Abstract
The response of synthetic substrates of sex steroid hormones—cholesterol (CHO), pregnenolone (PREG), and progesterone (PROG)—in the serum and testes of male tilapia (Oreochromis niloticus) to the environmental estrogen pesticide methomyl (0.2, 2, 20, and 200 μg·L−1) was evaluated using static-water contact toxicity tests. The results showed that low methomyl concentrations (0.2 and 2 μg·L−1) had no significant effects on the contents of CHO, PREG, and PROG in the serum and testes of male tilapia (p > 0.05). Consequently, the concentration of 2 μg·L−1 could be used as a preliminary reference threshold for the non-effective dose of methomyl in male tilapia. Exposure to high methomyl concentrations (20 and 200 μg·L−1) significantly inhibited the levels of CHO, PREG, and PROG in the serum and testes of male tilapia (p < 0.05) and showed a dose–response relationship. Sex steroid hormone synthesis substrate damage to male tilapia caused by less than 20 μg·L−1 methomyl was reversible, while the damage caused by equal to or greater than 200 μg·L−1 methomyl was irreversible when tilapia were transferred to methomyl-free water for 18 days. Thus, a concentration of 200 μg·L−1 could be used as a reference threshold for irreversible damage caused by methomyl in male tilapia.
1. Introduction
Methomyl (C5H10N2O2S) is a widely used carbamate pesticide that plays an important role in pest control and agricultural product harvest. Methomyl has been found in food crops and surface water due to its high solubility in water (57.9 g L−1 at 25 °C) and only weak or moderate adsorption in soil [1]. Methomyl residues in concentrations as high as 55.3 μg L−1 have been reported in natural water [2]. Because methomyl is often detected in natural water bodies and has the potential to disrupt the endocrine system in animals [3], its toxicity to aquatic animals has attracted extensive attention [4,5,6,7].
Recent evidence suggests that numerous compounds present in the environment act as hormone mimics and interfere with the reproductive status of vertebrates through effects ranging from alterations in plasma reproductive hormone levels to sterility [8]. An alteration in steroid hormone synthesis is an important biological response from a toxicological standpoint [9,10,11]. This is because the biological effects of pollutant exposure in organisms can be investigated through several molecular and biochemical biomarkers reflecting the onset of various cellular alterations [12,13]. These responses have been widely adopted as sensitive and reliable early warning signals for biological exposure to pollutants [14,15]. Therefore, this study aims to investigate the alterations in precursor molecules (cholesterol (CHO), pregnenolone (PREG), and progesterone (PROG)) in steroid hormone synthesis, concerning the methomyl dosage in the blood and testes of male tilapia (Oreochromis niloticus), to understand the toxic effects of methomyl on fish reproduction, and to protect the quality of natural water bodies.
2. Materials and Methods
2.1. Fish and Chemicals
Male Nile tilapia were obtained from a fish farm hatchery of Freshwater Fisheries Research Center of the Chinese Academy of Fishery Science (Wuxi, China), with an average weight of 112.24 ± 9.48 g and an average length of 17.06 ± 0.91 cm. Tilapia were farmed in 200-L glass aquaria to acclimatize to the laboratory conditions for 4 weeks before the experiment. Dechlorinated tap water (pH: 7.0 ± 0.5, temperature: 25 ± 0.5 °C, and dissolved oxygen: 6.3–7.0 mg L−1) was used in the experiment. A commercial feed purchased from Ningbo Tech-Bank Co., Ltd., China, was used to feed tilapia at a daily quantity of 2% of body weight. The photoperiod was 12-h light/12-h dark. Methomyl (CAS 16752-77-5) with a purity of 97% (w/w) was used. All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Sangon Biotech (Shanghai, China) and were of analytical grade.
2.2. Experimental Design
Thirty tilapia were randomly stocked in the glass aquaria with a volume of 300 L, and the volume of test water was 200 L. Fifteen glass aquaria were, in fact, used for the experiment. Five groups were set in the experiment. The nominal concentrations of methomyl were set as 0 (control), 0.2, 2, 20, and 200 μg L−1, respectively, and each group had three replicates. The concentration of methomyl ranges was chosen according to the following information: the 96-h LC50 of methomyl to tilapia (430 μg L−1) from our previous research [7]; the residue levels of methomyl reported in the natural environmental water was in the range of 0–55.3 μg L−1 [2]; the maximum permissible concentration for methomyl in drinking water of 200 μg L−1 published by the United States Environmental Protection Agency (EPA) in 2012. Half of the water in the tank was changed daily, and methomyl was added as needed to maintain the nominal concentrations. The experiment lasted for 48 days, during which the tilapia were exposed to methomyl for 30 days and recovered in methomyl-free water for 18 days.
2.3. Quantification of Methomyl
An improved method called the ULTRA-performance liquid chromatography tandem mass spectrometry method was established to determine actual methomyl concentrations in the test water. The water samples in the 0 (control), 0.2, 2, 20 and 200 μg L−1 groups were sampled, respectively, just after the fish were exposed and after exposure for 24 h, with 3 replicates per treatment. The water sample was filtered through HLB column. Methomyl on the HLB column was eluted using methanol, and the eluted solution was concentrated to 1 mL by rotary evaporation and was then blown to dryness by nitrogen gas. Then, the residue was dissolved with acetonitrile and water (v/v, 19:1). The detection limits, standard addition recoveries, and RSD of methomyl were 0.063 μg L−1, 87.9–109%, and 3.6–8.5%, respectively. The specified QA/QC procedures and methods are described in our previously published paper [16].
2.4. Sampling and Biochemical Analysis
Testes and serums (n = 6 per group) were sampled at 10 min (day 0) and on days 6, 12, 18, 24, and 30 in the methomyl exposure period (note: only the samples sampled at day 30 were used in this study) and at 18 days in the tilapia that recovered in methomyl-free water for 18 days. Tilapia were euthanized using 250 mg L−1 MS-222, and their length and weight were measured. Blood was collected from the caudal vein of the fish and then centrifuged at 1040× g for 15 min at 4 °C. The separated serum samples were stored at −80 °C for biochemical analysis. The testes were sampled immediately after sampling blood, snap-frozen using liquid nitrogen, and stored at −80 °C until analysis.
CHO, PREG, and PROG in the serum and testes of male tilapia were measured using ELISA kits following the manufacturer’s protocols. The kits were purchased from Shanghai Zhaorui Biotechnology Co., LTD. Protein levels were estimated by the Bradford method [17] using bovine serum albumin as a standard. An electric homogenizer (PRO200; PRO Scientific Bio-Gen, Oxford, CT, USA), a refrigerated centrifuge (Sigma 2–16K, Osterode am Harz, Germany), and an ultraviolet-visible spectrophotometer (UV-Vis 759S, Shanghai, China) were used for homogenization, centrifugation, and quantification, of the samples, respectively.
2.5. Statistical Analysis
The levels of CHO, PREG, and PROG in the serum and testes of tilapia were compared with the levels in the controls for each sampling day; results were expressed as a percentage of the control. Statistical analyses were performed using SPSS 15.0. All data are expressed as the mean ± SD (n = 6), and significant differences were analyzed with one-way analysis of variance (ANOVA). Data were tested for normality of distribution (Shapiroe-Wilk test) and homogeneity of variance (Levene’s test) prior to analysis. Data that did not meet assumptions of normality and homoscedasticity were transformed (lg) and then analyzed by one-way ANOVA. Tukey’s multiple comparisons and Student’s t-test were used for statistical comparisons, with p < 0.05 considered significant.
3. Results
The actual methomyl concentrations in the 0 (control), 0.2, 2, 20, and 200 μg L−1 groups were 0, 0.23 ± 0.02, 2.12 ± 0.13, 21.50 ± 1.21, and 182.0 ± 8.96 μg L−1, respectively, just after the fish were exposed and 0, 0.21 ± 0.03, 1.92 ± 0.14, 18.52 ± 1.43, and 179.0 ± 9.12 μg L−1, respectively, after exposure for 24 h. The results are discussed in relation to the nominal concentrations.
The effects of chronic exposure to pesticide methomyl on sex steroid hormones CHO, PREG, and PROG in the serum and testes of male tilapia and recovery patterns are shown in Figure 1. No significant changes (p > 0.05) were observed in the sex steroid hormones CHO, PREG, and PROG in the serum and testes of male tilapia exposed to 0.2 μg L−1 and 2 μg L−1 methomyl, whereas male tilapia exposed to 20 or 200 μg L−1 methomyl showed significant changes (p < 0.05): CHO, PREG, and PROG both in serum and testes were significantly decreased (p < 0.05). Moreover, dose–response relationships were found between methomyl and CHO, PREG, and PROG in both serum and testes. Specifically, CHO, PREG, and PROG, both in the serum and testes, decreased with an increase in methomyl concentration.
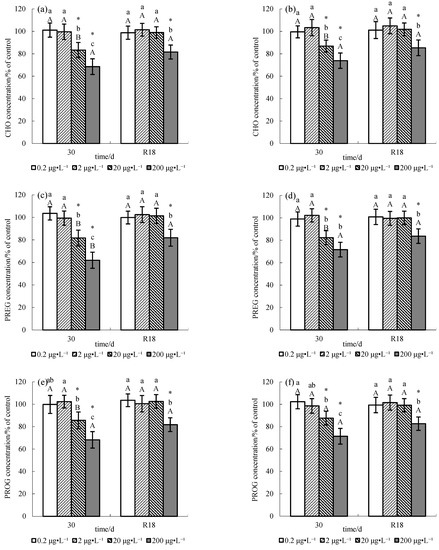
Figure 1.
Cholesterol (CHO), pregnenolone (PREG), and progesterone (PROG) levels in testes (a,c,e) and serum (b,d,f) of male O. niloticus exposed to methomyl for 30 days and after an 18-day recovery period in methomyl-free water (R18). (Note: All the data were expressed as Average ± SD (n = 6). “*” means significant difference from the control. Different lower-case letters indicate significant difference among concentrations at the same exposure period, and different upper-case letters indicate difference between exposure and recovery periods at the same methomyl concentration, with p < 0.05 considered significant).
When the tilapia were transferred to methomyl-free water for 18 days, CHO, PREG, and PROG in both the serum and testes of male tilapia in each group recovered comparatively. There were no significant differences in CHO, PREG, and PROG both in the serum and testes of male tilapia exposed to 2 and 20 μg L−1 methomyl compared with the controls. Conversely, significant differences (p < 0.05) in the 200 μg L−1 treatment group were observed compared to the control group.
4. Discussion
4.1. Effect of Methomyl on Sex Steroid Hormone Synthesis Substrates
CHO is a precursor of the sexual steroid hormones testosterone (T), 11-ketotestosterone (11-KT), and 17β -estradiol (E2). The basic process of CHO synthesis in fish is shown in Figure 2 [18]. CHO is transported from the mitochondrial outer membrane to the inner membrane under the action of steroidogenic acute regulatory protein (StAR) [19]. CHO is converted to PREG by the action of the CHO side-chain cleavage enzyme (CYP11A1) [20]. PREG is converted into PROG catalyzed by 3β-hydroxysteroid dehydrogenase (3β-HSD) in the smooth endoplasmic reticulum [18], and 17α hydroxylase (P45017α) catalyzes the conversion of PREG and PROG to androgen [18]. Androgen is converted into estradiol under the catalysis of aromatase [21].
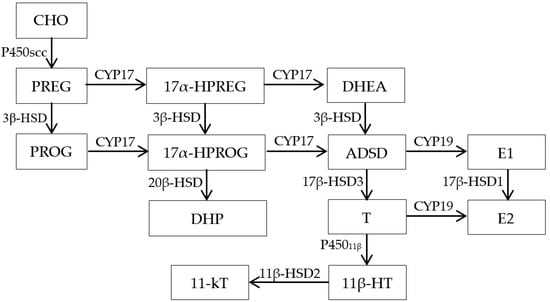
Figure 2.
The sex hormone biosynthesis pathway in fish. (Note: CHO (Cholesterol), PREG (Pregnenolone), PROG (Progesterone), P450scc (Cholesterol side chain cleavage enzyme/CYP11A1), 3β-HSD (3β-hydroxysteroid dehydrogenase), CYP17 (Cytochrome P450 17α-hydroxylase), 17α-HPREG (17α-Hydroxypregnenolone), 17α-HPROG (17α-Hydroxyprogesterone), DHEA (Dehydroepiandrosterone), ADSD (Androstenedione), 17β-HSD1 (17β-hydroxysteroid dehydrogenase type I), 17β-HSD2 (17β-hydroxysteroid dehydrogenase type II), 17β-HSD3 (17β-hydroxysteroid dehydrogenase type III), E1 (Estrone), E2 (17β-estradiol), T (Testosterone), 11-KT (11-ketotestosterone), 11β-HT (Hydroxytestosterone), DHP(17α,20β-dihydroxy Progesterone)).
In this study, methomyl had no significant effect on CHO content in serum and testes at low concentrations (0.2 and 2 μg L−1) (p > 0.05), but a significant (p < 0.05) decrease in CHO content in serum and testes at high methomyl concentrations (20 and 200 μg·L−1) was found. Mu et al. [22] studied the effect of difenoconazole on CHO in zebrafish and found that low concentrations of difenoconazole (0.1% and 10 μg L−1) had no significant effect on CHO content (p > 0.05). High concentrations (500 μg L−1) significantly reduced CHO content (p < 0.05), which was consistent with the change trends of CHO in our study. Other studies have also shown that environmental estrogen could alter gonadal steroid production at specific sites within the steroidogenic pathway, that is, downstream of CHO mobilization to side-chain cleavage of P450scc and/or conversion of CHO to PREG [23]. The decreased levels of PREG produced by the testes of methomyl-treated tilapia suggest that methomyl may affect CHO availability or reduce the activity of the side-chain cleavage enzyme P450scc, which mediates the conversion of CHO to PREG [8].
Similarly, the response of PREG and PROG to methomyl in serum and testes showed no effect at low concentrations (0.2 and 2 μg L−1) (p > 0.05) and significantly decreased at high concentrations (20 and 200 μg L−1). However, a significant (p < 0.05) decrease in the present experiment, consistent with that of CHO in the serum and testes, was observed. PREG is transformed from CHO under the catalysis of CYP11A1 [20], and PROG is transformed from PREG under the catalysis of 3β-HSD in the smooth endoplasmic reticulum [18]. Therefore, a decrease in CHO content inevitably leads to a decrease in PREG and PROG content. At the same time, our previous study showed that the expression of testes 3β-HSD mRNA was significantly inhibited at high concentrations (20 and 200 μg L−1) (p < 0.05), and the inhibition intensity increased with an increase in methomyl concentration [5]. This suggests that decreased levels of 3β-HSD in the testes further inhibited the conversion of PREG to PROG, thereby exacerbating the decline in PROG levels.
The liver is the main site of CHO synthesis in the body, as well as an important organ to maintain CHO demand and the stability of blood CHO content [24]. Therefore, the significant decrease in serum CHO content in this study indicates that its synthesis in the liver is blocked to some extent. Meanwhile, CHO, which is used as a steroid hormone synthesis substrate in teleost fish, is mainly exogenous CHO absorbed from plasma [25]. Under the action of lipoproteins, CHO is transported from the synthesis or absorption site to the functional site through blood circulation. The sex hormone synthesis pathway is initiated only when CHO is transported into the inner membrane of mitochondria [18]. Transmembrane transport of CHO from the mitochondrial outer membrane to the intima is a rate-limiting step in sex hormone synthesis [26]. The CHO transport process in fish is completed by high-density lipoprotein (HDL) [27], and StAR is an important CHO transporter involved in CHO metabolism. Therefore, damage to the StAR protein could lead to obstruction of the CHO transport pathway [18]. The simultaneous study of our experiment showed that methomyl had no significant (p > 0.05) effect on the expression of StAR mRNA in the testes at low concentrations (0.2 and 2 μg L−1) but significantly (p < 0.05) inhibited its expression at high concentrations (20 and 200 μg L−1) [5]. Consequently, the decreased CHO content in the testes induced by methomyl at high concentrations (20 and 200 μg L−1) was due to both the obstruction of CHO synthesis and transport pathways. CHO, PREG, and PROG are the primary precursors of sex steroid hormones. The synthesis of steroid hormones and their primary precursors is an important and critical factor in the physiological development of organisms, including growth, development, and reproduction [28]. Therefore, the decrease in CHO, PREG and PROG levels affects the synthesis of sex steroid hormones and ultimately affects the reproductive development of fish [18].
4.2. Recovery Pattern
Recovery tests showed that the levels of CHO, PREG, and PROG in the serum and testes of tilapia exposed to 2 and 20 μg L−1 methomyl recovered when tilapia were transferred to methomyl-free water for 18 days. However, the degree of recovery differed for different methomyl concentrations. The above parameters in the 20 μg L−1 recovery group were not significantly different than those in the control group (p > 0.05), and all of them returned to normal levels. However, in the 200 μg L−1 recovery group, their levels still significantly differed from those in the control group (p < 0.05) and were not restored to normal levels after 18 days. In conclusion, the damage to male tilapia steroid synthetic substrates caused by ≤20 μg L−1 methomyl could be recovered, while that caused by ≥200 μg L−1 methomyl could not be recovered within 18 days. Hence, 200 μg L−1 can be used as the reference threshold value for irreversible damage caused by methomyl in male tilapia.
5. Conclusions
Methomyl exposure at low concentrations (0.2 and 2 μg L−1) had no significant effects on the contents of cholesterol (CHO), pregnenolone (PREG), and progesterone (PROG) in the serum and testes of male tilapia (p > 0.05). An amount of 2 μg L−1 can be preliminarily used as the reference threshold value of a “no-effect dose” of methomyl in male tilapia.
Methomyl at high concentrations (20 and 200 μg L−1) significantly inhibited the contents of cholesterol (CHO), pregnenolone (PREG), and progesterone (PROG) in the serum and testes of male tilapia (p < 0.05) and showed a dose-response relationship. Since cholesterol is a synthetic substrate for sex steroid hormones, a decrease in cholesterol levels can affect the synthesis of sex steroid hormones, which, in turn, affects the reproductive function of tilapia.
The damage of steroid synthetic substrates in male tilapia caused by the lower methomyl levels of 20 μg L−1 was reversible within 18 days after exposure, but the damage caused by 200 μg L−1 methomyl was irreversible.
Author Contributions
Conceptualization, S.M. and X.C.; methodology, C.S.; software, L.F.; validation, J.C., P.X. and S.M.; formal analysis, L.Q. and G.H.; resources, J.C.; data curation, X.C.; writing—original draft preparation, S.M.; writing—review and editing, X.C.; visualization, S.M.; supervision, J.C. and P.X.; project administration, J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System of MOF and MARA (No.CARS-46) and the National Key Research and Development Program of China (No.2020YFD0900502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Ethic Statement
This study was approved by the Ethics Committee of the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Institutional Animal Care and Ethics Committee of Nanjing Agricultural University, Nanjing, China. [Permit number: SYXK (Su) 2011-0036]).
References
- Kongphonprom, K.; Burakham, R. Determination of carbamate insecticides in water, fruit, and vegetables by ultrasound-assisted dispersive liquid-liquid micro extraction and high-performance liquid chromatography. Anal. Lett. 2016, 49, 753–767. [Google Scholar] [CrossRef]
- Van Scoy, A.R.; Yue, M.; Deng, X.; Tjeerdema, R.S. Environmental fate and toxicology of methomyl. Rev. Environ. Contam. T 2013, 222, 93–109. [Google Scholar]
- Guo, X.B. Environmental Health; Peking University Medical Press: Beijing, China, 2006. [Google Scholar]
- Meng, S.L.; Hu, G.D.; Qiu, L.P.; Song, C.; Fan, L.M.; Chen, J.Z.; Xu, P. Effects of chronic exposure of methomyl on the antioxidant system in kidney of tilapia (Oreochromis niloticus) and recovery pattern. J. Toxicol. Environ. Health A 2013, 76, 937–943. [Google Scholar] [CrossRef]
- Meng, S.L.; Qiu, L.P.; Hu, G.D.; Fan, L.M.; Song, C.; Zheng, Y.; Wu, W.; Qu, J.H.; Li, D.D.; Chen, J.Z.; et al. Effects of methomyl on steroidogenic gene transcription of the hypothalamic-pituitary-gonad-liver axis in male tilapia. Chemosphere 2016, 165, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.L.; Qiu, L.P.; Hu, G.D.; Fan, L.M.; Song, C.; Zheng, Y.; Wu, W.; Qu, J.H.; Li, D.D.; Chen, J.Z.; et al. Effect of methomyl on sex steroid hormone and vitellogenin levels in serum of male tilapia (Oreochromis niloticus) and recovery pattern. Environ. Toxicol. 2017, 32, 1869–1877. [Google Scholar] [CrossRef]
- Meng, S.L.; Chen, X.; Song, C.; Fan, L.M.; Qiu, L.P.; Zheng, Y.; Chen, J.Z.; Xu, P. Effect of chronic exposure to pesticide methomyl on antioxidant defense system in testis of tilapia (Oreochromis niloticus) and its recovery pattern. Appl. Sci. 2021, 11, 3332. [Google Scholar] [CrossRef]
- Maclatchy, D.L.; Vanderkraak, G.J. The phytoestrogen β-sitosterol alters the reproductive endocrine status of goldfish-sciencedirect. Toxicol. Appl. Pharmacol. 1995, 134, 305–312. [Google Scholar] [CrossRef]
- Ibor, O.R.; Adeogun, A.O.; Chukwuka, A.V.; Arukwe, A. Gross pathology, physiological and toxicological responses in relation to metals and persistent organic pollutants (POPs) burden in tilapia species from ogun river, nigeria. Mar. Environ. Res. 2017, 129, 245–257. [Google Scholar] [CrossRef]
- Choi, J.; Lee, G.; Kim, S.; Choi, K. Investigation on sex hormone-disruption effects of two novel brominated flame retardants (DBDPE and BTBPE) in male zebrafish (Danio rerio) and two human cell lines (H295R and MVLN). Appl. Sci. 2021, 11, 3837. [Google Scholar] [CrossRef]
- Ferreira, C.; Oliveira, M.; Santos, M.A.; Pacheco, M. Effects of benzo[a]pyrene, cortisol, and 17-estradiol on liver microsomal erod activity of Anguilla anguilla: An In Vitro approach. Appl. Sci. 2021, 11, 2533. [Google Scholar] [CrossRef]
- Osman, A.G.M.; Reheem, A.E.; AbuelFadl, K.Y.; Gad El-Rab, A.G. Enzymatic and histopathologic biomarkers as indicators of aquatic pollution in fishes. Nat. Sci. 2010, 2, 1302–1311. [Google Scholar] [CrossRef][Green Version]
- Pereira, S.; Pinto, A.L.; Cortes, R.; Fontainhas-Fernandes, A.; Coimbra, A.M.; Monteiro, S.M. Gill histopathological and oxidative stress evaluation in native fish captured in Portuguese Northwestern Rivers. Ecotoxicol. Environ. Saf. 2013, 90, 157–166. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E.; Benedetti, M.; Arukwe, A. Molecular and biochemical biomarkers in environmental monitoring: A comparison of biotransformation and antioxidant defense systems in multiple tissues. Aquat. Toxicol. 2011, 105, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Meng, S.L.; Liu, T.; Song, C.; Zhang, C.; Qiu, L.P.; Chen, J.Z.; Xu, P. Determination of Methomyl Residue in Water by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Anhui Agric. Sci. 2018, 46, 166–167. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, H. Effects and Mechanisms of Monocrotophos Pesticide on Sex Hormone Synthesis and Transformation in the Male Goldfish (Carassius auratus); Ocean University of China: Qingdao, China, 2013. [Google Scholar]
- Thongbuakaew, T.; Suwansa-Ard, S.; Chaiyamoon, A.; Cummins, S.F.; Sobhon, P. Sex steroids and steroidogenesis-related genes in the sea cucumber, holothuria scabra and their potential role in gonad maturation. Sci. Rep. 2021, 11, 2194. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, X. Research progress in steroidogenic acute regulatory protein. J. Shanxi Norm. Univ. Nat. Sci. Ed. 2009, 37, 85–89. [Google Scholar]
- Barannikova, I.A.; Bayunova, L.V.; Semenkova, T.B. Serum levels of testosterone, 11-ketotestosterone and oestradiol-17β in three species of sturgeon during gonadal development and final maturation induced by hormonal treatment. J. Fish. Biol. 2004, 64, 1330–1338. [Google Scholar] [CrossRef]
- Mu, X.Y. The Toxicity Effect and Mechanism of Difenoconazole on Zebrafish (Danio rerio); Agricultural University: Beijing, China, 2015. [Google Scholar]
- Hogan, N.S.; Currie, S.; Le Blanc, S.; Hewitt, M.L.; MacLatchy, D.L. Modulation of steroidogenesis and estrogen signalling in the estuarine killifish (Fundulus heteroclitus) exposed to ethinylestradiol. Aquat. Toxicol. 2010, 98, 148–156. [Google Scholar] [CrossRef]
- Meng, X.X.; Wei, Y.L.; Liang, M.Q.; Xu, H.G. Progress in Cholesterol Nutritional Requirements of Fish. Chin. J. Anim. Nutr. 2021, 33, 719–728. [Google Scholar]
- Sharpe, R.L.; Woodhouse, A.; Moon, T.W.; Trudeau, V.L.; MacLacthy, D.L. β-Sitosterol and 17β-estradiol alter gonadal steroidogenic acute regulatory protein (StAR) expression in goldfish, Carassius auratus. Gen. Comp. Edocr. 2007, 151, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Jefcoate, C.R.; McNamara, B.C.; Artemenko, I.; Yamazaki, T. Regulation of cholesterol movement to mitochondrial cytochrome P450scc in steroid hormone synthesis. J. Steroid. Biochem. Mol. Biol. 1992, 43, 751–767. [Google Scholar] [CrossRef]
- Babin, P.J.; Vernier, J.M. Plasma lipoproteins in fish. J. Lipid Res. 1989, 30, 467–489. [Google Scholar] [CrossRef]
- Sayed, A.D.; Mahmoud, U.M.; Mekkawy, I.A. eproductive biomarkers to identify endocrine disruption in Clarias gariepinus exposed to 4-nonylphenol. Ecotoxicol. Environ. Saf. 2012, 78, 310–319. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).