Abstract
A simple and practical spray method is employed to prepare a PVDF/PFOTES-SiO2 superhydrophobic composite coating on the AZ31B Mg alloy substrate. The morphology, composition, and water contact angle (CA) were measured by Fourier transform infrared spectroscopy (FTIR), field emission scanning electron microscope (FESEM) and contact angle measuring instrument. Hydrophilic nano-SiO2 is modified by PFOTES to obtain hydrophobicity. The influence of the mass of PFOTES-SiO2 to PVDF on the hydrophobic properties was studied. The wear resistance and stability of the composite coating have been investigated by immersion test, cross-cut adhesion test and friction test. Additionally, the corrosion resistance was measured by electrochemical workstation and salt spray corrosion test. The CA of PVDF/PFOTES-SiO2 coating is 161.3° and the sliding angle (SAs) is less than 2°. After 10× the sandpaper friction test, the superhydrophobic contact angle of the coating remained above 155°, and the sliding angle was less than 5°, which indicated that the prepared coating is a strong superhydrophobic coating with good wear resistance. The results of the electrochemical tests show that the superhydrophobic coating improved the anti-corrosion performance of Mg alloy, and the water contact angle is greater than 150° after 168 h salt spray corrosion test. Due to its excellent superhydrophobicity, wear resistance and anti-corrosion properties, the robust PVDF/PFOTES-SiO2 coating is considered to have great potential for future applications in the automotive and marine industries.
1. Introduction
Magnesium (Mg) and its alloys have been widely used in the automotive industry, optical equipment, electronics industry, aerospace, and other fields due to their low density, high specific strength and specific rigidity, excellent electromagnetic shielding performance, low processing cost, easy forming, etc. However, poor corrosion resistance of Mg alloys has limited their larger-scale applications, especially in special environments (such as seawater, organic/inorganic salt solutions, etc.) [1]. Thus, an anti-corrosion coating on Mg alloy is one of the most extensive and effective ways to improve corrosion resistance [2,3]. Many techniques can be developed using to prepare anti-corrosion coatings, including chemical conversion, anodic oxidation, micro-arc oxidation, vapor deposition, electrodeposition, superhydrophobic coatings, etc. [4,5,6,7,8]. Among all the methods, building a superhydrophobic coating is considered an efficient and promising method, which can isolate the corrosive medium and the anti-corrosion coatings, and thereby significantly enhancing the corrosion resistance of Mg alloys and extending the service life of the coatings [9].
Numerous techniques have been developed and applied to develop of superhydrophobic coatings on Mg alloys, including the hydrothermal process, chemical deposition, electrodeposition techniques, conversion coatings, polymer coatings, sol-gel coatings, laser processing, etc. [10,11,12,13,14,15]. In general, two essential features are required to construct a superhydrophobic surface on Mg alloys, i.e., micro-nano hierarchical structure, and a low surface energy chemistry. However, many methods have many drawbacks, such as multi-step process, poor mechanical stability, low impact resistance, etc., which restrict their large-scale and practical application [16]. Both surface roughness and low surface energy should be obtained in one step to overcome these disadvantages and simplify the complexity of multi-step processing. Moreover, it is essential and inevitable to propose and construct a durable superhydrophobic coating with excellent wear resistance.
Polyvinylidene fluoride (PVDF) has excellent thermal stability, excellent chemical stability and good processing properties, which is a semi-crystalline polymer, including amorphous and crystalline phases [17]. Due to its low surface energy and good physical and chemical properties, PVDF is widely used to prepare superhydrophobic coatings [18]. However, the softness of PVDF causes the superhydrophobic coating to be easily damaged by the friction surface, which reduces the adhesion to most substrates, resulting in a decrease or loss of superhydrophobicity [19].
It is appropriate to use PVDF as the matrix of superhydrophobic coating, the finely dispersed SiO2 particles is employed to improve the performances of superhydrophobic coating. In this work, a simple one-step spray technique is employed to manufacture PVDF/PFOTES-SiO2 superhydrophobic films on AZ31B Mg alloy. The wettability of the prepared coating can be adjusted by the mass ratio of PFOTES-SiO2 to PVDF. The adhesion performance and wear resistance of the superhydrophobic nanocomposite coating are discussed. In addition, the corrosion behavior was evaluated by electrochemistry, long-time immersion, and salt spray corrosion. The as prepared superhydrophobic composite surface not only exhibited excellent wear resistance, but also showed good corrosion resistance. It is known that spraying coating is a straightforward method and can be applied to a large surface with low cost. Therefore, the goal of this investigation is to prepare corrosion-resistant PVDF/PFOTES-SiO2 coatings on a large scale.
2. Experimental
2.1. Materials and Reagents
The AZ31B Mg alloy (composition: Al 2.98 wt %, Zn 0.84 wt %, Mn 0.34 wt %, Si 0.01 wt %, with the balance being Mg) was purchased from Dongguan Hongxing Metal Materials Co., Ltd. The dimension of the AZ31B plates was 70 mm × 50 mm × 5 mm. Fumed nano-silica (nano-SiO2, 15 nm in diameter) and 1H, 1H, 2H, 2H-perfluorooctyltriethoxysilane (PFOTES, 97%) with a chemical formula of CF3(CF2)5(CH2)2-Si(OCH2CH3)3 were purchased from Shanghai Adamas Chemical Reagent Co., Ltd (Shanghai, China). All the other reagents, including N, N-Dimethylacetamide, ethanol, strong ammonia (25% NH3), HCl, NaCl, were analytically pure and were obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
2.2. Preparation of the Superhydrophobic Surfaces
First, all the plate samples were sequentially polished with 320, 800, and 2000 grit silicon carbide water abrasive papers prior to the construction of a superhydrophobic film on the AZ31B Mg alloy surface. After that, the plate sample was ultrasonically cleaned three times in ethanol and water, followed by air drying.
Then, 1 g of nano-silica was placed in a 1 wt % PFOTES ethanol solution (10 mL) and magnetically stirred for 12 h at room temperature. The obtained silica sol was centrifuged three times in sequence, washed with ethanol, dried, and ground into a fine powder with a mortar. Next, 0.1–0.6 g PVDF powder was magnetically stirred in 10 mL N,N-Dimethylacetamide (DMAc) until it was completely dissolved to form a transparent solution, respectively, and then fluorinated PFOTES-SiO2 particles of different masses were added to the solution and stirring was continued for 12 h to form a uniform suspension.
Superhydrophobic coatings are made using the spraying method. A spray gun (Infinity, Harder and Steenbeck, Germany) was used to spray 4 mL of the prepared suspension onto the surface of the AZ31B alloy at a pressure of 0.2 MPa N2. The spray gun’s tip is 15 cm away from the surface of the AZ31B alloy during the spraying process. To speed up solvent evaporation, a hot plate is used to heat the AZ31B alloy sample pretreated during spraying. The samples were then heated for 1 h at 100 °C after coating. The preparation process is shown in Scheme 1.
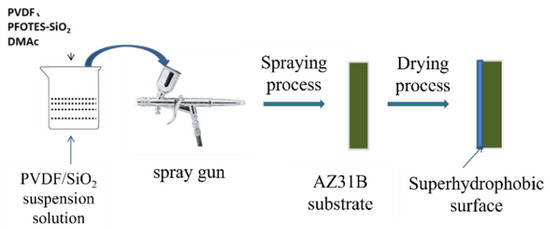
Scheme 1.
Schematic illustration of the preparation of superhydrophobic surface coatings.
2.3. Characterization
The morphologies and structures of the prepared materials were observed using field emission scanning electron microscopy (FESEM, Hitachi SU8010) and Fourier transform infrared (FTIR, Thermo Nicollet). The powder of the superhydrophobic surface coating scratched from the superhydrophobic plate surface was ground with KBr and pelletized for the measurement of FTIR spectra in transmittance mode. The measurement parameters were set as a wavenumber range from 4000 to 650 cm−1 with a resolution of 4 cm−1.
DSA25 optical contact angle measuring instrument (Kruss, Germany) was used to measure the static contact angle and sliding angle of different solution droplets with a volume of 5 μL on the superhydrophobic surface at room temperature according to the static drop method. In about 5 s, the contact angle value is determined. All measurements were repeated five times, with the average value being used for discussion. Before measuring the static contact angle and sliding angle of the coating after air exposure, immersion test or salt spray test, the sample was rinsed three times with deionized water and dried at 40 °C for half an hour.
2.4. Electrochemical Tests
For corrosion resistance evaluation of the obtained samples, electrochemical measurements including polarization curves and electrochemical impedance spectroscopy (EIS). All electrochemical measurements were performed using a CHI604E electrochemical workstation (Shanghai Chenghua Instrument Co., Ltd., Shanghai, China). A three-electrode device was used, with a platinum plate serving as an auxiliary electrode, a saturated calomel electrode serving as a reference electrode, and the test sample serving as a test electrode. The tested sample’s exposure area was 1 cm2. After immersing the sample in the corrosive medium for 30 min, open-circuit potential (OCP) was obtained for experiments.
Polarization curves were obtained in 3.5 wt % NaCl solutions within a potential range of open circuit potential (OCP) ± 250 mV at a scan rate of 1 mV s−1. The corrosion current density (Icorr), corrosion potential (Ecorr), and Tafel constant were determined using the extrapolation method. The polarization tests were repeated five times to confirm the dependability of the electrochemical parameters. EIS tests were conducted at open circuit potential (OCP) and the measuring frequency was ranged from 105 Hz down to 10−2 Hz, with a sinusoidal signal amplitude of 10 mV.
The samples were immersed in the corrosive medium for 30 min prior to the electrochemical tests.
2.5. Corrosion Tests
The samples were tested by immersing in 3.5 wt % NaCl solution, immersing in different pH solutions, exposing to air, and salt spray tests to investigate their corrosion resistance and stability in aqueous medium and atmospheric conditions.
For the corrosion test in atmospheric conditions, the samples were placed into a salt spray test chamber (LYW-015, Shanghai Lanbao Testing Equipment Manufacturing Co., Ltd., Shanghai, China), and the salt spray test is tested following ASTM B117. The sample was placed at a 30° angle with the plane of the corrosion box during the experiment, and the solution in the experiment box was fully atomized to simulate the slow deposition of salts on the surface of the sample in the actual environment. The salt spray chamber temperature is 35 °C, the pressure barrel temperature is 47 °C, and the 5 wt % NaCl solution has a pH value of 6.5–7.2. According to the requirements of the equipment, the inlet pressure is 0.35 MPa, the spray pressure is 0.05 MPa, and the salt spray precipitation is 1–2 mL/h. Every 24 h during the salt spray corrosion test, samples were taken out of the salt spray corrosion chamber to observe surface corrosion and test the wettability of the surface.
3. Results and Discussion
3.1. Surface Morphology and Composition
As we all know, the wettability of a solid surface is determined by the geometric microstructure and chemical composition of the surface [20]. Due to the presence of many hydroxyl groups, the surface of nano-SiO2 particles is hydrophilic. In addition, its surface energy is relatively high, which easily leads to agglomeration of SiO2 nanoparticles. Fluorosilane can effectively modify hydroxyl-rich surfaces, thereby reducing the surface energy of solids [21]. Figure 1 shows the FTIR spectra of unmodified SiO2 and PFOTES-SiO2. Compared with the infrared spectrum of unmodified SiO2, the characteristic peaks of 1120, 702 and 660 cm−1 appear in PFOTES-SiO2. This is attributed to the stretching vibration of CF, CF2 or CF3 [22], indicating that PFOTES has successfully modified SiO2 nanoparticles [21].
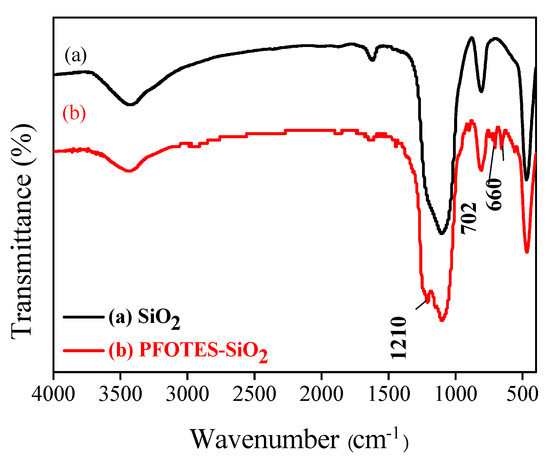
Figure 1.
FTIR spectra of (a) SiO2, (b) PFOTES–SiO2.
The influence of the mass ratio of PFOTES-SiO2 to PVDF on the wettability is investigated. The surface wettability of PVDF/PFOTES-SiO2 coatings with different mass ratios is tested with a contact angle measuring instrument, and the results are shown in Figure 2. Figure 2a shows the effect of PFOTES-SiO2 content on wettability (the amount of PVDF is determined to be 0.3 g). It can be seen from Figure 2a that when the coating contains only PVDF, the contact angle is 97.2°. As the content of PFOTES-SiO2 in the coating increases, the contact angle continues to increase. When the content of PFOTES-SiO2 is 0.10 g, the contact angle reaches the maximum value of 163.1°. However, with the increase of PFOTES-SiO2, the contact angle began to decrease, but it remained greater than 150°. This is because the increase of hydrophobic nanoparticles increases the roughness of the coating and reduce the surface energy, thereby improving the hydrophobicity of the magnesium alloy surface. However, with the excessive content of nanoparticles, PVDF as a film-forming agent cannot effectively combine the nanoparticles, thereby reducing the uniformity of the dispersion of nanoparticles in the coating, thus reducing the wettability of the coating surface. When the content of nanoparticles is excessive, PVDF as a film-forming agent cannot effectively bond the nanoparticles, thereby reducing the uniformity of the dispersion of nanoparticles in the coating, and thus reducing the wettability of the coating surface.
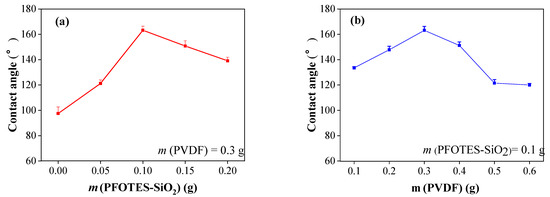
Figure 2.
Influence of each variable on wettability of the surface: (a) PFOTES-SiO2, (b) PVDF.
Figure 2b shows the effect of PVDF content in the coating on the surface wettability (the amount of PFOTES-SiO2 is determined to be 0.1 g). It can be seen that the contact angle in the coating increases first and then decreases with the increase of PVDF content. When the PVDF content is 0.3 g, the contact angle (CA) reaches the maximum value (163.1°). This is because when the PVDF content is low, PVDF cannot completely disperse the PFOTES-SiO2 in the coating during the phase separation and film formation process, resulting in the coating contact angle being less than 150°. As the content of PVDF increases, PVDF can wrap the entire nanoparticles, so the surface contact angle continues to increase. When the content of PVDF is too much (m(PVDF) > 0.5 g), the spraying solution is too viscous, resulting in uneven dispersion of the solution during the preparation process, thus reducing the hydrophobic performance. As mentioned above, by adjusting the mass of PVDF to PFOTES-SiO2 in the coating, a suitable surface microstructure can be obtained, thereby obtaining a stable superhydrophobic coating. Based on the results of water contact angle tests, the optimal value of coating preparation is obtained (m(PVDF) = 0.3 g and m(PFOTES-SiO2) = 0.1 g), so that the superhydrophobic surface prepared based on the optimal value is studying in stability performance, corrosion performance, etc.
Figure 3 displayed the SEM images of the bare AZ31B Mg alloy substrate and the superhydrophobic surface. It can be seen from Figure 3a that the magnesium alloy surfaces appear smooth except for several scratches by polishing. Figure 3b–d show the SEM images of the superhydrophobic surface at different magnification. Figure 3b show that the PVDF/PFOTES-SiO2 coating is uniformly and densely covered on the surface of the Mg alloy. It can be seen from Figure 3c,d that the aggregated PVDF and PFOTES-SiO2 form countless protrusions of different sizes, and the pore structure formed between these protrusions can trap air [23], thereby reducing the actual contact area with the droplets, thus covering the Mg alloy surface with PVDF/PFOTES-SiO2 coating exhibits superhydrophobicity, and the contact angle and sliding angle (SA) of the superhydrophobic surface reach 163.1° and 1.6°, respectively.
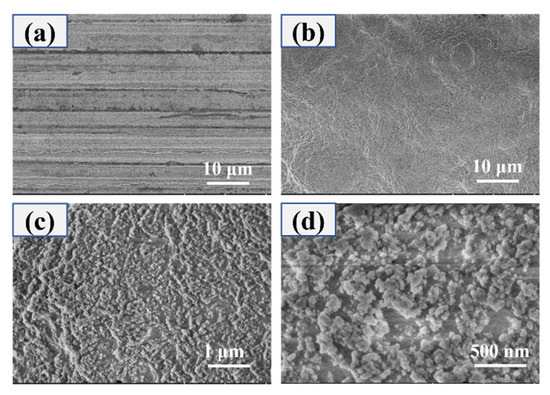
Figure 3.
FESEM images of (a) the bare AZ31B Mg alloy and (b–d) the super-hydrophobic surface under magnifications.
Based on the optimal solution ratio, the chemical composition of the composite coating was analyzed by FT-IR. It can be seen from Figure 4 that the peak at 3458 cm−1 is attributed to the stretching vibration peak of the OH bond, and the 2971 and 2871 cm−1 are attributed to the –CH2CH3 absorption peaks [24], 462, 866 and 1150 cm−1 are attributed to Si–O–Si symmetric stretching vibration, bending vibration and rocking vibration, respectively. The peaks at 574, 666, and 1206 cm−1 are attributed to CF, CF2 or CF3, respectively [25]. The above peaks are derived from PFOTES-SiO2, and the vibration peak at 1143 cm−1 is the –CH2 deformation swing vibration in PVDF [24]. The FTIR results indicate that the hydrophobically modified SiO2 nanoparticles are successfully compounded into the coating.
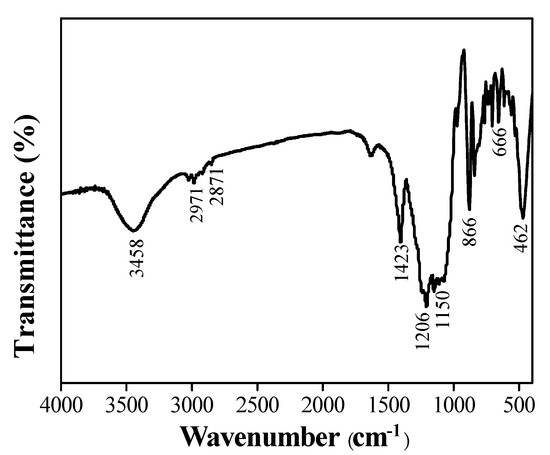
Figure 4.
FTIR spectrum of PVDF/PFOTES–SiO2 superhydrophobic surface.
3.2. Stability and Wear Resistance of Superhydrophobic Surfaces
The chemical stability of the superhydrophobic surface is a key factor that determines its feasibility in industrial applications. As a result, using an acid-based solution and air exposure method, the chemical stability of the obtained superhydrophobic surface was investigated.
Figure 5a shows the contact angle and sliding angle of water droplets of different pH solutions to the superhydrophobic surface. The contact angle is maintained above 154° and the sliding angle is below 10° in the pH range of 1–14, indicating that acidity and alkalinity have little effect on the superhydrophobic properties of the prepared superhydrophobic coating. To investigate the change of surface wettability of superhydrophobic samples in the air, the prepared samples were exposed to air at a room temperature of 20–25° and relative humidity of 30–50%, and the contact angle and sliding angle was tested every 10 days, as shown in Figure 5b. It can be found that the superhydrophobic surfaces maintain good superhydrophobicity after exposing it to air for 60 days, the contact angle remains above 155°, and the sliding angle remains below 5°, indicating that the superhydrophobic surface has stability under air conditions [26,27]. Figure 5c shows the contact angle and sliding angle of the superhydrophobic surface immersed in 3.5 wt % NaCl solution. The results show that the surface is still superhydrophobic after being immersed in 3.5 wt % NaCl for 7 days (contact angle is 155.2°, sliding angle is 3.2°).
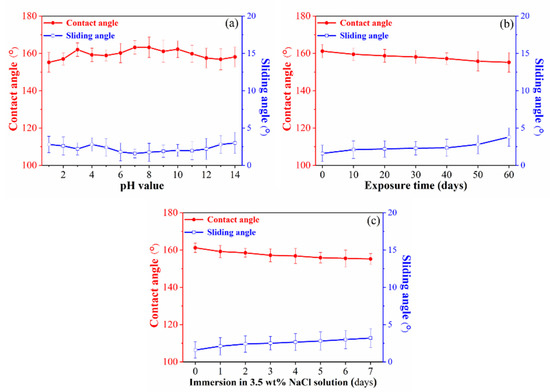
Figure 5.
The CA and the SA of the superhydrophobic surfaces (a) under pH value, (b) exposure to air, and (c) immersed in 3.5 wt % NaCl solution).
Most applications superhydrophobic coatings are based on their repellence with aqueous solutions. The functional properties of the superhydrophobic coatings are usually characterized by the value of the contact angle produced by the droplets on the surface of these materials in a short period of time. However, if the superhydrophobic surfaces are used in the industrial field, it is necessary to study the characteristics and stability of the wetting behavior of the prepared superhydrophobic surface in long-term contact with an aqueous medium [14]. Therefore, the chemical durability of superhydrophobic surfaces to acidic, neutral, or alkaline aqueous solutions has been investigated by measuring the static water contact angle and sliding angle of the superhydrophobic surface before and after immersion in aqueous solutions of different pH values, and the results are shown in the Figure 6. After immersion in the acidic solutions at pH 4.0 and neutral aqueous at pH 7.0, the static water contact angles and sliding angle of the superhydrophobic surfaces decreases slightly as the immersion time increases. The static water contact angles decreased from 161.0° to 157.0° and from 162.2° to 159.8° within 120 min after immersion in the acidic solution and the neutral solution, respectively. And the sliding angle increased 2.4° and 0.2° in pH 4.0 solutions and pH 7.0 solutions, respectively. However, within immersion for 100 min in alkaline solutions (pH 10.0), the static water contact angles gradually decreased to 150.2°. As the immersion time is further extended, the surface loses its superhydrophobicity. These results indicate that although the prepared superhydrophobic surface exhibits high chemical durability in neutral and acidic aqueous solutions, it has low chemical durability in alkaline aqueous solutions. This may be due to the degradation of the PVDF surface layer by prolonged contact with alkaline solution, resulting in a decrease in plasticity and loss of superhydrophobicity of the coating [28].
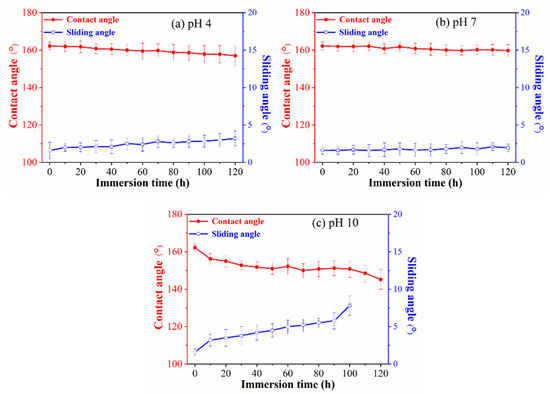
Figure 6.
Changes CA and the SA of the superhydrophobic surfaces as a function of immersion time in aqueous solutions at pHs (a) 4.0, (b) 7.0, and (c) 10.0, measured at five different points on the same samples. The error bars for each plot indicate the maximum and minimum water contact angles.
The coating adhesion cross-cut method test standard (ASTM D 3359) is used to assess the adhesion between the superhydrophobic film and the magnesium alloy substrate [29]. The experiment was carried out with Elcometer 107, which was used to make six parallel cuts with equal spacing (1 mm) on the coating, and then cut perpendicularly the cuts with the same number and spacing as the former, and the adhesion was evaluated by the peeling area of the coating surface. The crosshatch tape adhesion test for composite coatings is shown in photos in Figure 7. The lattice does not fall off and the cut edge is smooth after the cross-cut tape adhesion test. Therefore, the ASTM 5B rating for the superhydrophobic coating denotes the highest substrate adhesion.

Figure 7.
Crosshatch tape adhesion test for the composite coating.
The superhydrophobic coating’s abrasion resistance was tested using sandpaper. Scheme 2 illustrates the test device. A 50 g weight and 1200-grit sandpaper make up most of the test device. The superhydrophobic film is first placed in contact with the sandpaper, then the weight is placed on the magnesium alloy that has been prepared. The sample is then rotated 90° (the side of the film facing the sandpaper) and moved another 10 cm in the opposite direction after the superhydrophobic film has been pushed 10 cm in the opposite direction. A wear cycle is a term for the entire process described above. Following the abrasion test, the superhydrophobic film’s contact angle and sliding angle were measured. Figure 8 shows that after 10 cycles of abrasion test, the contact angle of the coating dropped from 161.3° to 154.2°, and the sliding angle increased from 1.6° to 4.5°, indicating that the composite coating still maintains superhydrophilicity.
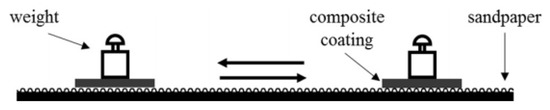
Scheme 2.
The schematic of the friction test.
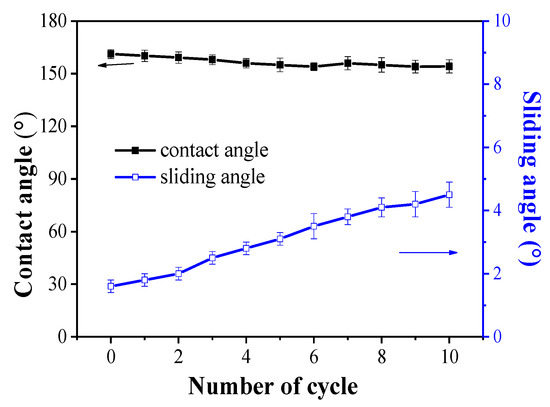
Figure 8.
The hydrophobic performance of superhydrophobic surface after 10× abrasion testing.
3.3. Corrosion Resistance Properties
The superhydrophobic surface is in the Cassie–Baxter state, and its surface micro/nano structure can trap air, thereby increasing the contact angle with water [30]. When the super-hydrophobic surface is immersed in the corrosive medium, the micro/nano structure is filled with a large amount of air, and the “air cushion layer” formed is similar to the dielectric between parallel plate capacitors, which can inhibit the electron transfer between the corrosive medium and the substrate. Figure 9 shows the polarization curve test results of AZ31B Mg alloy substrate and PVDF/PFOTES-SiO2 superhydrophobic coatings. It can be seen from Figure 9 that fitting the Tafel line according to the experimental data, the corrosion current densities (Icorr) of bare AZ31B Mg alloy and superhydrophobic coatings can be obtained 9.76 ± 1.05 × 10−6 A/cm2 and 6.18 ± 2.43 × 10−9 A/cm2, respectively, the corrosion voltage (Ecorr) is −1.54 ± 0.05 V and −1.32 ± 0.04 V, respectively.
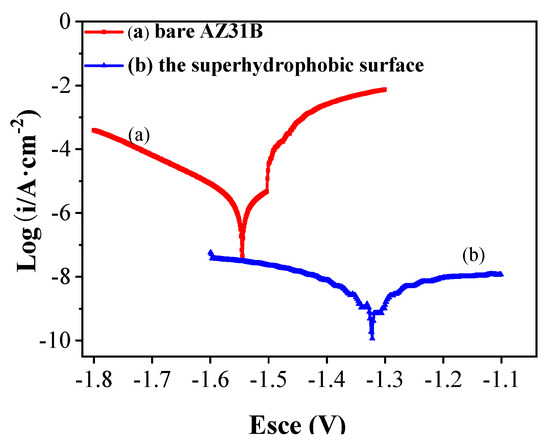
Figure 9.
Polarization curve of AZ31B Mg alloy surface: (a) pre–treated magnesium alloy, (b) the PVDF/PFOTES-SiO2 super-hydrophobic surface on Mg alloy.
However, it can be found from Figure 9a that the anode polarization curve does not show a broad Tafel area, and the anode curve has an active/passive transition, which may be the existence of the dissolution reaction and the initiation of the passivation reaction did not lead to a clear experimental anode Tafel area [31]. Compared with the polarization curve of bare AZ31B Mg alloy, the polarization curve of superhydrophobic coating (Figure 9b) is quite different. The anode curve and cathodic curve of superhydrophobic coating do not conform to Tafel’s law. This is maybe attribute to the air cushion between the coatings and the NaCl solution, which prevents the corrosive solution from the surface of the magnesium alloy [14]. Although the application of dynamic polarization curve to superhydrophobic surface has certain limitations, the fitting results also indicate that the superhydrophobic surface improves the corrosion resistance of Mg alloy.
To better understand the influence of superhydrophobic coating on the corrosion resistance of magnesium alloys, EIS test is considered as an effective way to analyze the corrosion characterization of coated or modified metals [32]. The corrosion resistances of the as-prepared super-hydrophobic surface were further explored by the Nyquist plots. Figure 10 shows the Nyquist plots of the bare AZ31B Mg alloy substrate and as-prepared super-hydrophobic surface in 3.5 wt % NaCl solution. The results show quite different capacitive loops that can be attributed to the charge transfer resistance of the corrosion process [33]. Compared with the bare AZ31B Mg alloy, the capacitive loops of the as prepared superhydrophobic surface have a widespread trend attributed to a protective surface film. The diameter of the capacitor circuit is usually proportional to the corrosion rate, that is, the larger the diameter, the smaller the corrosion rate, which shows that the super-hydrophobic surface has better corrosion resistance.
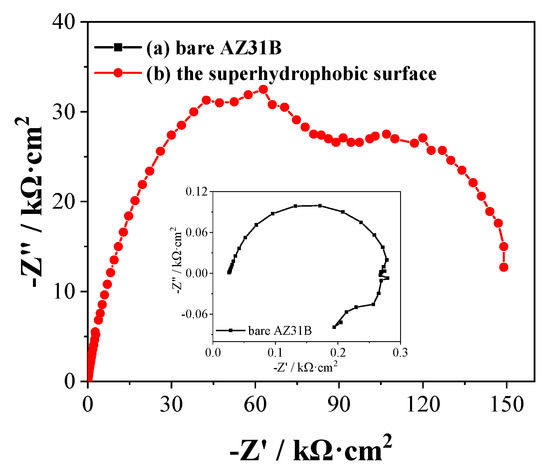
Figure 10.
The Nyquist plots obtained from (a) pretreated magnesium alloy, (b) the PVDF/PFOTES-SiO2 super-hydrophobic surface on Mg alloy after immersion in 3.5 wt % NaCl aqueous solution for 30 min.
A continuous neutral salt spray test was performed in a salt spray test box following the ASTM B117 standard. The samples were taken out every 24 h to observe the macroscopic changes on the surface of the samples and photograph them with a digital camera. The surface of the sample after the salt spray experiment is shown in Figure 11. The surface of the bare AZ31B has been completely corroded after a 24 h salt spray test. The depth of surface pitting pits continues to increase as corrosion time increases. However, after the 96 h salt spray test, the PVDF/PFOTES-SiO2 coating gradually showed salt spray deposition and pitting corrosion on the surface. The corrosion area on the surface continues to grow as the salt spray time is extended. Although the majority of the surface area retains the film after 168 h of salt spray corrosion, it can be seen that there are obvious bubbling on the surface. Although numerous studies have shown that superhydrophobic coatings can improve the corrosion resistance of metals [33,34], the durability of corrosion resistance during actual use is closely related to the adhesion between the superhydrophobic coating and the substrate [35].
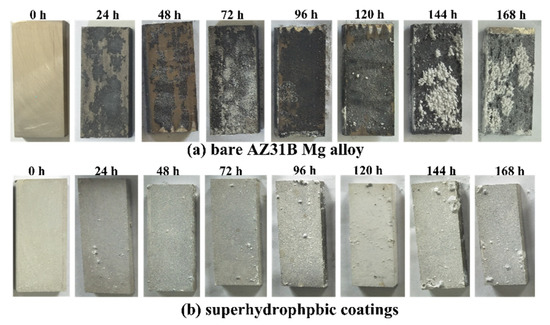
Figure 11.
The optical surface photo after salt spray experiment: (a) bare AZ31B Mg alloy, (b) superhydrophobic coatings. The dimensions for all samples were 70 × 50 × 5 mm).
SEM was used to characterize the sample at the micro-scale after observing the surface of the sample after the salt spray experiment at the macro level. The results are shown in Figure 12. After salt spray corrosion, the surface of bare AZ31B is composed of flakes that resemble petals, which is due to the contact between the corrosive medium and the magnesium alloy substrate to form a film containing MgO and Mg(OH)2 [11]. However, as shown in Figure 12d, the superhydrophobic coating surface morphology barely changed before and after corrosion, indicating that salt spray corrosion did not affect the structure of the superhydrophobic composite coating. The contact angle of the sample surface was measured after the salt spray experiment. Due to the composite coating maintains its surface integrity after 144 h of salt spray corrosion, the contact angle is still greater than 150°, as shown in Figure 13, which corresponds to the appearance of the sample in the digital photo of Figure 11.
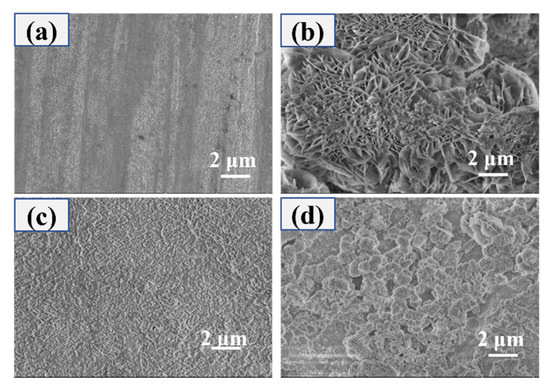
Figure 12.
The surface morphology before and after the salt spray experiment (a) bare AZ31B Mg alloy—0 h, (b) bare AZ31B Mg alloy—168 h, (c) PVDF/SiO2 coating—0 h, (d) PVDF/PFOTES-SiO2 coating—168 h.
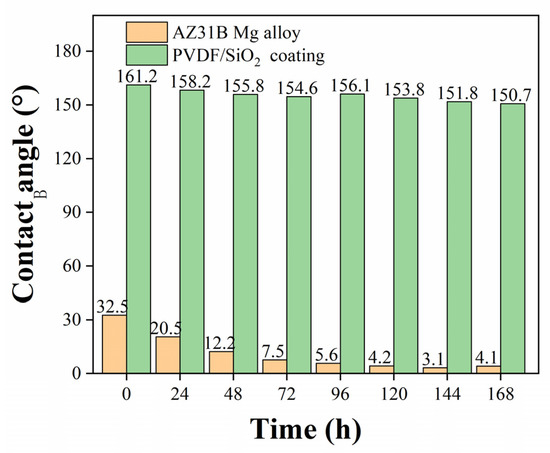
Figure 13.
The contact angle after salt spray experiment of superhydrophobic coating.
The long-term corrosion performance of the samples after salt spray corrosion was studied through the dynamic polarization curve [14,15], and the curves are recorded in Figure 14. Parameters of the potentiodynamic polarization were listed in Table 1. The parameters clearly showed that with the extension of salt spray corrosion time, the Icorr gradually decreases. The Icorr changed from (6.18 ± 2.34) × 10−9 A/cm2 to (5.58 ± 2.35) × 10−7 A/cm2 after salt spray for 168 h, but it is still an order of magnitude lower than Icorr of brae AZ31B Mg alloy, indicating that durability of the as prepared superhydrophobic.
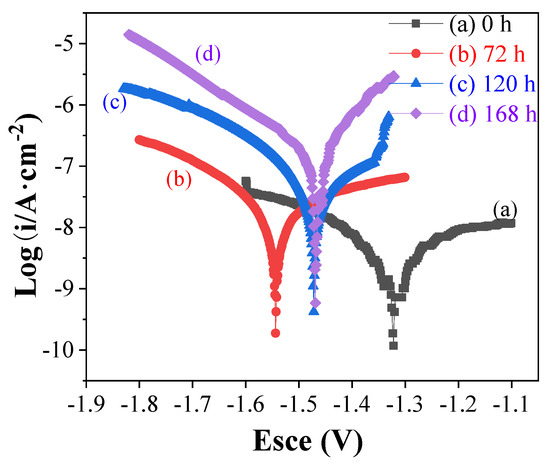
Figure 14.
Potentiodynamic polarization curves of the superhydrophobic coatings before and after salt spray experiment (a: 0 h, b: 72 h, c: 120 h, d: 168 h).

Table 1.
Corrosion parameters of the samples.
4. Conclusions
In summary, a stable superhydrophobic PVDF/PFOTES-SiO2 coatings on AZ31B Mg alloy were successfully fabricated by a simple and facile spraying method. Surface wettability can be adjusted by changing the mass ratio of PVDF to PFOTES-SiO2, and a coating with CA = 161° and SA < 2° is produced, which has good mechanical strength and superior corrosion resistance. This is owning to the “synergistic effect” of the matrix PVDF and the dispersed SiO2 particles to form a network structure and low surface energy coatings. In addition, the contact angle of the composite coating only decreased by 4% after 2 m sandpaper abrasion, and the results of the Tafel and EIS plots indicate that prepared coatings have superior corrosion protection ability to AZ31 Mg alloy. It is believed that the prepared coating can provide long-term protection for magnesium alloys. The method reported in this article will provide new ideas for the design of new anti-corrosion coatings.
Author Contributions
Z.Q.: Investigation, Methodology, Data curation, Funding acquisition, Writing—original draft preparation. Z.L.: Methodology, Formal analysis, Software. S.W.: Investigation, Methodology. X.Y.: Formal analysis, Software, Supervision, Project administration. Z.W.: Conceptualization, Validation, Resources, Writing—review & editing, Supervision, Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
Qinghai Provincial Science and Technology Project, China: 2019-ZJ-956Q; National Natural Science Foundation of China: U20A20147; Thousand Talent Program of Qinghai Province, China: 2019; the CAS “Light of West China” Program: 2018.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zhang, G.; Wu, L.; Tang, A.; Zhang, S.; Yuan, B.; Zheng, Z.; Pan, F. A Novel Approach to Fabricate Protective Layered Double Hydroxide Films on the Surface of Anodized Mg-Al Alloy. Adv. Mater. Interfaces 2017, 4, 1700163. [Google Scholar] [CrossRef]
- Chang, L.R.; Cao, F.H.; Cai, J.S.; Liu, W.J.; Zhang, Z.; Zhang, J.Q. Influence of electric parameters on MAO of AZ91D magnesium alloy using alternative square-wave power source. Trans. Nonferrous Met. Soc. China 2011, 21, 307–316. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, A.M.; Cao, F.Y.; Dargusch, M.S. Review of Recent Developments in the Field of Magnesium Corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liu, Z.J.; Wang, E.Q.; Yuan, R.X.; Gao, D.; Zhang, X.G.; Zhu, Y.J. A robust superhydrophobic PVDF composite coating with wear/corrosion-resistance properties. Appl. Surf. Sci. 2015, 332, 518–524. [Google Scholar] [CrossRef]
- Gray-Munro, J.; Campbell, J. Mimicking the hierarchical surface topography and superhydrophobicity of the lotus leaf on magnesium alloy AZ31. Mater. Lett. 2017, 189, 271–274. [Google Scholar] [CrossRef]
- Sonmez, S.; Aksakal, B.; Dikicic, B. Influence of hydroxyapatite coating thickness and powder particle size on corrosion performance of MA8M magnesium alloy. J. Alloy Compd. 2014, 456, 125–131. [Google Scholar] [CrossRef]
- Qiu, X.; Wan, P.; Tan, L.L.; Fan, X.M.; Yang, K. Preliminary research on a novel bioactive silicon doped calcium phosphate coating on AZ31 magnesium alloy via electrodeposition. Mater. Sci. Eng. 2014, 36, 65–76. [Google Scholar] [CrossRef]
- Wu, Y.; Hang, T.; Yu, Z.; Xu, L.; Li, M. Lotus leaf-like dual-scale silver film applied as a superhydrophobic and self-cleaning substrate. Chem. Commun. 2014, 50, 8405–8407. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Q.; Wang, S.D.; Ye, X.S.; Liu, Z.; Wu, Z.J. Corrosion resistance and wetting properties of silica-based superhydrophobic coatings on AZ31B Mg alloy surfaces. Appl. Surf. Sci. 2018, 453, 1–10. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Song, G.L.; Wu, P.P.; Huang, J.F.; Zheng, D.J. A protective superhydrophobic Mg-Zn-Al LDH film on Surface-Alloyed Magnesium. J. Alloys Compd. 2021, 855, 157550–157562. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.Y.; Zhu, Y.; Li, Z.H.; Shen, T.; Yang, D.Q. An environment-friendly fabrication of superhydrophobic surfaces on steel and magnesium alloy. Mater. Lett. 2016, 171, 297–299. [Google Scholar] [CrossRef]
- Wan, H.R.; Hu, X.F. One-step solve-thermal process for the construction of anticorrosion bionic superhydrophobic surfaces on magnesium alloy. Mater. Lett. 2016, 174, 209–212. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, Q.; Wang, Y.L.; Chen, R.R.; Takahashi, K.; Li, R.M.; Liu, L.H.; Wang, J. Formation of a corrosion-resistant and anti-icing superhydrophobic surface on magnesium alloy via a single-step method. J. Electrochem. Soc. 2016, 163, 62–69. [Google Scholar] [CrossRef]
- Wei, J.F.; Li, B.C.; Jing, L.Y.; Tian, N.; Zhao, X.; Zhang, J.P. Efficient protection of Mg alloy enabled by combination of a conventional anti-corrosion coating and a superamphiphobic coating. Chem. Eng. J. 2020, 390, 124562–124575. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Domantovsky, A.G.; Chulkova, E.V.; Emelyanenko, A.M.; Boinovich, L.B. Thermally induced gradient of properties on a superhydrophobic magnesium alloy surface. Metals 2021, 11, 41. [Google Scholar] [CrossRef]
- Viswanathan, S. Recent progress in superhydrophobic and superamphiphobic coatings for magnesium and its alloys. J. Magnes. Alloy. 2021, 9, 748–778. [Google Scholar]
- Wang, S.; Li, Y.; Fei, X.; Sun, M.; Zhang, C.; Li, Y.; Yang, Q.B.; Hong, X. Preparation of adurable superhydrophobic membrane by electrospinning poly(vinylidenefluoride)(PVDF) mixed with epoxy-siloxane modified SiO2 nanoparticles: A possible route to superhydrophobic surfaces with low water sliding angle and high water contact angle. J. Colloid Interface Sci. 2011, 359, 380–388. [Google Scholar]
- Liu, T.; Li, X.; Wang, D.; Huang, Q.; Liu, Z.; Li, N.; Xiao, C. Superhydrophobicity and regeneration of PVDF/SiO2 composite films. Appl. Surf. Sci. 2017, 396, 1443–1449. [Google Scholar] [CrossRef]
- Gao, J.; Wu, L.; Zheng, G. A hierarchical carbon nanotube/SiO2 nanoparticle network induced superhydrophobic and conductive coating for wearable strain sensors with superior sensitivity and ultra-low detection limit. J. Mater. Chem. C. 2019, 7, 4199–4209. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid fabrication of large-area, corrosion-resistant superhydrophobic mg alloy surfaces. ACS Appl. Mater. Interf. 2011, 3, 4404–4414. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, U.; Cansoy, C.E. Applicability of Cassie-Baxter equation for superhydrophobic fluoropolymer-silica composite films. Appl. Surf. Sci. 2015, 335, 99–106. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Yu, N.; Shang, Y.; Sun, Y.Z.; Wang, L.S.; Liu, C.S. Superhydrophobic TiO2/polyvinylidene fluoride composite surface with reversible wettability switching and corrosion resistance. Chem. Eng. J. 2016, 290, 37–44. [Google Scholar] [CrossRef]
- Oberli, L.; Caruso, D.; Hall, C.; Fabretto, M.; Murphy, P.J.; Evans, D. Condensation and freezing of droplets on superhydrophobic surfaces. Adv. Colloid Interf. 2013, 210, 47–57. [Google Scholar] [CrossRef]
- Kumar, D.; Li, L.; Chen, Z. Mechanically robust polyvinylidene fluoride (PVDF) based superhydrophobic coatings for self-cleaning applications. Prog. Org. Coat. 2016, 101, 385–390. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Si, X.; He, Z.J.; Qi, J.L.; Feng, J.F.; Cao, J. Mechanical durable ceria superhydrophobic coating fabricated by simple hot-press sintering. Appl. Surf. Sci. 2020, 529, 147113–147122. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Wu, M.; Wei, W.C. Durable superhydrophobic surface with hierarchical microstructures for efficient water collection. Surf. Coat. Technol. 2021, 419, 127279–127288. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Sulaiman, N.M.N.; Aroua, M.K.; Hashim, N.A. Effects of alkaline environments at mild conditions on the stability of PVDF membrane: An experimental study. Ind. Eng. Chem. Res. 2013, 52, 15874–15882. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, H.; Yu, Y.; Chen, S.G. Corrosion protection of silane coatings modified by carbon nanotubes on stainless steel. Int. J. Electrochem. Sci. 2015, 10, 3497–3509. [Google Scholar]
- Liu, Q.; Chen, D.; Kang, Z. One-step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.; Zhang, J.; Han, Z.; Ren, L. A electro-deposition process for fabrication of biomimeticsuper-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, W.G.; Yin, X.M.; Wang, H.Y.; Han, Z.W.; Ren, L.Q. Controlling wettability for improved corrosion inhibition on magnesium alloy as biomedical implant materials. Adv. Mater. Internsh. 2016, 3, 1500723–1500732. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Zhang, J.H.; Liu, Y.; Hang, T.; Ling, H.Q.; Li, M. Chemical grafting of the superhydrophobic surface on copper with hierarchical microstructure and its formation mechanism. Appl. Surf. Sci. 2018, 436, 950–956. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, X.; Yan, Q. One step electrochemical deposition to achieve superhydrophobic cobalt incorporated amorphous carbon-based film with self-cleaning and anti-corrosion. Surf. Interface Anal. 2017, 50, 290–296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).