A Prospective, Randomised, Controlled, Split-Face Clinical Trial to Assess the Safety and the Efficacy of Cold Atmospheric Plasma in the Treatment of Acne Vulgaris
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Size Rationale
2.2. Ethical Considerations and Study Registration
2.3. Inclusion and Exclusion Criteria
2.4. Study Procedures
2.5. Patient Enrolment
2.6. Primary Endpoints
2.7. Secondary Endpoints
2.8. Statistical Analyses
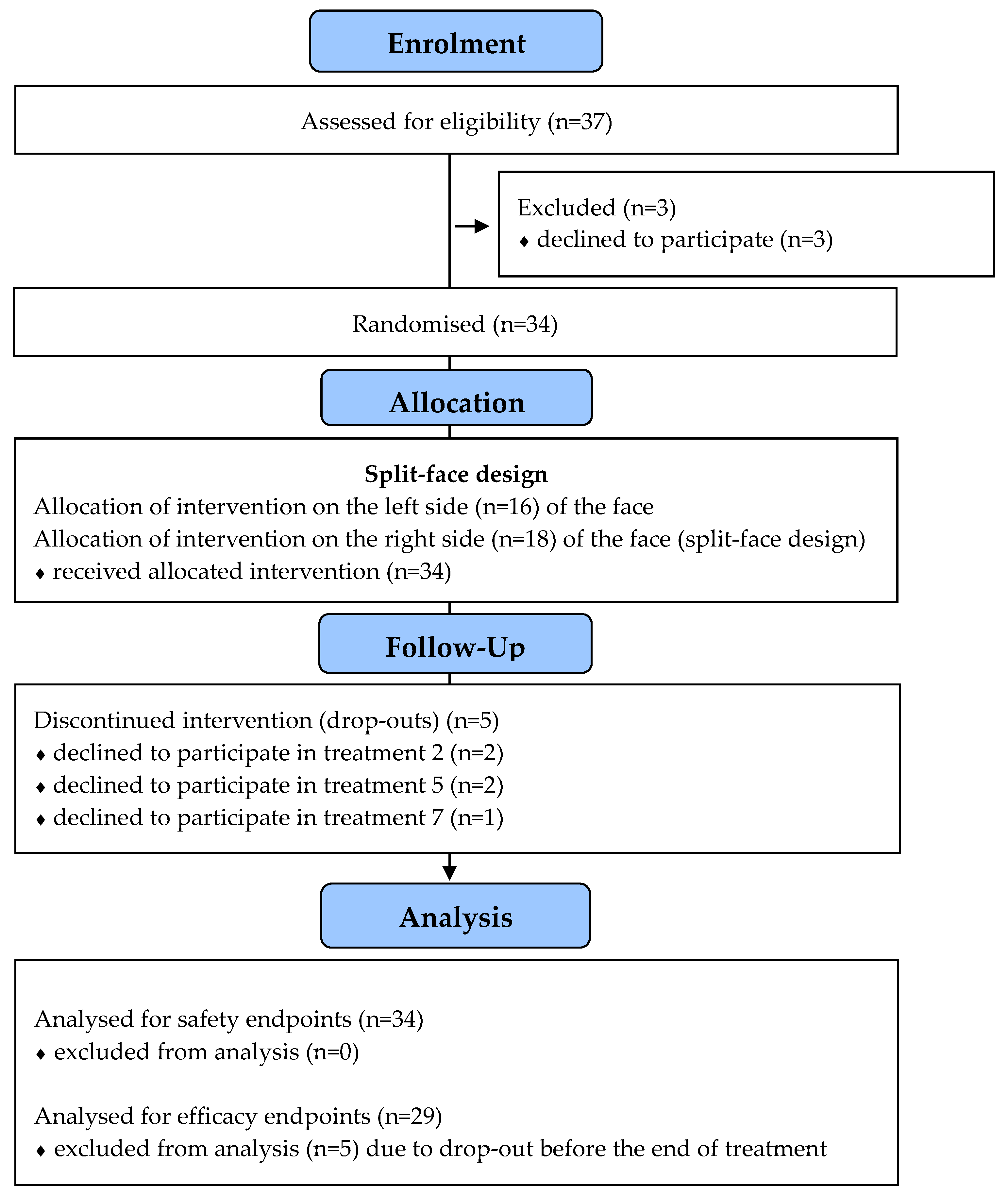
3. Results
3.1. Demographic and Treatment-Related Data
3.2. Primary Safety Endpoints
3.3. Secondary Safety Endpoint
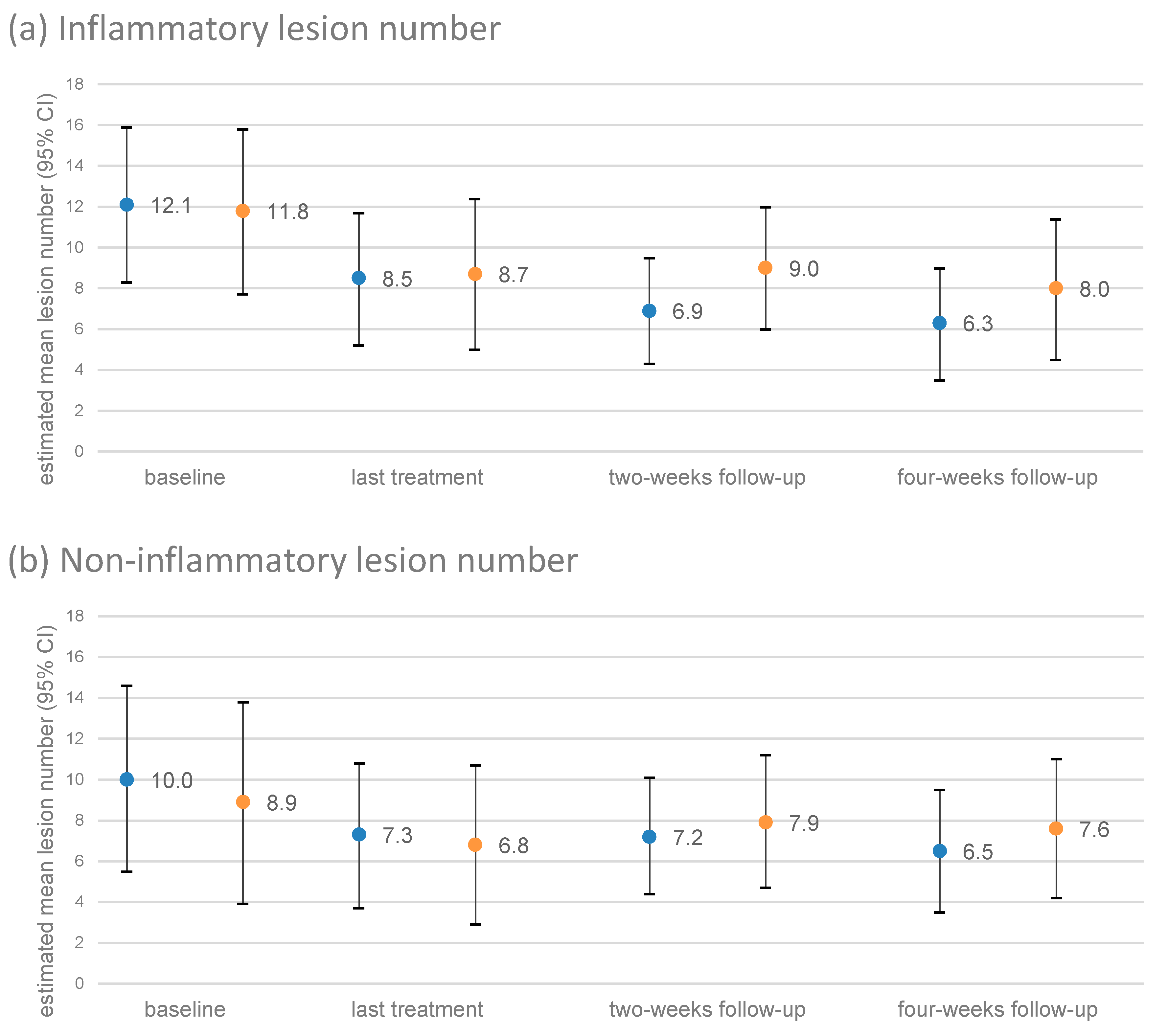
3.4. Course of the Number of Lesions
3.5. Primary Efficacy Endpoints
3.6. Secondary Efficacy Endpoints
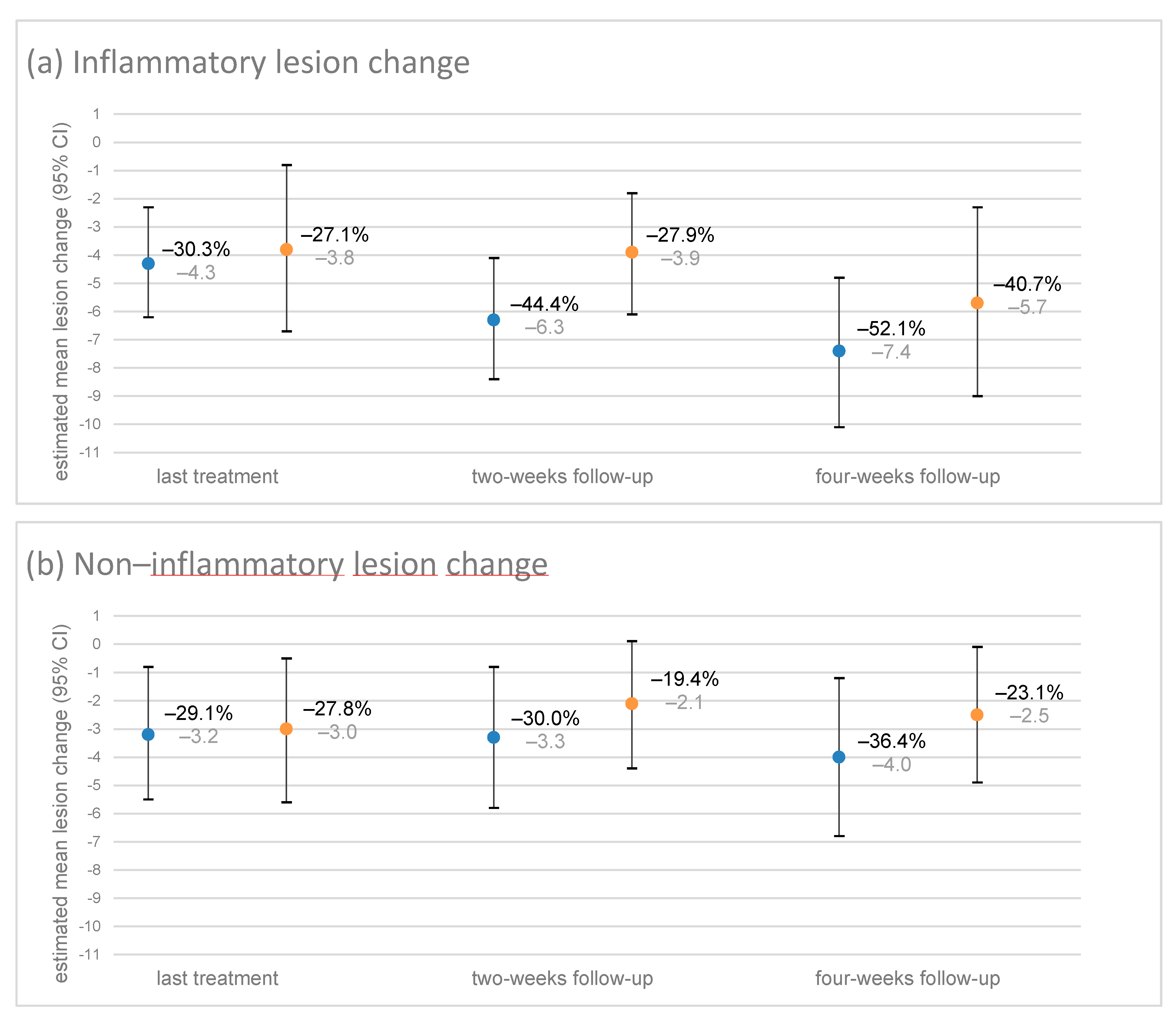
3.6.1. Change in Lesions until the Two- and Four-Week Follow-Ups
3.6.2. Subjective Aesthetic Change
3.6.3. Acne Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nast, A.; Dreno, B.; Bettoli, V.; Bukvic Mokos, Z.; Degitz, K.; Dressler, C.; Finlay, A.Y.; Haedersdal, M.; Lambert, J.; Layton, A.; et al. European evidence-based (S3) guideline for the treatment of acne—Update 2016—Short version. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1261–1268. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Barankin, B. Dermatology: How to manage acne vulgaris. Drugs Context. 2021, 10. [Google Scholar] [CrossRef]
- Heng, A.H.S.; Chew, F.T. Systematic review of the epidemiology of acne vulgaris. Sci. Rep. 2020, 10, 5754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, T.R.; Efthimiou, J.; Dreno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect. Dis. 2016, 16, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Brany, D.; Dvorska, D.; Halasova, E.; Skovierova, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [Green Version]
- Heinlin, J.; Isbary, G.; Stolz, W.; Morfill, G.; Landthaler, M.; Shimizu, T.; Steffes, B.; Nosenko, T.; Zimmermann, J.; Karrer, S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical applications of cold atmospheric plasma: State of the science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Heinlin, J.; Maisch, T.; Zimmermann, J.L.; Shimizu, T.; Holzmann, T.; Simon, M.; Heider, J.; Landthaler, M.; Morfill, G.; Karrer, S. Contact-free inactivation of Trichophyton rubrum and Microsporum canis by cold atmospheric plasma treatment. Future Microbiol. 2013, 8, 1097–1106. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Isbary, G.; Heinlin, J.; Karrer, S.; Klampfl, T.G.; Li, Y.F.; Morfill, G.; Zimmermann, J.L. Contact-free inactivation of Candida albicans biofilms by cold atmospheric air plasma. Appl. Environ. Microbiol. 2012, 78, 4242–4247. [Google Scholar] [CrossRef] [Green Version]
- Maisch, T.; Shimizu, T.; Li, Y.F.; Heinlin, J.; Karrer, S.; Morfill, G.; Zimmermann, J.L. Decolonisation of MRSA, S-aureus and E-coli by Cold-Atmospheric Plasma Using a Porcine Skin Model In Vitro. PLoS ONE 2012, 7, e34610. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Wiegand, C.; Beier, O.; Horn, K.; Pfuch, A.; Tolke, T.; Hipler, U.C.; Schimanski, A. Antimicrobial impact of cold atmospheric pressure plasma on medical critical yeasts and bacteria cultures. Skin Pharmacol. Physiol. 2014, 27, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.E.; Klämpfl, T.; Steffes, B.; Thomas, H. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Moelleken, M.; Jockenhofer, F.; Wiegand, C.; Buer, J.; Benson, S.; Dissemond, J. Pilot study on the influence of cold atmospheric plasma on bacterial contamination and healing tendency of chronic wounds. J. Dtsch. Dermatol. Ges. 2020, 18, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs. Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, V.A.; Richter, H.; Bob, A.; von Hutten, J.; Painsi, C.; Hugel, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef]
- Heinlin, J.; Zimmermann, J.L.; Zeman, F.; Bunk, W.; Isbary, G.; Landthaler, M.; Maisch, T.; Monetti, R.; Morfill, G.; Shimizu, T.; et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013, 21, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Shimizu, T.; Zimmermann, J.; Heinlin, J.; Al-Zaabi, S.; Rechfeld, M.; Morfill, G.; Karrer, S.; Stolz, W. Randomized placebo-controlled clinical trial showed cold atmospheric argon plasma relieved acute pain and accelerated healing in herpes zoster. Clin. Plasma Med. 2014, 2, 50–55. [Google Scholar] [CrossRef]
- Bogle, M.A.; Arndt, K.A.; Dover, J.S. Evaluation of plasma skin regeneration technology in low-energy full-facial rejuvenation. Arch. Dermatol. 2007, 143, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, M.; Stoffels, I.; Dissemond, J.; Schadendorf, D.; Roesch, A. Actinic keratoses treated with cold atmospheric plasma. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 37–39. [Google Scholar] [CrossRef]
- Boxhammer, V.; Li, Y.F.; Koritzer, J.; Shimizu, T.; Maisch, T.; Thomas, H.M.; Schlegel, J.; Morfill, G.E.; Zimmermann, J.L. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutat. Res.-Genet. Toxicol. Environ. 2013, 753, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Bosserhoff, A.; Unger, P.; Heider, J.; Shimizu, T.; Zimmermann, J.; Morfill, G.; Landthaler, M.; Karrer, S. Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environ. Mol. Mutagen. 2017, 58, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.; Masur, K.; von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res.-Genet. Toxicol. Environ. 2016, 798, 48–54. [Google Scholar] [CrossRef]
- Lademann, O.; Kramer, A.; Richter, H.; Patzelt, A.; Meinke, M.C.; Roewert-Huber, J.; Czaika, V.; Weltmann, K.D.; Hartmann, B.; Koch, S. Antisepsis of the follicular reservoir by treatment with tissue-tolerable plasma (TTP). Laser Phys. Lett. 2011, 8, 313–317. [Google Scholar] [CrossRef]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef]
- Graham, G.M.; Farrar, M.D.; Cruse-Sawyer, J.E.; Holland, K.T.; Ingham, E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br. J. Dermatol. 2004, 150, 421–428. [Google Scholar] [CrossRef]
- Leyden, J. Current issues in antimicrobial therapy for the treatment of acne. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 51–55. [Google Scholar] [CrossRef]
- Patel, D.J.; Bhatia, N. Oral Antibiotics for Acne. Am. J. Clin. Dermatol. 2021, 22, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kim, Y.H.; Lee, J.Y.; Lee, S.; Uhm, H.S.; Cho, G.; Park, B.J.; Choi, E.H. Inactivation of Propionibacterium acnes and its biofilm by non-thermal plasma. Curr. Appl. Phys. 2014, 14, S142–S148. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.J.; Kim, H.K.; Hong, J.Y.; Cho, S.B. Effects of argon and nitrogen plasma pulses on the skin and skin appendages in an in vivo animal model. Skin Res. Technol. 2020, 26, 81–90. [Google Scholar] [CrossRef]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.-F.; Morfill, G.E.; Bosserhoff, A.-K.; Karrer, S. Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS ONE 2015, 10, 0120041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arndt, S.; Schmidt, A.; Karrer, S.; von Woedtke, T. Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clin. Plasma Med. 2018, 9, 24–33. [Google Scholar] [CrossRef]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.-K. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, 79325. [Google Scholar] [CrossRef] [Green Version]
- Barton, A.; Hasse, S.; Bundscherer, L.; Wende, K.; Weltmann, K.; Lindequist, U.; Masur, K. Growth factors and cytokines are regulated by non-thermal atmospheric pressure plasma: P034. Exp. Dermatol. 2014, 23, e2–e52. [Google Scholar]
- Chutsirimongkol, C.; Boonyawan, D.; Polnikorn, N.; Techawatthanawisan, W.; Kundilokchai, T. Non-thermal plasma for acne and aesthetic skin improvement. Plasma Med. 2014, 4, 79–88. [Google Scholar] [CrossRef]
- Mariachiara, A.; Anna, V.; Alessandra, G.; Edoardo, G.P.; Stefania, B.; Mariateresa, R.; Piergiacomo, C.-P. Cold atmospheric plasma (CAP) as a promising therapeutic option for mild to moderate acne vulgaris: Clinical and non-invasive evaluation of two cases. Clin. Plasma Med. 2020, 19, 100110. [Google Scholar] [CrossRef]
- Conforti, C.; Chello, C.; Giuffrida, R.; di Meo, N.; Zalaudek, I.; Dianzani, C. An overview of treatment options for mild-to-moderate acne based on American Academy of Dermatology, European Academy of Dermatology and Venereology, and Italian Society of Dermatology and Venereology guidelines. Dermatol. Ther. 2020, 33, 13548. [Google Scholar] [CrossRef]
- Baldwin, H. Oral Antibiotic Treatment Options for Acne Vulgaris. J. Clin. Aesthet. Dermatol. 2020, 13, 26–32. [Google Scholar] [PubMed]
- Garner, S.E.; Eady, A.; Bennett, C.; Newton, J.N.; Thomas, K.; Popescu, C.M. Minocycline for acne vulgaris: Efficacy and safety. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ochsendorf, F.R. New developments in acne treatment: Role of combination adapalene–benzoylperoxide. Ther. Clin. Risk Manag. 2016, 12, 1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metelmann, H.-R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Nguyen, T.T.H.; Nguyen, T.H.M.; Nguyen, T.L. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clin. Plasma Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]

| Global Aesthetic Improvement Scale (GAIS) | Investigator Global Assessment (IGA) |
|---|---|
| 3 = very much improved: Optimal cosmetic result for the treatment in this subject. | 0 = clear skin with no inflammatory or non-inflammatory lesion |
| 2 = much improved: Marked improvement in appearance from the initial condition but not completely optimal for this subject. | 1 = almost clear, rare non-inflammatory lesions with no more than one small inflammatory lesion |
| 1 = improved: Obvious improvement in appearance from the initial condition. | 2 = mild, some non-inflammatory lesions, no more than a few inflammatory lesions |
| 0 = no change: The appearance is essentially the same as baseline. | 3 = moderate, up to many non-inflammatory lesions and may have some inflammatory lesions, but no more than one small nodular lesion |
| −1 = worse: The appearance is worse than the original condition. | 4 = severe, up to many non-inflammatory and inflammatory lesions, but no more than a few nodular lesions. |
| −2 = much worse: Marked worsening in appearance from the initial condition. | |
| −3 = very much worse: Obvious worsening in appearance from the initial condition. |
| Safety Population | Primary Efficacy Endpoint Population | |
|---|---|---|
| Number of patients | 34 | 29 |
| Age in years mean ± sd (range) | 26.1 ± 8.3 (18–54) | 26.9 ± 9.0 (18–54) |
| Sex | ||
| men | 5 (14.7%) | 4 (13.8%) |
| women | 29 (85.3%) | 25 (86.2%) |
| Fitzpatrick type | ||
| II | 19 (55.9%) | 15 (51.7%) |
| III | 14 (41.2%) | 13 (44.8%) |
| IV | 1 (2.9%) | 1 (3.4%) |
| Ethnic origin | ||
| Caucasian | 34 (100%) | 29 (100%) |
| Change | Lesions | Treatment Number | Treated Side | Untreated Side | Δ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | 95% CI | m | 95% CI | m | 95% CI | p | ||||||
| Last treatment—baseline | inflammatory | –4.3 (–30.3%) | –6.2 | –2.3 | –3.8 (–27.1%) | –6.7 | –0.8 | 0.5 | –1.8 | 2.8 | 0.669 | |
| 8 | –4.8 | –8.5 | –1.1 | –5.9 | –11.5 | –0.4 | –1.2 | –5.5 | 3.2 | 0.590 | ||
| 10 | –3.8 | –5.2 | –2.3 | –1.6 | –3.8 | 0.6 | 2.1 | 0.4 | 3.9 | 0.017 | ||
| non-inflammatory | –3.2 (–29.1%) | –5.5 | –0.8 | –3.0 (–27.8%) | –5.6 | –0.5 | 0.14 | –1.7 | 2.0 | 0.879 | ||
| 8 | –3.5 | –7.9 | 0.9 | –4.2 | –8.9 | 0.5 | –0.7 | –4.2 | 2.8 | 0.685 | ||
| 10 | –2.8 | –4.6 | –1.1 | –1.9 | –3.7 | 0.0 | 1.0 | –0.4 | 2.4 | 0.159 | ||
| Two-week follow-up to baseline | inflammatory | –6.3 (–44.4%) | –8.4 | –4.1 | –3.9 (–27.9%) | –6.1 | –1.8 | 2.3 | –0.1 | 4.8 | 0.061 | |
| 8 | –7.0 | –11.0 | –3.0 | –4.8 | –8.7 | –0.8 | 2.2 | –2.3 | 6.8 | 0.323 | ||
| 10 | –5.5 | –7.1 | –3.9 | –3.1 | –4.7 | –1.5 | 2.4 | 0.7 | 4.3 | 0.010 | ||
| non-inflammatory | –3.3 (–30.0%) | –5.8 | –0.8 | –2.1 (–19.4%) | –4.4 | 0.1 | 2.3 | –1.9 | 4.2 | 0.442 | ||
| 8 | –3.8 | –8.4 | 0.9 | –2.7 | –7.0 | 1.5 | 1.0 | –4.6 | 6.6 | 0.711 | ||
| 10 | –2.8 | –4.7 | –1.0 | –1.5 | –3.2 | 0.1 | 1.3 | –1.0 | 3.5 | 0.254 | ||
| Four-week follow-up to baseline | inflammatory | –7.4 (–52.1%) | –10.1 | –4.8 | –5.7 (–40.7%) | –9.0 | –2.3 | 1.8 | –0.1 | 3.7 | 0.068 | |
| 8 | –8.7 | –13.6 | –3.7 | –7.3 | –13.5 | –1.1 | 1.3 | –2.2 | 4.9 | 0.449 | ||
| 10 | –6.2 | –8.2 | –4.3 | –4.0 | –6.5 | –1.6 | 2.2 | –3.7 | –0.8 | 0.003 | ||
| non-inflammatory | –4.0 (–36.4%) | –6.8 | –1.2 | –2.5 (–23.1%) | –4.9 | –0.1 | 1.5 | –1.2 | 4.3 | 0.263 | ||
| 8 | –4.5 | –9.8 | 0.7 | –3.5 | –8.0 | 1.0 | 1.0 | –4.1 | 6.1 | 0.693 | ||
| 10 | –3.5 | –5.6 | –1.4 | –1.4 | –3.2 | 0.4 | 2.1 | 0.0 | 4.1 | 0.047 | ||
| Untreated Side | ||||||
|---|---|---|---|---|---|---|
| Treated Side | Worse | No Change | Improved | Total | p | |
| last treatment | worse | 1 | 0 | 0 | 1 | 0.004 |
| no change | 0 | 4 | 1 | 5 | ||
| improved | 0 | 11 | 12 | 23 | ||
| total | 1 | 15 | 13 | 29 | ||
| two-week follow-up | worse | 1 | 0 | 0 | 1 | 0.008 |
| no change | 1 | 5 | 1 | 7 | ||
| improved | 1 | 8 | 12 | 21 | ||
| total | 3 | 13 | 13 | 29 | ||
| four-week follow-up | worse | 1 | 0 | 0 | 1 | 0.013 |
| no change | 0 | 4 | 1 | 5 | ||
| improved | 1 | 8 | 14 | 23 | ||
| total | 2 | 12 | 11 | 29 | ||
| Untreated Side | ||||||
|---|---|---|---|---|---|---|
| Treated Side | Almost Clear Skin | Mild | Moderate | Total | p | |
| last treatment | almost clear skin | 0 | 1 | 0 | 1 | 0.132 |
| mild | 0 | 9 | 7 | 16 | ||
| moderate | 0 | 3 | 8 | 11 | ||
| total | 0 | 13 | 15 | 28 | ||
| two-week follow-up | almost clear skin | 1 | 2 | 0 | 3 | 0.132 |
| mild | 0 | 7 | 6 | 13 | ||
| moderate | 0 | 3 | 10 | 13 | ||
| total | 1 | 12 | 16 | 29 | ||
| four-week follow-up | almost clear skin | 0 | 0 | 0 | 0 | 0.157 |
| mild | 1 | 13 | 6 | 20 | ||
| moderate | 0 | 1 | 8 | 9 | ||
| total | 1 | 14 | 14 | 29 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karrer, S.; Berneburg, M.; Zeman, F.; Koller, M.; Müller, K. A Prospective, Randomised, Controlled, Split-Face Clinical Trial to Assess the Safety and the Efficacy of Cold Atmospheric Plasma in the Treatment of Acne Vulgaris. Appl. Sci. 2021, 11, 11181. https://doi.org/10.3390/app112311181
Karrer S, Berneburg M, Zeman F, Koller M, Müller K. A Prospective, Randomised, Controlled, Split-Face Clinical Trial to Assess the Safety and the Efficacy of Cold Atmospheric Plasma in the Treatment of Acne Vulgaris. Applied Sciences. 2021; 11(23):11181. https://doi.org/10.3390/app112311181
Chicago/Turabian StyleKarrer, Sigrid, Mark Berneburg, Florian Zeman, Michael Koller, and Karolina Müller. 2021. "A Prospective, Randomised, Controlled, Split-Face Clinical Trial to Assess the Safety and the Efficacy of Cold Atmospheric Plasma in the Treatment of Acne Vulgaris" Applied Sciences 11, no. 23: 11181. https://doi.org/10.3390/app112311181
APA StyleKarrer, S., Berneburg, M., Zeman, F., Koller, M., & Müller, K. (2021). A Prospective, Randomised, Controlled, Split-Face Clinical Trial to Assess the Safety and the Efficacy of Cold Atmospheric Plasma in the Treatment of Acne Vulgaris. Applied Sciences, 11(23), 11181. https://doi.org/10.3390/app112311181






