Radiation Dose Assessment of the Fog Lead Acrylic Shields during Coronary Angiography: A Phantom Study
Abstract
:1. Introduction
2. Material and Methods
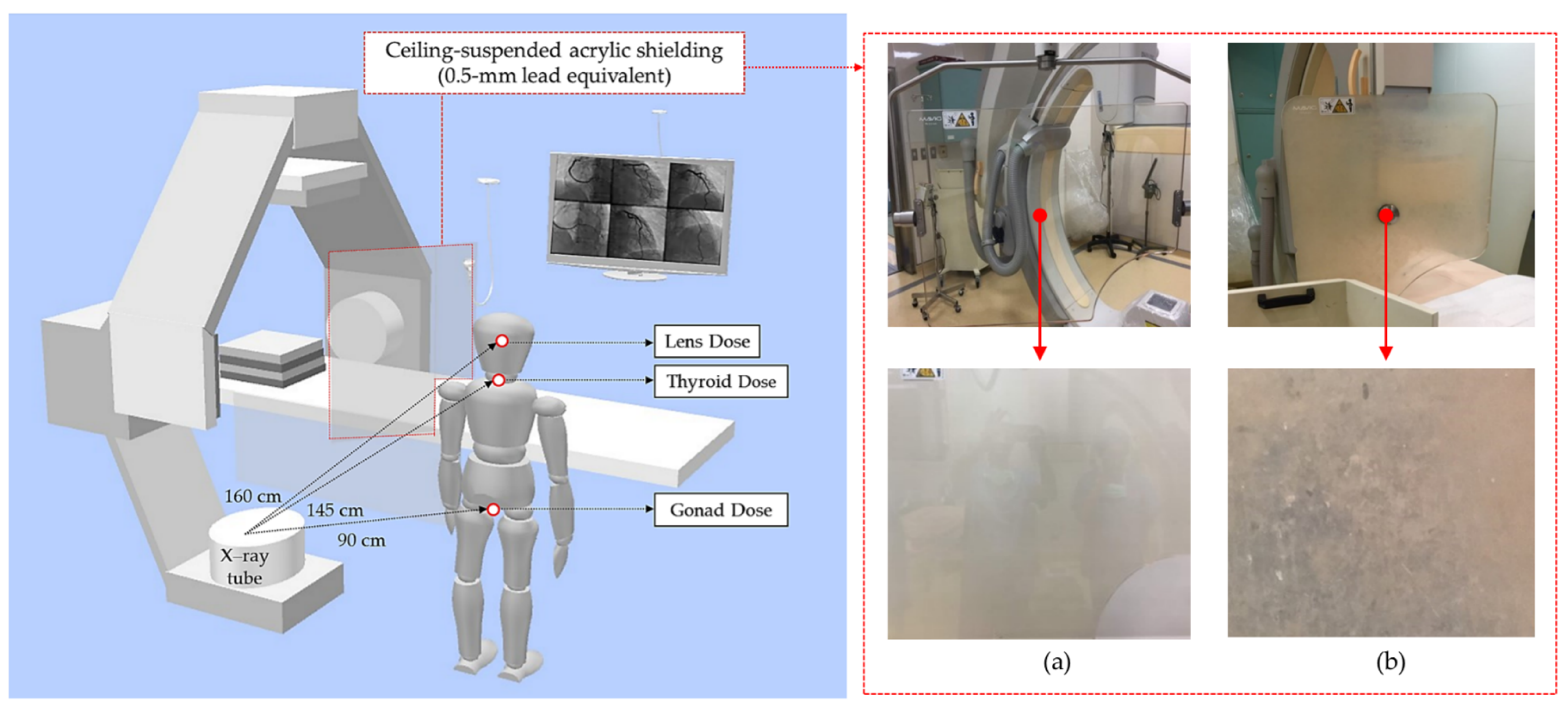
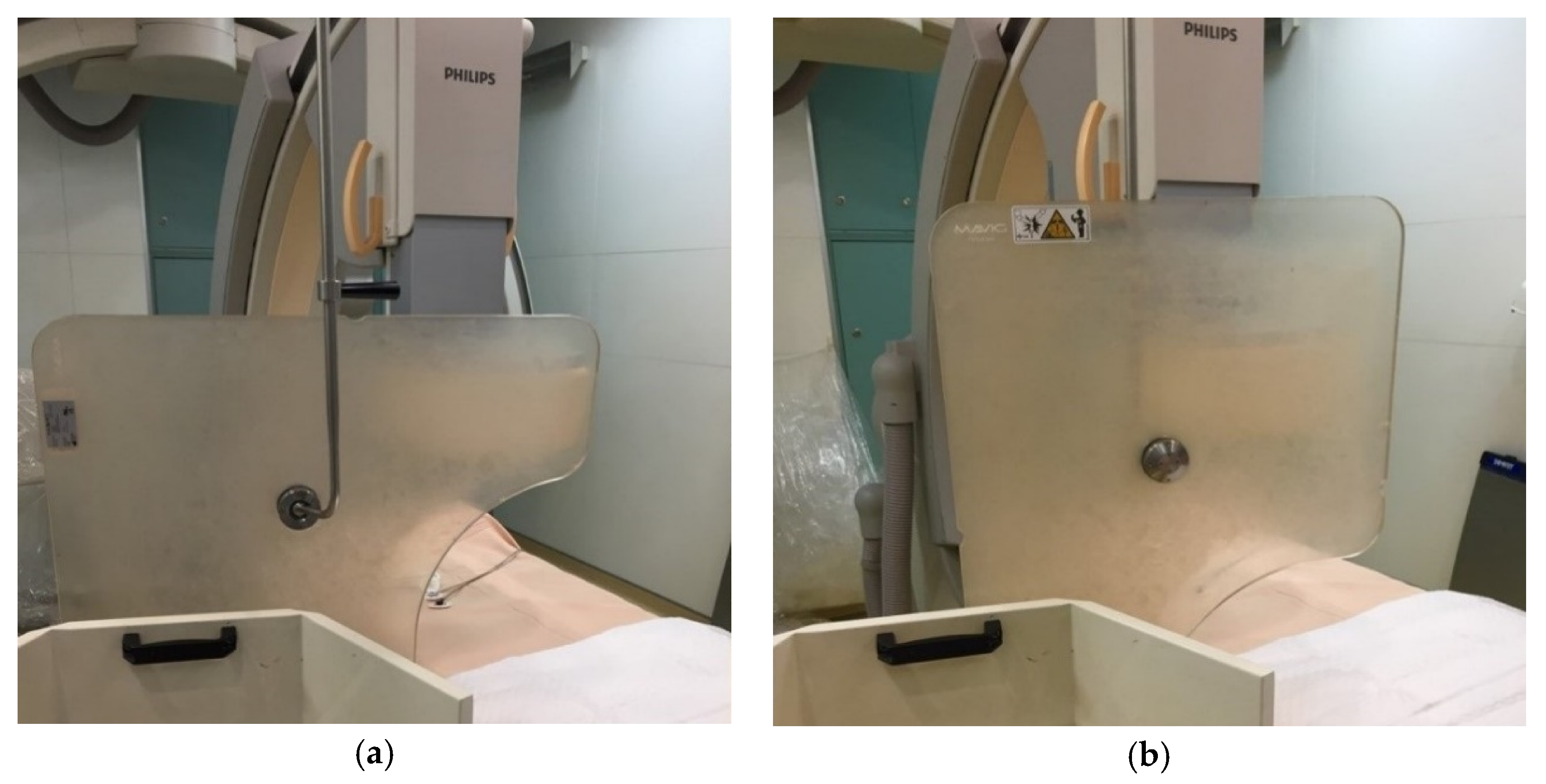
2.1. Study Design and Equipment
2.2. Radiation Dose Measurement
2.3. OSLDs
2.4. Statistical Analysis
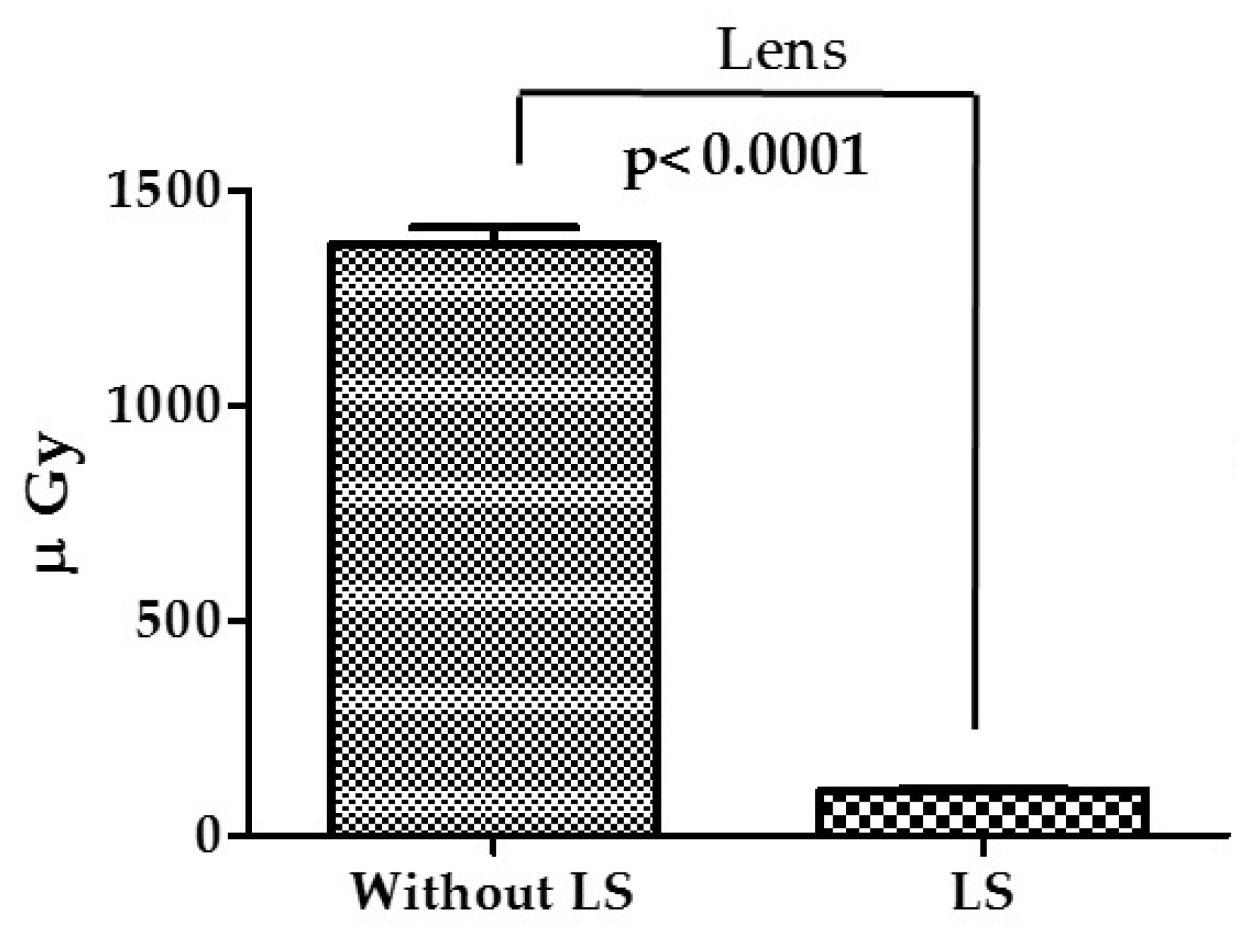
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heer, T.; Hochadel, M.; Schmidt, K.; Mehilli, J.; Zahn, R.; Kuck, K.H.; Zeymer, U. Sex differences in percutaneous coronary intervention—insights from the coronary angiography and PCI registry of the German Society of Cardiology. J. Am. Heart Assoc. 2017, 6, e004972. [Google Scholar] [CrossRef]
- Navarese, E.P.; Schulze, V.; Andreotti, F.; Kowalewski, M.; Kołodziejczak, M.; Kandzari, D.E.; Valgimigli, M. Comprehensive meta-analysis of safety and efficacy of bivalirudin versus heparin with or without routine glycoprotein IIb/IIIa inhibitors in patients with acute coronary syndrome. JACC: Cardiovasc. Interv. 2015, 8, 201–213. [Google Scholar] [CrossRef]
- Dowling, A.; Gallagher, A.; O’connor, U.; Larkin, A.; Gorman, D.; Gray, L.; Malone, J. Acceptance testing and QA of interventional cardiology systems. Radiat. Prot. Dosim. 2008, 129, 291–294. [Google Scholar] [CrossRef]
- Vano, E.; Ubeda, C.; Miranda, P.; Leyton, F.; Durán, A.; Nader, A. Radiation protection in pediatric interventional cardiology: An IAEA PILOT program in Latin America. Health Phys. 2011, 101, 233–237. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Piccaluga, E.; Guagliumi, G.; Del Greco, M.; Gaita, F.; Picano, E. Occupational health risks in cardiac catheterization laboratory workers. Circ. Cardiovasc. Interv. 2016, 9, e003273. [Google Scholar] [CrossRef] [Green Version]
- Khaleghi Fard, A.; Alian, A.H.M.; Pourafkari, L.; Ghojazadeh, M.; Tarighatnia, A.; Farajollahi, A. Impact of pelvic and rad-board lead shields on operator and patient radiation dose in trans-radial coronary procedures. Radiat. Prot. Dosim. 2019, 187, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Kaga, Y.; Haga, Y.; Kataoka, N.; Kumasaka, E.; Meguro, T.; Zuguchi, M. Occupational dose in interventional radiology procedures. Am. J. Roentgenol. 2013, 200, 138–141. [Google Scholar] [CrossRef]
- Kim, K.P.; Miller, D.L.; Balter, S.; Kleinerman, R.A.; Linet, M.S.; Kwon, D.; Simon, S.L. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Phys. 2008, 94, 211–227. [Google Scholar] [CrossRef]
- Miller, S.W.; Castronovo, F.P., Jr. Radiation exposure and protection in cardiac catheterization laboratories. Am. J. Cardiol. 1985, 55, 171–176. [Google Scholar] [CrossRef]
- Dash, H.; Leaman, D.M. Operator radiation exposure during percutaneous transluminal coronary angioplasty. J. Am. Coll. Cardiol. 1984, 4, 725–728. [Google Scholar] [CrossRef] [Green Version]
- Finci, L.; Meier, B.; Steffenino, G.; Roy, P.; Rutishauser, W. Radiation exposure during diagnostic catheterization and single-and double-vessel percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 1987, 60, 1401–1403. [Google Scholar] [CrossRef]
- Madder, R.D.; LaCombe, A.; VanOosterhout, S.; Mulder, A.; Elmore, M.; Parker, J.L.; Wohns, D. Radiation exposure among scrub technologists and nurse circulators during cardiac catheterization: The impact of accessory lead shields. JACC Cardiovasc. Interv. 2018, 11, 206–212. [Google Scholar]
- Patel, C.; Grossman, M.; Shabanova, V.; Asnes, J. Reducing radiation exposure in cardiac Catheterizations for congenital heart disease. Pediatric Cardiol. 2019, 40, 638–649. [Google Scholar] [CrossRef]
- Hirshfeld, J.W.; Balter, S.; Brinker, J.A.; Kern, M.J.; Klein, L.W.; Lindsay, B.D.; Weitz, H.H. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures: A report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J. Am. Coll. Cardiol. 2004, 44, 2259–2282. [Google Scholar] [PubMed] [Green Version]
- Stewart, F.A.; Akleyev, A.V.; Hauer-Jensen, M.; Hendry, J.H.; Kleiman, N.J.; Macvittie, T.J.; Wallace, W.H. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef]
- Pantelić, G.; Čeliković, I.; Živanović, M.; Vukanac, I.; Nikolić, J.K.; Cinelli, G.; Gruber, V. Qualitative overview of indoor radon surveys in Europe. J. Environ. Radioact. 2019, 204, 163–174. [Google Scholar] [CrossRef]
- Venneri, L.; Rossi, F.; Botto, N.; Andreassi, M.G.; Salcone, N.; Emad, A.; Picano, E. Cancer risk from professional exposure in staff working in cardiac catheterization laboratory: Insights from the National Research Council’s Biological Effects of Ionizing Radiation VII Report. Am. Heart J. 2009, 157, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNSCEAR (United Nation Scientific Committee on the Effects of Atomic Radiation Report). Sources and Effects of Ionizing Radiation, Report to the General Assembly; United Nations: New York, NY, USA, 2008. [Google Scholar]
- Rajabi, A.B.; Noohi, F.; Hashemi, H.; Haghjoo, M.; Miraftab, M.; Yaghoobi, N.; Khabazkhoob, M. Ionizing radiation-induced cataract in interventional cardiology staff. Res. Cardiovasc. Med. 2015, 4, e25148. [Google Scholar]
- Kry, S.F.; Alvarez, P.; Cygler, J.E.; DeWerd, L.A.; Howell, R.M.; Meeks, S.; Mihailidis, D. AAPM TG 191: Clinical use of luminescent dosimeters: TLDs and OSLDs. Med. Phys. 2020, 47, 19–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Senan, R.M.; Hatab, M.R. Characteristics of an OSLD in the diagnostic energy range. Med. Phys. 2011, 38, 4396–4405. [Google Scholar]
- Matsubara, K.; Yoshida, S.; Hirosawa, A.; Chusin, T.; Furukawa, Y. Characterization of Small Dosimeters Used for Measurement of Eye Lens Dose for Medical Staff during Fluoroscopic Examination. Diagnostics 2021, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Bohari, A.; Hashim, S.; Mustafa, S.N.M. Occupational radiation dose during fluoroscopy guided interventional procedures at Institut Kanser Negara. J. Phys. Conf. Ser. 2019, 1248, 012052. [Google Scholar] [CrossRef]
- Jursinic, P.A. Characterization of optically stimulated luminescent dosimeters, OSLDs, for clinical dosimetric measurements. Med. Phys. 2007, 34, 4594–4604. [Google Scholar] [CrossRef] [PubMed]
- Fetterly, K.A.; Magnuson, D.J.; Tannahill, G.M.; Hindal, M.D.; Mathew, V. Effective use of radiation shields to minimize operator dose during invasive cardiology procedures. JACC Cardiovasc. Interv. 2011, 4, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Koukorava, C.; Farah, J.; Struelens, L.; Clairand, I.; Donadille, L.; Vanhavere, F.; Dimitriou, P. Efficiency of radiation protection equipment in interventional radiology: A systematic Monte Carlo study of eye lens and whole body doses. J. Radiol. Prot. 2014, 34, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Chen, Z.; Jiang, X.; Zhao, Z.; Huang, M.; Li, J.; Zhuang, J.; Liu, X.; Hu, T.; Liang, W. Operator radiation and the efficacy of ceiling-suspended lead screen shielding during coronary angiography: An anthropomorphic phantom study using real-time dosimeters. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]

| Position | Shields | CAG Projections | |
|---|---|---|---|
| Types | Orientation | ||
| 70 cm | CLS | Horizontal/Vertical | CAU 30°/RAO 10° + CRA 30°/LAO 40° + CAU 30°/LAO 60°/LAO 10° + CRA 30° |
| FLS | |||
| 20 cm | CLS | Horizontal | CAU 30°/RAO 10° + CRA 30°/LAO 40° + CAU 30°/LAO 60°/LAO 10° + CRA 30° |
| FLS | |||
| Organs | Horizontal Placement | p-Value | |
|---|---|---|---|
| FLS (μGy) | CLS (μGy) | ||
| Lens | 106.7 ± 5.84 | 103.00 ± 4.77 | 0.63 |
| Thyroid | 91.33 ± 3.02 | 91.00 ± 3.30 | 0.94 |
| Gonad | 134.7 ± 4.08 | 132.7 ± 3.16 | 0.61 |
| Organs | Vertical Placement | p-Value | |
| FLS (μGy) | CLS (μGy) | ||
| Lens | 109.30 ± 4.45 | 104.7 ± 3.76 | 0.43 |
| Thyroid | 97.00 ± 2.45 | 95.00 ± 2.61 | 0.78 |
| Gonad | 135.30 ± 3.42 | 133.3 ± 2.73 | 0.65 |
| Organs | Horizontal Placement | p-Value | |
|---|---|---|---|
| FLS (μGy) | CLS (μGy) | ||
| Lens | 106.7± 5.83 | 104.0 ± 4.64 | 0.72 |
| Thyroid | 89.67 ± 2.93 | 88.0 ± 4.35 | 0.75 |
| Gonad | 133.2 ± 2.03 | 132.3 ± 3.09 | 0.65 |
| Rotation | Angulation | DAP (cGy·cm2) | AK (mGy) |
|---|---|---|---|
| LAO 60° | 633.6 ± 5.62 | 114.1 ± 1.02 | |
| LAO 10° | CRA 30° | 299.6 ± 1.85 | 41.96 ± 0.26 |
| CAU 30° | 490.0 ± 0.82 | 78.44 ± 0.13 | |
| RAO 10° | CRA 30° | 402.7 ± 9.01 | 65.81 ± 0.71 |
| LAO 40° | CAU 30° | 652.1 ± 3.31 | 117.30 ± 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-H.; Lai, L.-H.; Tang, K.-T.; Ting, C.-Y.; Lai, C.-S. Radiation Dose Assessment of the Fog Lead Acrylic Shields during Coronary Angiography: A Phantom Study. Appl. Sci. 2021, 11, 10743. https://doi.org/10.3390/app112210743
Lin H-H, Lai L-H, Tang K-T, Ting C-Y, Lai C-S. Radiation Dose Assessment of the Fog Lead Acrylic Shields during Coronary Angiography: A Phantom Study. Applied Sciences. 2021; 11(22):10743. https://doi.org/10.3390/app112210743
Chicago/Turabian StyleLin, Hsin-Hon, Lu-Han Lai, Kuo-Ting Tang, Chien-Yi Ting, and Cheng-Shih Lai. 2021. "Radiation Dose Assessment of the Fog Lead Acrylic Shields during Coronary Angiography: A Phantom Study" Applied Sciences 11, no. 22: 10743. https://doi.org/10.3390/app112210743
APA StyleLin, H.-H., Lai, L.-H., Tang, K.-T., Ting, C.-Y., & Lai, C.-S. (2021). Radiation Dose Assessment of the Fog Lead Acrylic Shields during Coronary Angiography: A Phantom Study. Applied Sciences, 11(22), 10743. https://doi.org/10.3390/app112210743






