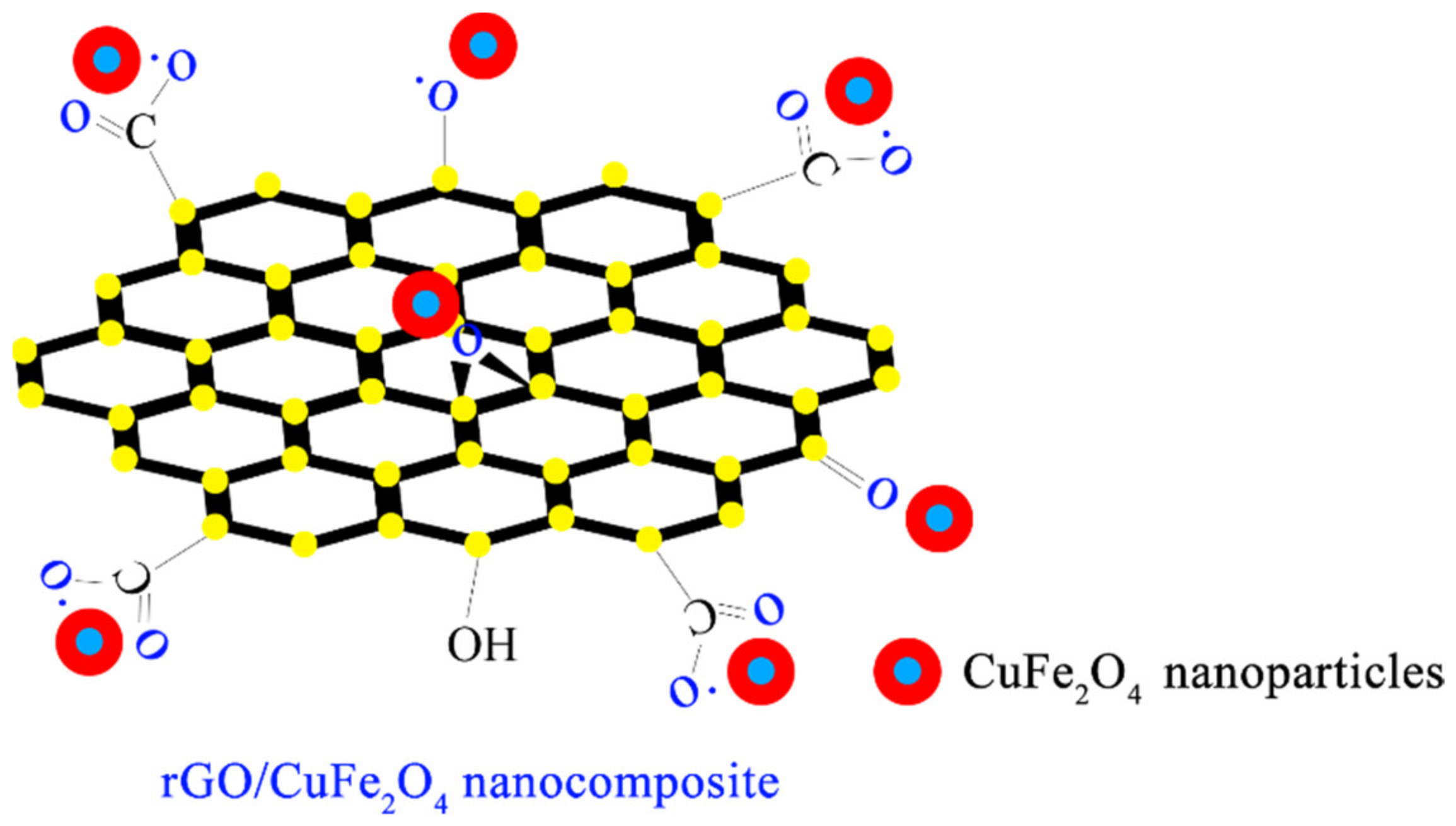
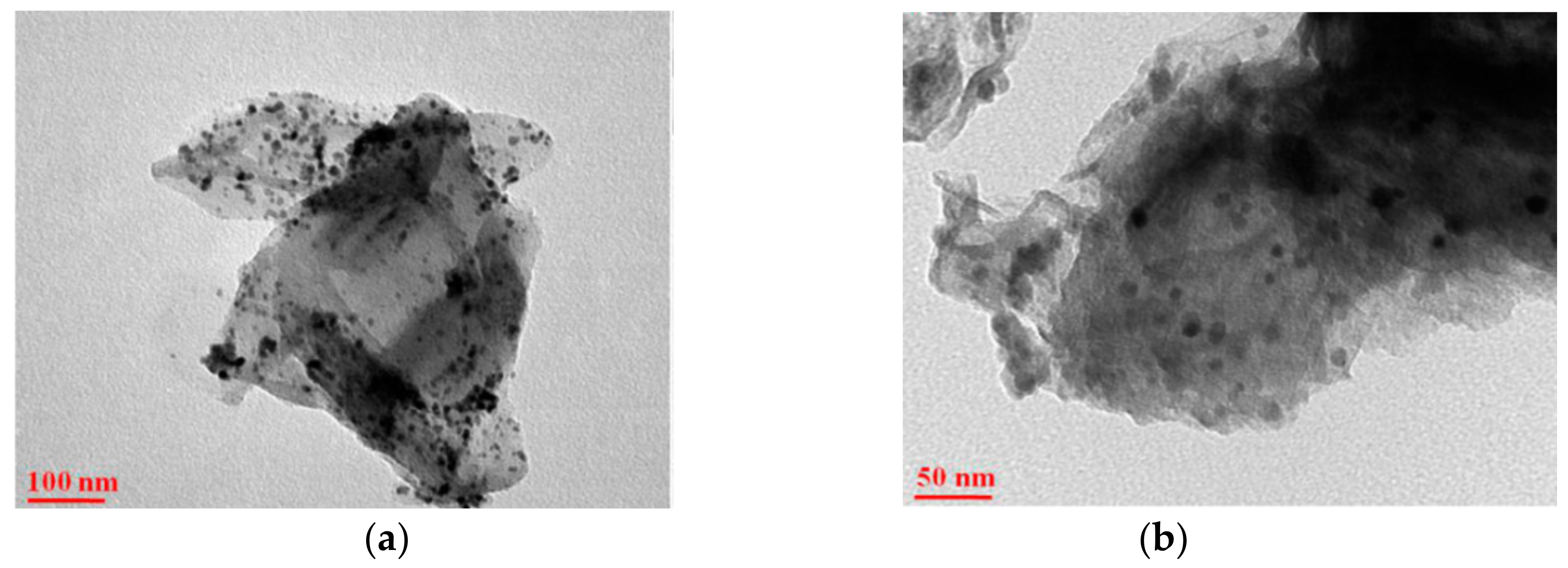



| Catalyst | Pollution | Main Mechanism | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate/% | Number of Cycles | Synthesis Techniques | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PbFe2O4 | Thionine | 1O2 | 10 μM | 0.4 g/L | PMS | 400 μM | 20 | 100 | Not mentioned | Solution combustion | [51] |
| CoFe2O4–loaded quartz sand | Sulfachloropyridazine sodium | ·SO4− ·OH | 2 g/L | 10 g | PMS | 75 mg/L | 150 | 90 | Not mentioned | Citrate combustion | [52] |
| CoFe2O4-SAC | Norfloxacin (NOF) | ·SO4− ·OH | 10 mg/L | 0.1 g/L | PMS | 0.15 g/L | 120 | TOC reduction 81 | 5 (>80%) | Hydrothermal | [47] |
| The biochar loaded with CoFe2O4 nanoparticles | Bisphenol A (BPA) | ·SO4− ·OH | 10 mg/L | 0.05 g/L | PMS | 0.5 g/L | 8 | 93 | Not mentioned | Hydrothermal | [39] |
| C3N4@MnFe2O4-graphene | Metronidazole | ·SO4− ·OH | 20 mg/L | 1.0 g/L | PS | 0.01 M | 90 | 94.5 | 5 (>80%) | Solvothermal | [40] |
| Zn0.8Cu0.2Fe2O4 | Atrazine | ·SO4− | 4.4 μM | 200 mg/L | PS | 0.5 mM | 30 | 95 | Not mentioned | Sol–gel | [41] |
| CuFe2O4/O3 | 2,4-Dichlorophenoxyacetic acid (2,4-D) | Not mentioned | 20 mg/L | 0.20 g/L | PMS O3 | PMS 2.0 mM; O3 16.0 mg/L; | 40 | 88.9 | 5 (>80%) | Coprecipitation | [42] |
| CoFe2O4 | Atrazine (ATZ) | ·SO4− | 10 mg/L | 0.4 g/L | PMS | 0.8 mM | 30 | >99 | 5 (>60%) | Hydrothermal | [53] |
| Catalyst | Pollution | Main Mechanism | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate /% | Number of Cycles | Synthesis Techniques | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CuFe2O4- 20%rGO | Methylparaben | SO4−· ·OH | 10 mg/L | 0.2 mg/L | PS | 5 mM | 120 | 96 | Not mentioned | Sol-gel | [64] |
| CuFe2O4- 1% (w/w) rGO | Phenol | ·OH | 20 ppm | 5 mL | 30% H2O2 | 6 mg/L | 240 | 100 | Not mentioned | Coprecipitation | [61] |
| CuFe2O4/g-C3N4 | Propranolol | SO4−· | 0.02 mM | 1 g/L | PS | 1 mM | 120 | 82.2 | Not mentioned | Sol-gel | [65] |
| CoFe2O4/CCNF | Dimethyl phthalate | SO4−· | 0.05 mM | 0.5 g/L | PMS | 1.5 mM | 60 | >90 | 5 (>90%) | Sol-gel | [66] |
| TiO2@CuFe2O4/UV | 2,4-D | SO4−· | 20 mg/L | 0.1 g/L | PMS | 0.3 mM | 60 | 97.2 | 5 (>90%) | Sol-gel | [67] |
| ZnS-ZnFe2O4 | Rhodamine B | SO4−· | 20 mg/L | 20 mg | PS | 5 mg | 90 | 97.67 | 3 (>95%) | Hydrothermal | [68] |
| Fe2O3@CoFe2O4 | NOF | SO4−· ·OH | 15 μM | 0.3 g/L | PMS | 0.4 mM | 25 | 89.8 | 4 (90%) | Hydrothermal | [69] |
| Nitrogen and sulfur codoped CNTs-COOH loaded CuFe2O4 | 2-Phenylbenzimidazole-5-sulfonic acid | SO4−· | 5 mg/L | 50 mg/L | PMS | 1:100 (molar ratio) | 40 | 98 | 5 (>95%) | Coprecipitation | [70] |
| Catalyst | Pollution | Main Mechanism | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate /% | Number of Cycles | Synthesis Techniques | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nZVI | Sulfamethazine | ·OH ·SO4− | 50 mg/L | 2 mM | PS H2O2 | 1 mM 0.5 mM | 30 | 96 | Not mentioned | Sol-gel | [88] |
| CN-Fe | Sulfamethazine | ·SO4− ·OH 1O2 | 50 μM | 0.5 g/L | PMS | 1 mM | 15 | 82 | Not mentioned | Carbothermal | [87] |
| Carbon-coated nZVI | 4-chlorophenol | ·SO4− ·OH | 150 μM | 0.25 g/L | PMS | 1 mM | 120 | 96 | Not mentioned | Commercially available | [86] |
| US-nZVI | Chloramphenicol | ·SO4− ·OH | 5 mg/L | 0.5 g/L | PMS | 1 mM | 90 | 98.1 | Not mentioned | Liquid phase reduction | [85] |
| Fe0@Fe3O4 | Dibutyl phthalate | ·OH ·SO4− | 18 μM | 0.5 g L−1 | PS | 1.8 mM | 180 | 94.7 | 6 (>68%) | Calcination | [89] |
| Fe0@Fe3O4 | Atrazine | ·OH ·SO4− | 500 μg/L | 25 mg/L | PMS | 1 mM | 2 | 100 | Not mentioned | Reduction | [90] |
| Fe@C | Bisphenol S | ·OH ·SO4− | 5 mg/L | 0.5 g/L | PMS | 1.0 mM | 60 | 92.8 | Not mentioned | Resin carbonization | [91] |
| Fe@C/PB | 2,4-DichloroPhenol | ·OH ·SO4− | 20 mg/L | 0.6 g/L | PMS | 2.0 g/L | 50 | 99.4 | Not mentioned | Calcination | [92] |
| Catalyst | Pollution | Main Mechanism | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate /% | Number of Cycles | Synthesis Techniques | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4 | BPA | ·SO4− ·OH | 20 mg/L | 2.0 g/L | PMS | 5 mM | 30 | 27.53 | Not mentioned | Commercially available | [95] |
| CuO-Fe3O4-BC | BPA | ·SO4− ·OH | 20 mg/L | 2.0 g/L | PMS | 5 mM | 30 | 100 | 4 (>85%) | Coprecipitation | [96] |
| rGO-Fe3O4 | NOF | 1O2 ·OH ·SO4− | 20 mg/L | 0.5 g/L | PS | 1 g/L | 30 | 89.69 | Not mentioned | Coprecipitation | [96] |
| Fe3O4 | Sulfamonomethoxine | ·SO4− | 0.06 mM | 2.4 mM | PS | 1.2 mM | 15 | 100 | Not mentioned | Coprecipitation | [97] |
| Fe3O4@Zn/Co-ZIFs | Carbamazepine | ·SO4− | 5 mg/L | 25 mg/L | PMS | 0.4 mM | 30 | 100 | Not mentioned | Solvothermal | [98] |
| Fe3O4/microwave irradiation (3 kW/L) | p-Nitrophenol | ·SO4− | 20 mg/L | 2.5 g/L | PS | 15:1 (molar ratio) | 28 | 94.2 | Not mentioned | Not mentioned | [99] |
| Fe3O4/MC | p-Hydroxybenzoic acid | ·SO4− | 1.0 g/L | 0.2 g/L | PS | 1.0 g/L | 30 | 100 | Not mentioned | Sol-gel | [100] |
| Fe3O4/graphene aerogels | Malachite green | Not mentioned | 20 mg/L | 0.2 g/L | PS | 1.0 mM | 12 | 91.7 | Not mentioned | Sol-gel | [101] |
| Catalyst | Pollution | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate /% | Number of Cycles | Synthesis Techniques | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| UV/Fe2+ | Lindane | 3.43 mM | 50 mM | PMS | 250 mM | 180 | 92.2 | Not mentioned | Commercially available | [110] |
| CuO-UV/Fe2O3 | 2,4-D | 50 mg/L | 0.5 g/L | PMS | 3 mM | 60 | 90.2 | Not mentioned | Hydrothermal | [111] |
| UV–Vis /Fe(II) | Carbamazepine | 0.05 mM | 0.1 mM | PMS | 0.2 mM | 30 | 100 | Not mentioned | Commercially available | [112] |
| UV/Fe2+ | Lindane | 3.43 mM | 0.25 mM | PMS | 0.25 mM | 720 | 78.4 | Not mentioned | Commercially available | [110] |
| UV/Fe2+ | Atrazine | 18.56 μM | 17.91 μM | PS | 1856 μM | Not mentioned | 62.94 | Not mentioned | Commercially available | [107] |
| UV-Vis/Fe(II) | Diclofenac, Sulfamethoxazole | Compound = 50 μM | 1 mM | PMS | 2 mM | 60 | >70 | Not mentioned | Commercially available | [113] |
| Vis/ZnFe2O4 | Orange II | 20 mg L−1 | 0.1 g L−1 | PMS | 0.5 g L−1 | 80 | 100 | Not mentioned | Commercially available | [114] |
| Vis/ZnFe2O4 | Orange II | 100 mg L−1 | 0.5 g L−1 | PS | 1.0 g L−1 | 300 | 50.5 | 5 (95%) | Sol-gel | [115] |
| Catalyst | Condition of US | Pollution | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate /% | Number of Cycles | Synthesis Techniques | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| US/PS/ Fe0f | 30 W L−1 28 kHz | TmpFG | 50 μM | 0.214 mM | PS | 1.45 mM | 40 | 100 | Not mentioned | Commercially available | [123] |
| US/ Fe0 | 140 W L−1 | SD | 20 mg/L | 1.3 mM | PS | 1.3 mM | 30 | 97.4 | Not mentioned | Hydrothermal | [124] |
| US/ Fe0 | 60 W L−1 | SMT | 0.05 mM | 0.1 mM | PS | 1 mM | 30 | 100 | Not mentioned | Magnetization | [125] |
| US/Fe2+ (pH = 3.5) | 40 kHz | Azorubine | 20 mg L−1 | 4 mM | PS | 4 mM | 60 | 66.5 | Not mentioned | Commercially available | [126] |
| US/Fe3O4 | 20 kHz | Azo dye | 0.06 mM | 0.4 g/L | PMS | 3 mM | 30 | 90 | Not mentioned | Hydrothermal | [127] |
| US/nZVI | 360 W L−1 40 kHz | Chloramphenicol | 5 mg/L | 0.5 g/L | PS | 1 mM | 90 | 98.1 | Not mentioned | Hydrothermal | [88] |
| US/Fe3O4@MOF-2 | 200 W L−1 | Diazinon | 30 mg/L | 0.7 g/L | PS | 10 mM | 120 | 98 | Not mentioned | Commercially available | [128] |
| Fe0/US | 40 kHz | Carbamazepine | 1.0 mg L−1 | 0.4 g L−1 | PDS | 0.4 g L−1 | 60 | 98.4 | Not mentioned | Commercially available | [37] |
| Catalyst | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| CoFe2O4 | CoFe2O4 exhibited an excellent performance for ATZ removal (over 99%). | It has a good effect in activating PMS, but in activating PS and H2O2; its recycling rate is not good due to the leaching of metal ions and loss of active sites. | [53] |
| CuFe2O4-20%rGO | Increase of specific surface area and chemical reaction activation sites. | The most suitable pH is 6.5; the application is limited. | [64] |
| Fe0@Fe3O4 | It has high reactivity for atrazine degradation (near 100% removal in 2 min) and is highly stable in air. | The synthetic route is complex. It has a low stoichiometric efficiency (10.3%) because most PMS are ineffectively consumed during activation. | [90] |
| UV/Fe2+ | Under the action of UV light, it shows an improved regeneration of Fe2+, causing a fast generation of highly reactive ·SO4− and ·OH. | Its applicable pH range is low (under 4). Too much catalyst will also reduce the reaction rate, so the use of catalyst needs to be strictly controlled. | [110] |
| US/ Fe0 | The reaction rate was improved by coupling activation. Compared with pure catalyst, the degradation rate is also improved. | It is easily affected by the action of other anions in the solution (Cl−, NO3−). Moreover, the effect of PMS alone is not good, and additional H2O2 is needed to better degrade pollutants. | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, K.; Li, Z.; Ma, P.; Tan, Y.; Zhou, Y.; Zhang, W.; Zhang, J. A Review of Activation Persulfate by Iron-Based Catalysts for Degrading Wastewater. Appl. Sci. 2021, 11, 11314. https://doi.org/10.3390/app112311314
Zhi K, Li Z, Ma P, Tan Y, Zhou Y, Zhang W, Zhang J. A Review of Activation Persulfate by Iron-Based Catalysts for Degrading Wastewater. Applied Sciences. 2021; 11(23):11314. https://doi.org/10.3390/app112311314
Chicago/Turabian StyleZhi, Keke, Zhe Li, Pengfei Ma, Yongxiang Tan, Yuefeng Zhou, Weikang Zhang, and Jingxing Zhang. 2021. "A Review of Activation Persulfate by Iron-Based Catalysts for Degrading Wastewater" Applied Sciences 11, no. 23: 11314. https://doi.org/10.3390/app112311314
APA StyleZhi, K., Li, Z., Ma, P., Tan, Y., Zhou, Y., Zhang, W., & Zhang, J. (2021). A Review of Activation Persulfate by Iron-Based Catalysts for Degrading Wastewater. Applied Sciences, 11(23), 11314. https://doi.org/10.3390/app112311314