Nutritional and Health Potential of Probiotics: A Review
Abstract
1. Introduction
1.1. Nutritional Impacts of Probiotics
1.2. Nutritional Impacts of Probiotics in Inflammation
1.3. Nutritional Impacts of Probiotics in Dental Carries
1.4. Nutritional Impacts of Probiotics in Obesity, Diabetes and Associated Issues
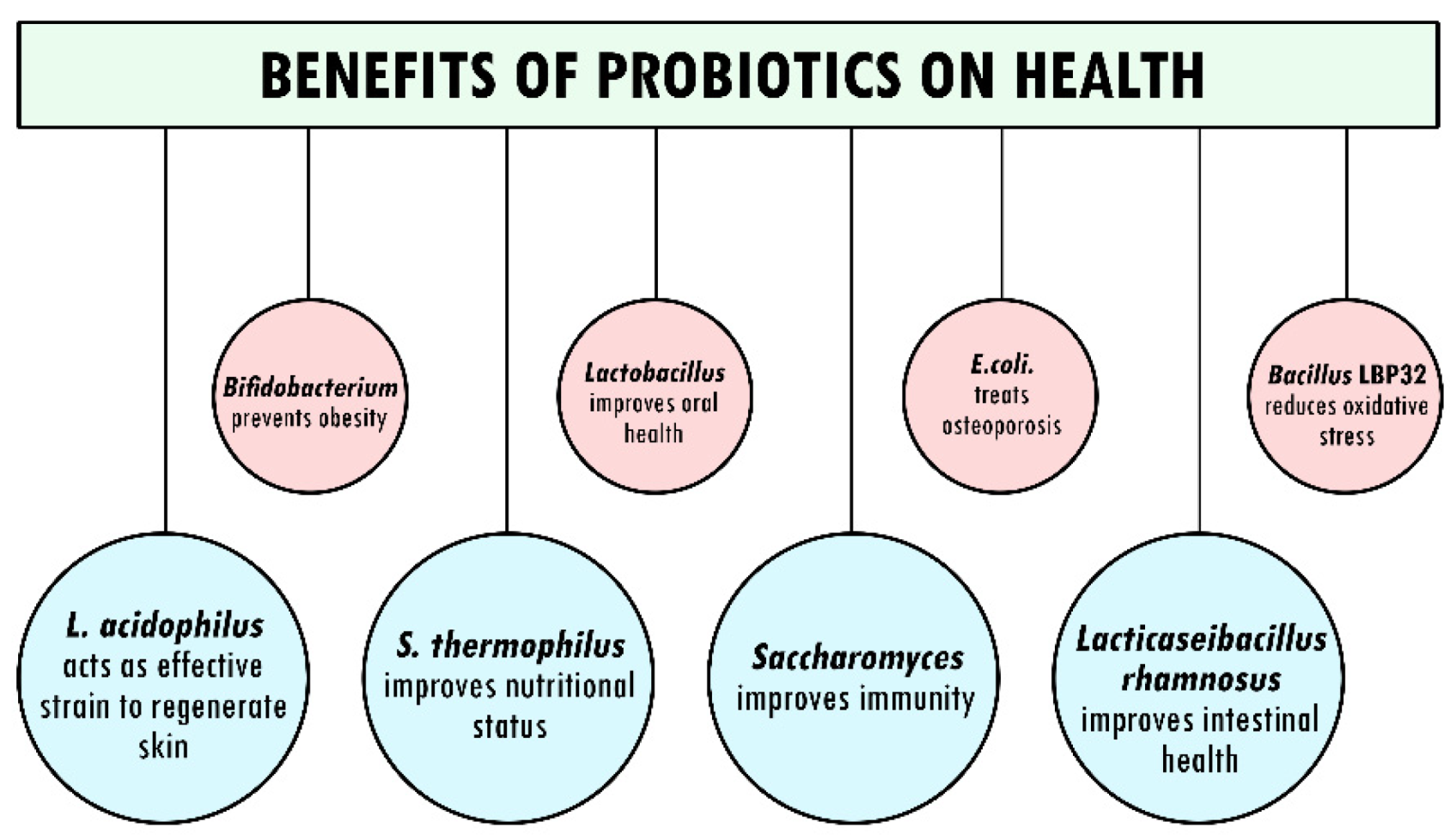
2. Probiotics and Their Benefits
2.1. Significant Effects of Probiotics on Oral Health Status
2.2. Improvement of Intestinal Health through Probiotics
2.3. Role of Probiotics in Development of Immunity
3. Association of Probiotics in Prevention of Diseases
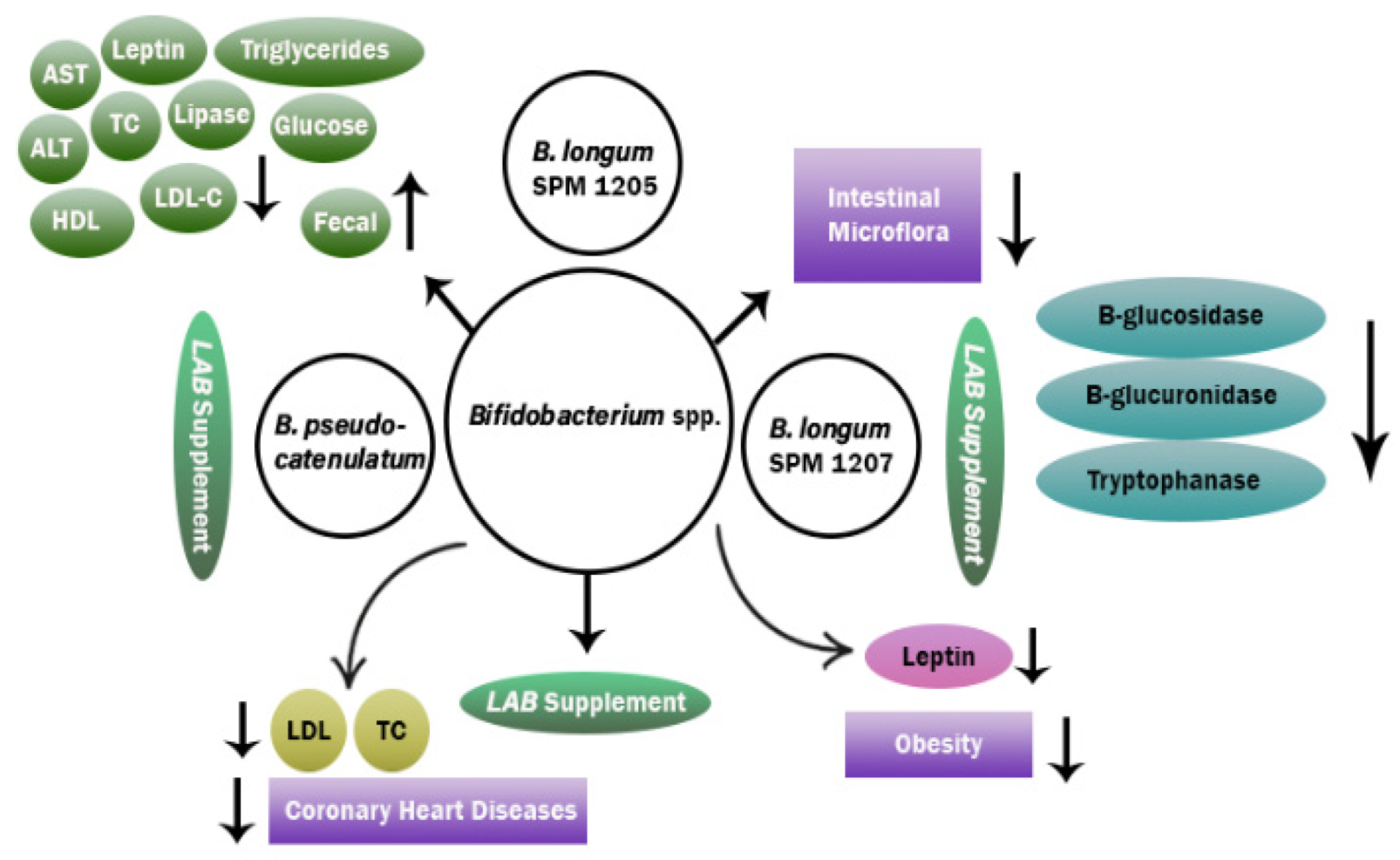
3.1. Action Mechanism of Probiotics in Reduction of Obesity
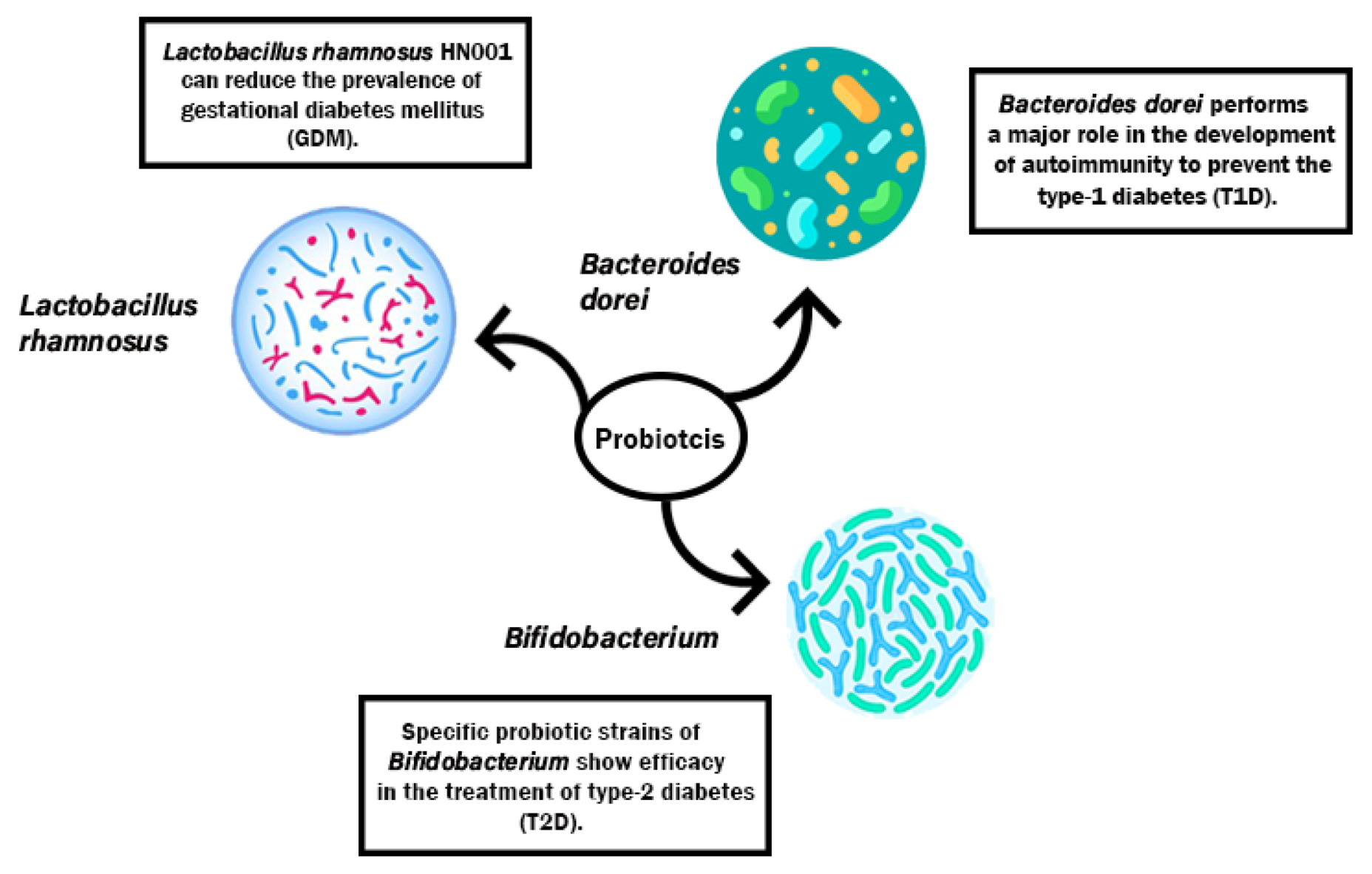
3.2. Action Mechanism of Probiotics in Minimizing T1D, T2D, GDM
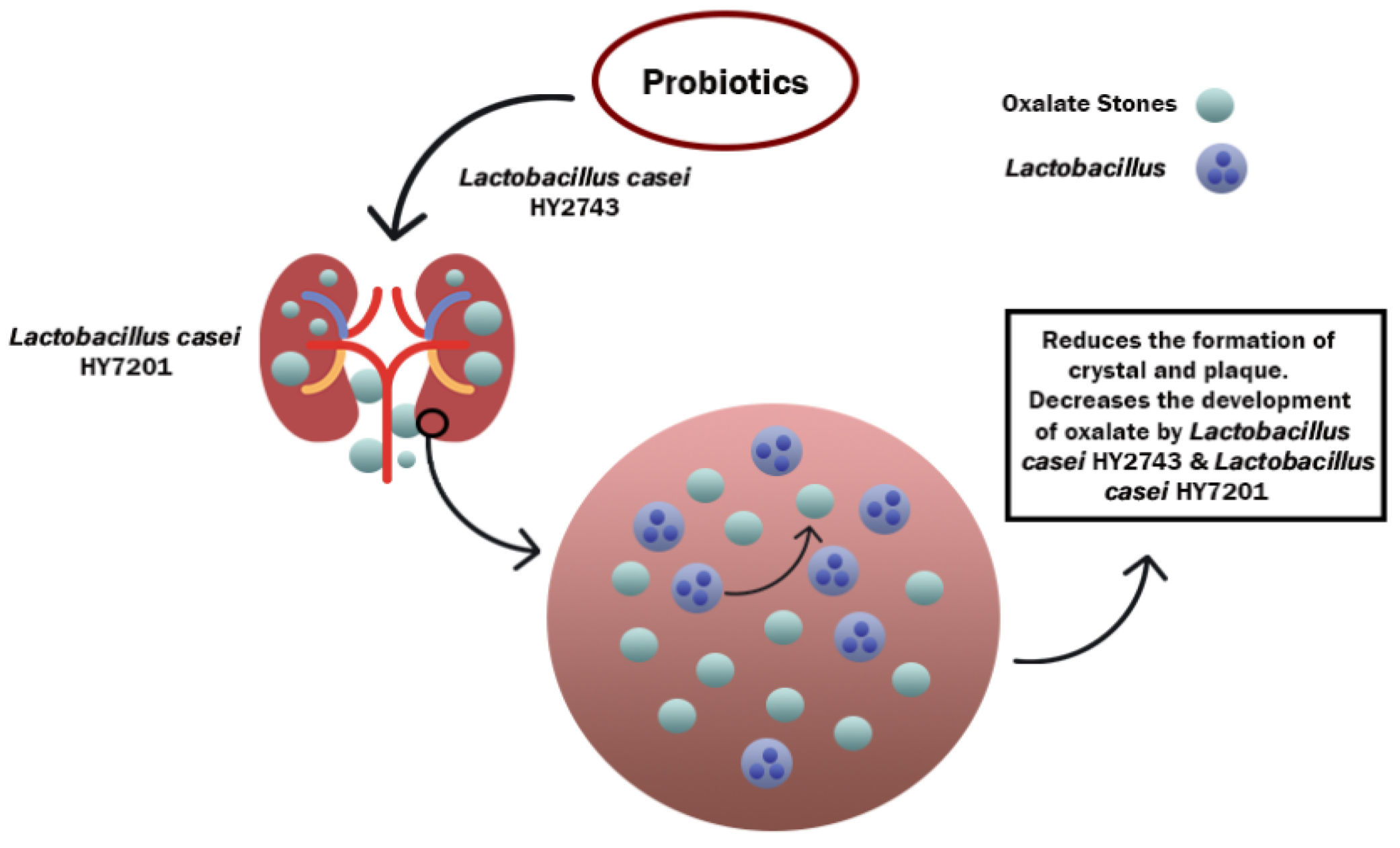
3.3. Mechanism of Action of Probiotics against CKD
4. Probiotics for Animal Health
5. Safety of Probiotics
6. Conclusions
7. Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guidance on Community Mental Health Services: Promoting Person-Centred and Rights-Based Approaches. Available online: https://www.who.int/publications/i/item/9789240025707 (accessed on 30 October 2021).
- Ranjha, M.M.A.N.; Amjad, S.; Ashraf, S.; Khawar, L.; Safdar, M.N.; Jabbar, S.; Nadeem, M.; Mahmood, S.; Murtaza, M.A. Extraction of polyphenols from apple and pomegranate peels employing different extraction techniques for the development of functional date bars. Int. J. Fruit Sci. 2020, 20, S1201–S1221. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Process 2021, 9, 1406. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.M.; Ranjha, M.M.A.N.; Jooste, L.; Hano, C.; Aadil, R.M. Heterocyclic aromatic amines in meat: Formation, isolation, risk assessment, and inhibitory effect of plant extracts. Foods 2021, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.N.; Irfan, S.; Nadeem, M.; Mahmood, S. A comprehensive review on nutritional value, medicinal uses, and processing of banana. Food Rev. Int. 2020, 1–27. [Google Scholar] [CrossRef]
- Salvatori, G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front. Cell. Infect. Microbiol. 2012, 2, 1–4. [Google Scholar] [CrossRef]
- Grover, S.; Rashmi, H.M.; Srivastava, A.K.; Batish, V.K. Probiotics for human health—New innovations and emerging trends. Gut Pathog. 2012, 4, 15. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, R. Probiotics. Indian J. Med. Microbiol. 2009, 27, 202–210. [Google Scholar] [CrossRef]
- Stambler, I. A History of Life-Extensionism in the Twentieth Century. Ph.D. Thesis, Bar Ilan University, Ramat Gan, Israel, 2017. [Google Scholar]
- Amara, A.A.; Shibl, A. Role of probiotics in health improvement, infection control and disease treatment and management. SAUDI Pharm. J. 2013, 23, 107–114. [Google Scholar] [CrossRef]
- Suskovic, J.; Kos, B.; Beganovic, J.; Pavunc, A.L. Antimicrobial activity—The most important property of probiotic and starter lactic acid bacteria. Food Technol. Biotechnol. 2010, 9862, 296–307. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Mei, G.; Carey, C.M.; Tosh, S.; Kostrzynska, M. Utilization of different types of dietary fibres by potential probiotics. Can. J. Microbiol. 2011, 57, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, J.; Swami, G.; Kumar, M. Probiotics and their effects on metabolic diseases: An update. J. Clin. Diagn. Res. 2012, 7, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khani, S.; Hosseini, H.M.; Taheri, M.; Nourani, M.R.; Fooladi, A.A.I. Probiotics as an alternative strategy for prevention and treatment of human diseases: A review. Inflamm. Allergy-Drug Targets 2012, 11, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Veerappan, G.R.; Betteridge, J.; Young, P.E. Probiotics for the treatment of inflammatory bowel disease. Curr. Gastroenterol. Rep. 2012, 14, 324–333. [Google Scholar] [CrossRef]
- Roobab, U.; Aadil, R.M.; Zeng, X. Ultrasound. In Advances in Noninvasive Food Analysis; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Haukioja, A. Probiotics and oral health. Eur. J. Dent. 2010, 4, 348–355. [Google Scholar] [CrossRef]
- Pagnini, C.; Saeed, R.; Bamias, G.; Arseneau, K.O.; Pizarro, T.T. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 1–6. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Rzepkowska, A.; Szydłowska, A.; Kołożyn-Krajewska, D. Trends and possibilities of the use of probiotics in food production. In Alternative and Replacement Foods; Academic Press: Amsterdam, The Netherlands, 2018; ISBN 9780128114469. [Google Scholar]
- Kim, K.M.; Yu, K.W.; Kang, D.H.; Suh, H.J. Anti-stress and anti-fatigue effect of fermented rice bran. Phyther. Res. 2002, 16, 700–702. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Soares, S.C.; Freire, C.J. An introduction of the role of probiotics in human infections and autoimmune diseases. Crit. Rev. Microbiol. 2019, 45, 413–432. [Google Scholar] [CrossRef]
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Hemati, M.; Nejad, Z.R.; Arabkari, V.; Namdar, A. Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell. Physiol. 2019, 234, 17127–17143. [Google Scholar] [CrossRef]
- Behnsen, J.; Deriu, E.; Sassone-Corsi, M.; Raffatellu, M. Probiotics: Properties, examples, and specific applications. Cold Spring Harb. Perspect. Med. 2013, 3, a010074. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The role of probiotics in cancer prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Altonsy, M.O.; Andrews, S.C.; Tuohy, K.M. Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: Mediation by the mitochondrial pathway. Int. J. Food Microbiol. 2010, 137, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S.; Keller, M.K. Advances in dental research probiotics for caries prevention and control. Adv. Dent. Res. 2012, 24, 98–102. [Google Scholar] [CrossRef]
- Mahasneh, S.A.; Mahasneh, A.M. Probiotics: A promising role in dental health. Dent. J. 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Velayudham, A.; Dolganiuc, A.; Ellis, M.; Petrasek, J.; Kodys, K.; Mandrekar, P.; Szabo, G. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced NASH model in mice. Hepatology 2009, 49, 989. [Google Scholar] [CrossRef]
- Eslamparast, T.; Eghtesad, S.; Hekmatdoost, A.; Poustchi, H. Probiotics and nonalcoholic fatty liver disease. Middle East J. Dig. Dis. 2013, 5, 129. [Google Scholar] [PubMed]
- Schrumpf, E.; Kummen, M.; Valestrand, L.; Greiner, T.U.; Holm, K.; Arulampalam, V.; Reims, H.M.; Baines, J.; Bäckhed, F.; Karlsen, T.H.; et al. The gut microbiota contributes to a mouse model of spontaneous bile duct inflammation. J. Hepatol. 2016, 66, 382–389. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Niafar, M.; Mofid, V. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 2011, 94, 3288–3294. [Google Scholar] [CrossRef]
- Da Silva, C.C.; Monteil, M.A.; Davis, E.M. Overweight and Obesity in children are associated with an abundance of firmicutes and reduction of bifidobacterium in their gastrointestinal microbiota. Child. Obes. 2020, 16, 204–210. [Google Scholar] [CrossRef]
- Wang, H.; Ren, P.; Mang, L.; Shen, N.; Chen, J.; Zhang, Y. In vitro fermentation of novel microwave-synthesized non-digestible oligosaccharides and their impact on the composition and metabolites of human gut microbiota. J. Funct. Foods 2019, 55, 156–166. [Google Scholar] [CrossRef]
- Slevin, M.M.; Allsopp, P.J.; Magee, P.J.; Bonham, M.P.; Naughton, V.R.; Strain, J.J.; Duffy, M.E.; Wallace, J.M.; Sorley, E.M.M. Supplementation with calcium and short-chain fructo-oligosaccharides affects markers of bone turnover but not bone mineral density in postmenopausal women. J. Nutr. 2014, 144, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Lucak, S. Use of probiotics in gastrointestinal disorders: What to recommend? Therap. Adv. Gastroenterol. 2010, 3, 307. [Google Scholar] [CrossRef] [PubMed]
- Tortora, A.; Gabrielli, M. The role of intestinal microbiota and the immune system. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 323–333. [Google Scholar]
- Wolff, B.J.; Price, T.K.; Joyce, C.J.; Wolfe, A.J.; Mueller, E.R. Oral probiotics and the female urinary microbiome: A double-blinded randomized placebo-controlled trial. Int. Urol. Nephrol. 2019, 51, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A. Gut microbiota and obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Marques, A.M. Preclinical relevance of probiotics in type 2 diabetes: A systematic review. Int. J. Exp. Pathol. 2020, 101, 68–79. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Borresen, E.C.; Henderson, A.J.; Kumar, A.; Weir, T.L.; Ryan, E.P. Fermented foods: Patented approaches and formulations for nutritional supplementation and health promotion. Recent Pat. Food Nutr. Agric. 2012, 4, 134–140. [Google Scholar] [CrossRef]
- Dekumpitiya, N.; Gamlakshe, D.; Indrika, S.; Jayaratne, D.L. Identification of the microbial consortium in Sri Lankan buffalo milk curd and their growth in the presence of prebiotics. J. Food Sci. Technol. 2016, 9, 20–30. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Faria, A.F.; Cruz, A.G. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compre. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Sullivan, J.N.O.; Rea, M.C.; Sullivan, J.N.O.; Rea, M.C.; Hill, C.; Ross, R.P. Protecting the outside: Biological tools to manipulate the skin microbiota. Microbiol. Ecol. 2020, 96. [Google Scholar] [CrossRef]
- Ghishan, F.K.; Kiela, P.R. From probiotics to therapeutics: Another step forward? J. Clin. Invest. 2011, 121, 2149–2152. [Google Scholar] [CrossRef]
- Islam, S.U. Clinical uses of probiotics. Medicine 2016, 95, e2658. [Google Scholar] [CrossRef] [PubMed]
- Harneet Singh, H.S. Probiotics—An emerging concept. Int. J. Sci. Res. Publ. 2014, 4, 1–3. [Google Scholar]
- Salvetti, E.; Torriani, S.; Felis, G.E. The genus Lactobacillus: A taxonomic update related papers. Probiotics Antimicrob. Proteins 2012, 4, 217–226. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Micro-organisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Jahromi, M.F.; Liang, J.B.; Wan, Y.; Shokryazdan, P.; Faseleh, M.; Boo, J.; Wan, Y. Probiotics: From isolation to application. J. Am. Coll. Nutr. 2017, 36, 666–676. [Google Scholar] [CrossRef]
- Shewale, R.N.; Sawale, P.D.; Khedkar, C.D.; Singh, A. Selection criteria for probiotics: A review Department of Dairy Microbiology College of Dairy Technology, Pusad, India. Int. J. Probiotics Prebiotics 2014, 9, 17–22. [Google Scholar]
- Jiang, T.; Li, H.-S.; Han, G.G.; Singh, B.; Kang, S.-K. Oral delivery of probiotics in poultry using pH-sensitive tablets. J. Microbiol. Biotechnol. 2017, 27, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Verma, V.; Sawant, P.M.; Tiwari, R.; Vaid, R.K.; Chauhan, R.S. Applications of probiotics in poultry: Enhancing immunity and beneficial effects on production performances and health—A Review. J. Immunol. Immunopathol. 2011, 13, 1–19. [Google Scholar]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Int. Sch. Res. Not. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; Shriner, K.A.; Wong-Beringer, A. Regulatory oversight and safety of probiotic use. Emerg. Infect. Dis. 2010, 16, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert Rev. Anti. Infect. Ther. 2019, 17, 635–645. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Fern, L.A.; Siti, D.; Rashidah, N.; Hj, P.; Chaiyasut, C. The influence of probiotics on bile acids in diseases and aging. Biomed. Pharmacother. 2020, 128, 110310. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Fonty, G. Establishment of cellulolytic bacteria and development of fermentative activities in the rumen of gnotobiotically-reared lambs receiving the microbial additive Saccharomyces cerevisiae CNCM I-1077. Reprod. Nutr. Dev. 2001, 41, 57–68. [Google Scholar] [CrossRef]
- Galanis, A.; Kourkoutas, Y.; Tassou, C.C.; Chorianopoulos, N. Detection and identification of probiotic Lactobacillus plantarum strains by multiplex PCR using RAPD-derived primers. Int. J. Mol. Sci. 2015, 16, 25141–25153. [Google Scholar] [CrossRef]
- Sattler, V.A.; Mohnl, M.; Klose, V. Development of a strain-specific real-time PCR Assay for enumeration of a probiotic Lactobacillus reuteri in chicken feed and intestine. PLoS ONE 2014, 9, e90208. [Google Scholar] [CrossRef]
- Collins, J.; Van Pijkeren, J.-P.; Svensson, L.; Claesson, M.; Sturme, M.; Li, Y.; Cooney, J.; Van Sinderen, D.; Walker, A.; Parkhill, J.; et al. Fibrinogen-binding and platelet-aggregation activities of a Lactobacillus salivarius septicaemia isolate are mediated by a novel fibrinogen-binding protein. Mol. Microbiol. 2012, 85, 862–877. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R.; Ouwehand, A.C. The production and delivery of probiotics: A review of a practical approach. Micro-organisms 2019, 7, 83. [Google Scholar] [CrossRef]
- Allaker, R.P.; Stephen, A.S. Use of probiotics and oral health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef]
- Hung, A. Probiotics and Oral health: A Sytematic Review. Int. J. Sci. Eng. Res. 2011, 2. [Google Scholar]
- Lin, T.; Lin, C.; Pan, T.; Lin, T. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 2017, 102, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; Souza, S.D.; Aucamp, M. Recent advances in the development of antimicrobial and antifouling biocompatible materials for dental applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Saraf, K.; Shashikanth, M.C.; Priya, T.; Sultana, N.; Chaitanya, N.C.S.K. Probiotics—Do they have a role in medicine and dentistry? J. Assoc. Phys. India 2010, 58, 488–490. [Google Scholar]
- Hashemi, A.; Villa, C.R.; Comelli, E.M. Probiotics in early life: A preventative and treatment approach. Food Funct. 2016, 7, 1752–1768. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Lobach, A.R.; Simon, R.; Pélerin, F.; Jüsten, P.; Urdaci, C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2016, 83, 54–65. [Google Scholar] [CrossRef]
- Vera, R.; Visitacion, D.; Guzman, G.; Acids, D.; Nutrition, M. Probiotics prevent dysbiosis and the raise in blood pressure in genetic hypertension: Role of short-chain fatty acids. Mol. Nutr. Food Res. 2020, 64, e1900616. [Google Scholar] [CrossRef]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A review on prebiotics and probiotics for the control of dysbiosis: Present status and future perspectives. Anim. Int. J. Anim. Biosci. 2015, 9, 43–48. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Mosi-Roa, Y.; Rubilar, M. Probiotics as an adjunct therapy for the treatment of halitosis, dental caries and periodontitis. Probiotics Antimicrob. Proteins 2019, 12, 325–334. [Google Scholar] [CrossRef]
- Shringeri, P.I.; Fareed, N.; Battur, H.; Khanagar, S. Role of probiotics in the treatment and prevention of oral malodor/halitosis: A systematic review. J. Indian Assoc. Public Health Dent. 2019, 17, 90. [Google Scholar] [CrossRef]
- Nagamine, Y.; Hasibul, K.; Ogawa, T.; Tadab, A.; Kamitoric, K.; Hossainc, A.; Yamaguchid, F.; Tokudae, M.; Kuwaharab, T.; Miyakea, M. D-tagatose effectively reduces the number of streptococcus mutans and oral bacteria in healthy adult subjects: A chewing gum pilot study and randomized clinical trial. Acta Med. Okayama 2020, 74, 307–317. [Google Scholar] [PubMed]
- Faujdar, S.S.; Mehrishi, P.; Sharma, A. Role of probiotics in human health and disease: An update role of probiotics in human health and disease: An update. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 328–344. [Google Scholar] [CrossRef]
- Reddy, R.S.; Swapna, L.A.; Ramesh, T.; Singh, T.R.; Vijayalaxmi, N.; Lavanya, R. Bacteria in oral health—Probiotics and prebiotics: A review. Int. J. Biol. Med. Res. 2011, 2, 1226–1233. [Google Scholar]
- Sharma, V.; Sharma, N.; Sheikh, I.; Kumar, V.; Sehrawat, N.; Yadav, M. Probiotics and prebiotics having broad spectrum anticancer therapeutic potential: Recent trends and future perspectives. Curr. Pharmacol. Rep. 2021, 7, 67–79. [Google Scholar] [CrossRef]
- Manuel, J.; Rodrı, M.A.; Macı, M.G.M.F.A.; Microflora, D.Á.E.Á. Soy isoflavones and their relationship with microflora: Beneficial effects on human health in equol producers. Phtochem. Rev. 2013, 12, 979–1000. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. Review the impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Team, R.; Guarner, F.; Khan, A.G.; Garisch, J.; Africa, S.; Eliakim, R.; Gangl, A.; Thomson, A.; France, J.K.; Lemair, T.; et al. World gastroenterology organisation global guidelines probiotics and prebiotics October 2011. J. Clin. Gastroenterol. 2012, 46, 119–129. [Google Scholar]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Salonen, A.; Philippou, E. Impact of diet on human intestinal microbiota and health. Annu. Rev. Food Sci. Technol. 2014, 5, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Expert review of endocrinology & metabolism a brief overview on the use of probiotics to treat overweight and obese patients. Expert Rev. Endocrinol. Metab. 2020, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.R.; Jack, A.A.; Masetti, G.; Davies, T.S.; Loxley, K.E.; Kerry-Smith, J.; Plummer, J.F.; Marchesi, J.R.; Mullish, B.H.; McDonald, J.; et al. A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Toole, P.W.O. Impact of diet on the human intestinal microbiota. Curr. Opin. Food Sci. 2014, 2, 71–77. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Gerardi, V.; Lopetuso, L.R.; Del Zompo, F.; Mangiola, F.; Boškoski, I.; Bruno, G.; Petito, V.; Laterza, L.; Cammarota, G.; et al. Gut microbial flora, prebiotics, and probiotics in IBD: Their current usage and utility. Bio Med Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Lee, E.; Song, E.; Nam, Y.; Lee, S. Probiotics in human health and disease: From nutribiotics to pharmabiotics. J. Microbiol. 2018, 56, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Ushida, K. Health-beneficial effects of probiotics: Its mode of action. Anim. Sci. J. 2009, 80, 361–371. [Google Scholar] [CrossRef]
- Martin, R.; Makino, H.; Yavuz, A.C.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef]
- Ozyurt, V.H.; Otles, S. Properties of probiotics and encapsulated probiotics in food. Acta Sci. Pol. Technol. Aliment. 2014, 13, 413–424. [Google Scholar] [CrossRef]
- Delgado, S.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Oryan, A.; Jalili, M.; Kamali, A.; Nikahval, B. The concurrent use of probiotic micro-organism and collagen hydrogel/scaffold enhances burn wound healing: An in vivo evaluation. Burns 2018, 44, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-tov, H.; Davis, C.; Tomic-canic, M.; Pastar, I. Probiotics or pro-healers the role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 912–922. [Google Scholar] [CrossRef]
- Salonen, A.; De Vos, W.M.; Palva, A. Gastrointestinal microbiota in irritable bowel syndrome: Present state and review gastrointestinal microbiota in irritable bowel syndrome: Present state and perspectives. Microbiology 2010, 156, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Iacono, A.; Mattace, G.; Berni, R.; Calignano, A.; Meli, R. Probiotics as an emerging therapeutic strategy to treat NAFLD: Focus on molecular and biochemical mechanisms. J. Nutr. Biochem. 2011, 22, 699–711. [Google Scholar] [CrossRef]
- Nole, K.L.B.; Mph, E.Y.; Keri, J.E. Probiotics and prebiotics in dermatology. J. Am. Dermatol. 2014, 22, 699–711. [Google Scholar] [CrossRef]
- Holmes, C.J.; Plichta, J.; Gamelli, R.L.; Radek, K.A. Dynamic role of host stress responses in modulating the cutaneous microbiome: Implications for wound healing and infection. Wound Health Soc. 2014, 4, 24–37. [Google Scholar] [CrossRef]
- Tsiouri, M.G. Human microflora, probiotics and wound healing. Biochem. Pharmacol. 2017, 19, 33–38. [Google Scholar] [CrossRef]
- Moratalla, A.Z.; De Lagrán, M.M.; Dierssen, M. Neurodevelopmental disorders: 2021 update. Neuropathology 2021, 6. [Google Scholar] [CrossRef]
- Navarro, F.; Liu, Y.; Rhoads, J.M.; Navarro, F.; Liu, Y.; Rhoads, J.M. Can probiotics benefit children with autism spectrum disorders? World J. Gasrtoenterol. 2016, 22, 10093–10102. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem. Res. 2014, 39, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.P.; Abalo, R.; Marco, E.M.; Cardona, D. Probiotics in digestive, emotional and pain-related disorders. Behav. Pharmacol. 2018, 29, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Larroya-García, A.; Navas-Carrillo, D.; Orenes-Piñero, E. Impact of gut microbiota on neurological diseases: Diet composition and novel treatments. Critical 2019, 59, 3102–3116. [Google Scholar] [CrossRef]
- Hori, T.; Matsuda, K. Probiotics: A dietary factor to modulate the gut microbiome, host immune system, and gut-brain interaction. Micro-organisms 2020, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, M.; Yi, L. Identifying children with autism spectrum disorder based on their face processing abnormality: A machine learning framework. Autrism Res. 2016, 9, 888–898. [Google Scholar] [CrossRef]
- Collins, F.L.; Rios-Arce, N.D.; Schepper, J.D.; Parameswaran, N.; McCabe, L.R. The potential of probiotics as a therapy for osteoporosis. Microbiol. Spectr. 2017, 5, 213–233. [Google Scholar] [CrossRef]
- Svensson, H. Finding Ways Forward with Pain as a Fellow Traveler Older Women’ S Experience of Living with Osteoporotic Vertebral Compression Fractures and Back Pain; Institute of Health and Care Sciences Sahlgrenska Academy at the University of Gothenburg: Gothenburg, Sweden, 2018; ISBN 9789162904647. [Google Scholar]
- Han, B. Therapy of Social Medicine; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9789812877475. [Google Scholar]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Li, P.; Sundh, D.; Lorentzon, M. Metabolic alterations in older women with low bone mineral density supplemented with Lactobacillus reuteri. JBMR Plus 2021, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wong, H.S.; Cheung, W.-H. The role of gut microbiota in bone homeostasis a systematic review of preclinical animal studies. Bone Jt. Res. 2021, 10, 51–59. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fåk, F.; Andersson, A.; Windahl, S.H.; Farman, H.H.; Movérare-Skrtic, S.; Islander, U.; Sjögren, K. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS ONE 2014, 9, e92368. [Google Scholar] [CrossRef]
- Anderson, J.L.; Miles, C.; Tierney, A.C. Effect of probiotics on respiratory, gastrointestinal and nutritional outcomes in patients with cystic fi brosis: A systematic review. J. Cyst. Fibros. 2016, 16, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.; Møller, K.; Svendsen, K.D.; Jakobsen, M.; Pedersen, B.K. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Britton, R.A.; Irwin, R.; Quach, D.; Schaefer, L.; Zhang, J.; Lee, T.; Parameswaran, N. ProbioticL. reuteriTreatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Romeo, M.G.; Romeo, D.M.; Trovato, L.; Oliveri, S.; Palermo, F.; Cota, F.; Betta, P. Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: Incidence of late-onset sepsis and neurological outcome. J. Perinatol. 2011, 31, 63–69. [Google Scholar] [CrossRef]
- Grin, P.M.; Kowalewska, P.M.; Alhazzani, W.; Fox-robichaud, A.E. Lactobacillus for preventing recurrent urinary tract infections in women: Meta-analysis. Can. J. Urol. 2013, 20, 6607–6614. [Google Scholar]
- Kang, J.; Yun, S.; Park, H. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J. Microbiol. 2010, 48, 712–714. [Google Scholar] [CrossRef]
- Keubler, L.M.; Buettner, M.; Häger, C.; Bleich, A. A multihit model: Colitis lessons from the interleukin-10–deficient mouse. Inflamm. Bowel Dis. 2015, 21, 1967–1975. [Google Scholar] [CrossRef]
- Liu, F. Porcine Small Intestinal Epithelial Cell Line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010, 23, 135–149. [Google Scholar] [CrossRef]
- Stenman, L.K.; Waget, A.; Garret, C.; Klopp, P.; Burcelin, R.; Lahtinen, S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef. Microbes 2014, 5, 437–445. [Google Scholar] [CrossRef]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitu: A randomised controlled trial. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef]
- Chang, H.H.T. Antioxidative properties and inhibitory effect of Bifidobacterium adolescentis on melanogenesis. J. Microbiol. Biotechnol. 2012, 28, 2903–2912. [Google Scholar] [CrossRef]
- Elian, S.; Souza, E.; Vieira, A.; Teixeira, M.; Arantes, R.; Nicoli, J.; Martins, F. Bifidobacterium longum subsp. infantis BB-02 attenuates acute murine experimental model of inflammatory bowel disease. Benef. Microbes 2015, 6, 277–286. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuhara, T.; Oki, M.; Xiao, J. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial Abstract. Benef. Microbes 2019, 10, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Khailova, L.; Petrie, B.; Baird, C.H.; Dominguez Rieg, J.A.; Wischmeyer, P.E. Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis. PLoS ONE 2014, 9, e97861. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W.; Chrubasik, S.; Boehm, S.; Stange, C.; Schulze, J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int. J. Colorectal Dis. 2012, 27, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Tims, S.; Roelofs, M.; Roug, C.; Rakza, T.; Chirico, G.; Roeselers, G.; Knol, J.; Christophe, J.; Turck, D. Fermented infant formula (with Bi fi dobacterium breve C50 and Streptococcus thermophilus O65) with prebiotic oligosaccharides is safe and modulates the gut microbiota towards a microbiota closer to that of breastfed infants. Clin. Nutr. 2020, 40, 778–787. [Google Scholar] [CrossRef]
- Guo, M.; Wu, F.; Hao, G.; Qi, Q.; Li, R.; Li, N.; Chai, T. Bacillus subtilis improves immunity and disease resistance in rabbits. Front. Immunol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Korf, H.; Van Belle, T.L.; Robert, S.; Grieco, F.A.; Caluwaerts, S.; Galleri, L.; Spagnuolo, I.; Steidler, L.; Van Huynegem, K.; et al. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J. Clin. Invest. 2012, 122, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Joffin, N.; Jaubert, A.M.; Durant, S.; Barouki, R.; Forest, C.; Noirez, P. Citrulline counteracts overweight- and aging-related effects on adiponectin and leptin gene expression in rat white adipose tissue. Biochim. Open 2015, 1, 1–5. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Wici, M. Probiotics for the treatment of overweight and obesity in humans—A review of clinical trials. Micro-organisms 2020, 8, 1148. [Google Scholar]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesæth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Etiol. Pathophysiol. 2018, 19, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Howarth, G.S.; Wang, H. Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients 2013, 5, 58–81. [Google Scholar] [CrossRef] [PubMed]
- Obesity, T.; Brusaferro, A.; Cozzali, R.; Orabona, C.; Biscarini, A.; Farinelli, E.; Cavalli, E.; Grohmann, U.; Principi, N.; Esposito, S. Is it time to use probiotics to prevent or treat obesity? Nutrients 2018, 10, 1613. [Google Scholar] [CrossRef]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9, 228S–233S. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- An, H.M.; Park, S.Y.; Lee, D.K.; Kim, J.R.; Cha, M.K.; Lee, S.W.; Lim, H.T.; Kim, K.J.; Ha, N.J. Antiobesity and lipid-lowering effects of Bifidobacterium spp. in high fat diet-induced obese rats. Lipids Health Dis. 2011, 10, 116–118. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Wang, L.; Irfan, S.; Safdar, M.N.; Murtaza, M.A.; Nadeem, M.; Mahmood, S.; Mueen-ud-Din, G.; Nadeem, H.R. A comprehensive review on phytochemistry, bioactivity and medicinal value of bioactive compounds of pomegranate (Punica granatum). Adv. Tradit. Med. 2021, 1–21. [Google Scholar] [CrossRef]
- Mishra, S.; Wang, S.; Nagpal, R.; Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and prebiotics for the amelioration of type 1 diabetes: Present and future perspectives. Micro-organisms 2019, 7, 67. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Javadi, M.; Ejtahed, H.; Mirmiran, P. Probiotics as beneficial agents in the management of diabetes mellitus: A systematic review. Diabetes. Metab. Res. Rev. 2016, 32, 143–168. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, X.; Li, J.; Li, Z.; Hu, Q.; Li, L. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit. Rev. Food Sci. Nutr. 2020, 60, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Salgaço, M.K.; Garcia, L.; Oliveira, S.; Costa, G.N.; Bianchi, F.; Sivieri, K. Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl. Microbiol. Biotechnol. 2019, 103, 9229–9238. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Tabatabaei-malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. J. Pharm. Sci. 2019, 27, 827–837. [Google Scholar] [CrossRef]
- Alokail, M.S.; Sabico, S.; Al-Saleh, Y.; Al-Daghri, N.M.; Alkharfy, K.M.; Vanhoutte, P.M.; Mcternan, P.G. Effects of probiotics in patients with diabetes mellitus type 2: Study protocol for a randomized, double-blind, placebo-controlled trial. Tirals 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hulston, C.J.; Churnside, A.A.; Venables, M.C. Probiotic supplementation prevents high-fat, overfeeding-induced insulin resistance in human subjects. Br. J. Nutr. 2015, 113, 596–602. [Google Scholar] [CrossRef]
- Callaway, L.K.; Mcintyre, H.D.; Barrett, H.L.; Foxcroft, K.; Tremellen, A.; Lingwood, B.E.; Tobin, J.M.; Wilkinson, S.; Kothari, A.; Morrison, M.; et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: Findings from the SPRING double-blind randomized controlled trial. Diabetes Care 2019, 42, 364–371. [Google Scholar] [CrossRef]
- Khraishi, M.; MacDonald, D.; Rampakakis, E.; Vaillancourt, J.; Sampalis, J.S. Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin. Rheumatol. 2011, 30, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Santos, A.; Bermudez, M.G. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity; NCBI: Bethesda, MD, USA, 2019; ISBN 3462930869.
- Watanabe, H.; Katsura, T.; Takahara, M.; Miyashita, K.; Katakami, N.; Matsuoka, T.A.; Kawamori, D.; Shimomura, I. Plasma lipopolysaccharide binding protein level statistically mediates between body mass index and chronic microinflammation in Japanese patients with type 1 diabetes. Diabetol. Int. 2020, 11, 293. [Google Scholar] [CrossRef]
- Adeshirlarijaney, A.; Gewirtz, A.T. Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes 2020, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and chronic kidney disease. Nat. Publ. Gr. 2015, 88, 958–966. [Google Scholar] [CrossRef]
- Dehghani, H.; Heidari, F.; Mozaffari-khosravi, H. Synbiotic supplementations for azotemia in patients with chronic kidney disease: A randomized controlled trial. Iran. J. Kidney Dis. 2016, 10, 351–357. [Google Scholar] [PubMed]
- Lieske, J.C. Probiotics for prevention of urinary stones. Ann. Transl. Med. 2017, 5, 29. [Google Scholar] [CrossRef]
- Le Bon, M.; Davies, H.E.; Glynn, C.; Thompson, C.; Madden, M.; Wiseman, J.; Dodd, C.E.; Hurdidge, L.; Payne, G.; Le Treut, Y.; et al. Influence of probiotics on gut health in the weaned pig. Livest. Sci. 2010, 133, 179–181. [Google Scholar] [CrossRef]
- Klimesova, K.; Whittamore, J.M.; Hatch, M. Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 2014, 43, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef]
- Zommiti, M.; Chikindas, M.L.; Ferchichi, M. Probiotics—Live Biotherapeutics: A story of success, limitations, and future prospects—Not only for humans. Probiotics Antimicrob. Proteins 2019, 12, 1266–1289. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigón, G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 2004, 97, 673–681. [Google Scholar] [CrossRef]
- Lee, Y.K.; Salminen, S. Handbook of Probiotics and Prebiotics, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–596. [Google Scholar] [CrossRef]
- Fefana Discusses Probiotics in Animal Feed—All about Feed. Available online: https://www.allaboutfeed.net/animal-feed/feed-additives/fefana-discusses-probiotics-in-animal-feed/ (accessed on 31 October 2021).
| Probiotics | Type of Probiotics | Subjects of Administration | Duration of Intervention | Diseases | Potential Effects | Mechanism of Therapeutic Action | References |
|---|---|---|---|---|---|---|---|
| Lactobacillus | L. acidophilus | Humans | 4 weeks | T2D | Improved the homoeostasis of glucose | Preservation and reduction of insulin sensitivity | [118] |
| Limosilactobacillus reuteri | Ovx Mice | 4 weeks | Menopausal bone loss | Loss in vertebral and femur bone was prevented, improvement in the density of bone | T-cells induced signals of osteoclast suppression by causing the reduction in osteoclastogenesis | [119] | |
| Lacticaseibacillus casei | Neonates | 12 months | Enteric Colonization | Fungal diseases such as enteric colonization is preventable with the consumption of probiotic | Modification in the ecology of fungi carries out the application of mechanisms potentially by LCC in the gut. Increased mucosal responses of IgA include a significant fungi exclusion and reduction in colonization ability as well | [120] | |
| L. crispatus | Women | 4 weeks | Recurrent UTI | Prevention of urinary tract infection such as vaginal flora infection and pregnancy issues due to urinary tract infection could be resolved | Probiotic was given as a vaginal suppository to indicate the reduction of infection in the urinary tract and enhanced the IgA | [121] | |
| L. gasseri | Rats | 12 weeks | Obesity | Obesity and other weight gain problems could be resolved by oral uptake | Probiotic extracted from the milk of human breast effectively imposed influence on adipose tissues either by destroying cells or reducing their number | [122] | |
| Lactiplantibacillus plantarum | Mice | 4 weeks | Spontaneous Colitis | Development of colitis due to the deficiency of IL usually under SPF conditions was prevented | Decrease the establishment of inflammatory colony inflammation due to mucosal IL | [123] | |
| Lacticaseibacillus rhamnosus | Epithelial cells | NR | Rotavirus | The development of an efficient immune system prevented rotavirus | Inoculation of IPEC-J2 cells with probiotics reduce the risk of rotavirus due to the anti-inflammatory properties | [124] | |
| Bifidobacterium | B. lactis | Mice | 12 weeks | Obesity | Reduced fat mass and weight gain | Significantly reduces adherence of mucosal bacteria in caecum and ileum | [125] |
| B. bifidum | Mice | 4 weeks | Inflammatory bowel disease | Assisted in controlling unusual responses of immunity in the tissues of intestine | Reduces lymphocyte infiltration and ameliorated the goblet cells reduction | [126] | |
| B. adolescentis | Cells | NR | Melanogenesis | Acted as antioxidant and inhibitory properties of melanoma made the B. adolescentis, a novel whitening agent for skin | Inhibition of tyrosinase action would lead to the reduction in melanogenesis, such as the melanoma process of cells | [127] | |
| B. infantis | Mice | 7 days | Inflammatory bowel disease | The permeability of intestine could be reduced to treat IBD | The reduction in the infiltration of neutrophils and the inflammatory colon is reported | [128] | |
| B. breve | Mice | NR | Alzheimer’s disease | Exhibit beneficial effects on peripheral tissues and the central nervous system and manages the neurodegenerative disorders | Non-viable bacterium metabolite or its components partially treat the cognitive decline while the specific probiotic suppresses the expressions and inflammation in the hippocampus | [129] | |
| B. longum | Mice | 10 days | Gut derived sepsis | Treat opportunistic infection and significantly treat immunocompromised patients | Less P. aeruginosa viable count in the jejunum is reported and significantly repressed the P. aeruginosa adherence to the cell’s monolayers | [130] | |
| Other Species | Escherichia coli | Humans | 12 weeks | Irritable bowel syndrome | Demonstrates beneficial effects to reduce the action of the syndrome of irritable bowel | Shows its action particularly in patients with enteric microflora having alteration, for instance, after antibiotics’ administration or gastroenterocolitis | [131] |
| Streptococcus thermophilus | Infants | 5 months | Diarrhea | The formula that is obtained by fermentation can cause a reduction in the severe diarrhea | A combination of Streptococcus thermophilus and Bifidobacterium breve are fermented and interact with the immune system of the intestine to prevent acute diarrhea | [132] | |
| Bacillus subtilis | Rabbits | 7 weeks | Immunodeficiency | Improvement in the mechanism of defense and immunity | Increases the weight of spleen and thymus prominently, provide innate immunity and induction of immunity on RK-13 cells | [133] | |
| Lactococcus lactis | Mice | 5 days | Colitis | Acute and chronic colitis could be effectively managed to prevent damage to epithelial tissues | L. lactis which releases TFF are involved in the Intragastric administration at the colonic mucosa, in distinction to the administration of TFF which is purified, demonstrated to heal and prevent acute colitis induced by DSS | [134] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjha, M.M.A.N.; Shafique, B.; Batool, M.; Kowalczewski, P.Ł.; Shehzad, Q.; Usman, M.; Manzoor, M.F.; Zahra, S.M.; Yaqub, S.; Aadil, R.M. Nutritional and Health Potential of Probiotics: A Review. Appl. Sci. 2021, 11, 11204. https://doi.org/10.3390/app112311204
Ranjha MMAN, Shafique B, Batool M, Kowalczewski PŁ, Shehzad Q, Usman M, Manzoor MF, Zahra SM, Yaqub S, Aadil RM. Nutritional and Health Potential of Probiotics: A Review. Applied Sciences. 2021; 11(23):11204. https://doi.org/10.3390/app112311204
Chicago/Turabian StyleRanjha, Muhammad Modassar Ali Nawaz, Bakhtawar Shafique, Maria Batool, Przemysław Łukasz Kowalczewski, Qayyum Shehzad, Muhammad Usman, Muhammad Faisal Manzoor, Syeda Mahvish Zahra, Shazia Yaqub, and Rana Muhammad Aadil. 2021. "Nutritional and Health Potential of Probiotics: A Review" Applied Sciences 11, no. 23: 11204. https://doi.org/10.3390/app112311204
APA StyleRanjha, M. M. A. N., Shafique, B., Batool, M., Kowalczewski, P. Ł., Shehzad, Q., Usman, M., Manzoor, M. F., Zahra, S. M., Yaqub, S., & Aadil, R. M. (2021). Nutritional and Health Potential of Probiotics: A Review. Applied Sciences, 11(23), 11204. https://doi.org/10.3390/app112311204










