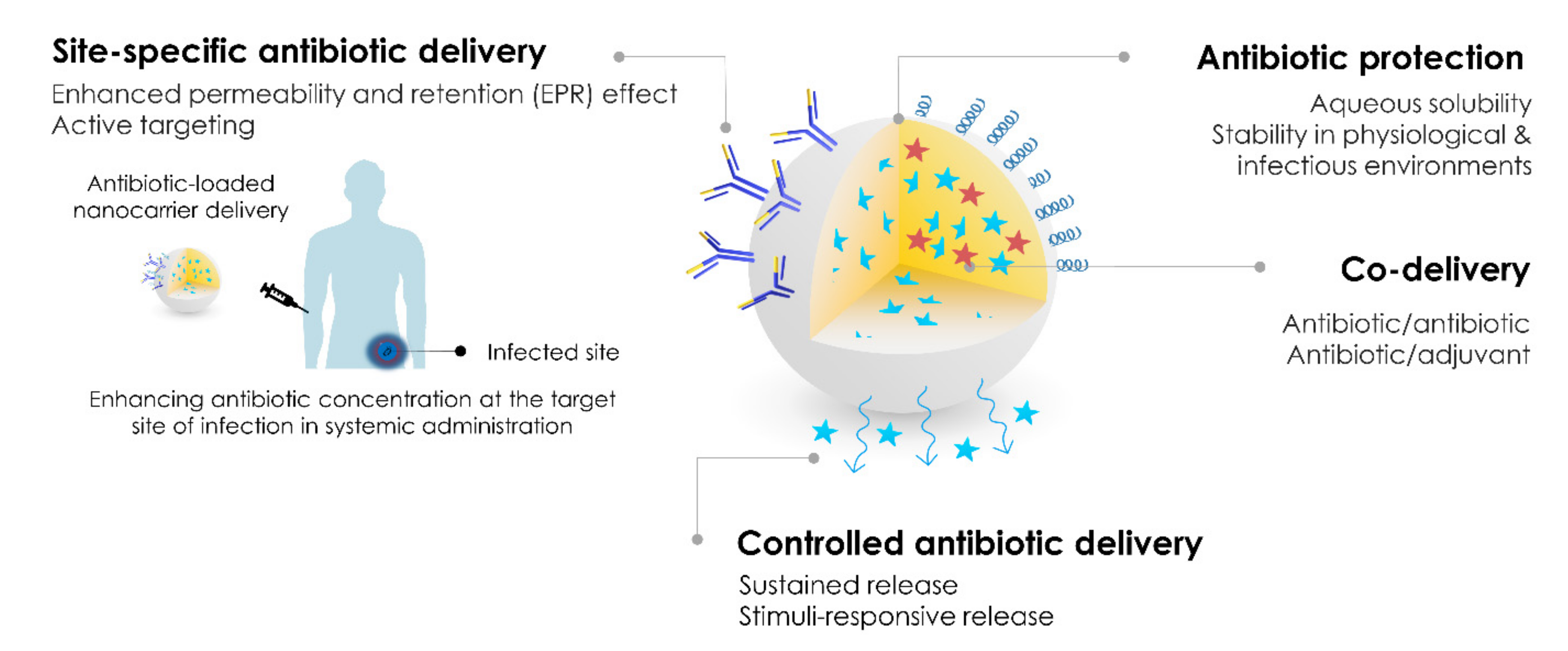
Application of Polymeric Nanocarriers for Enhancing the Bioavailability of Antibiotics at the Target Site and Overcoming Antimicrobial Resistance
Abstract
:1. Introduction
2. Dissolution and Stability Enhancement of Antimicrobial Compounds
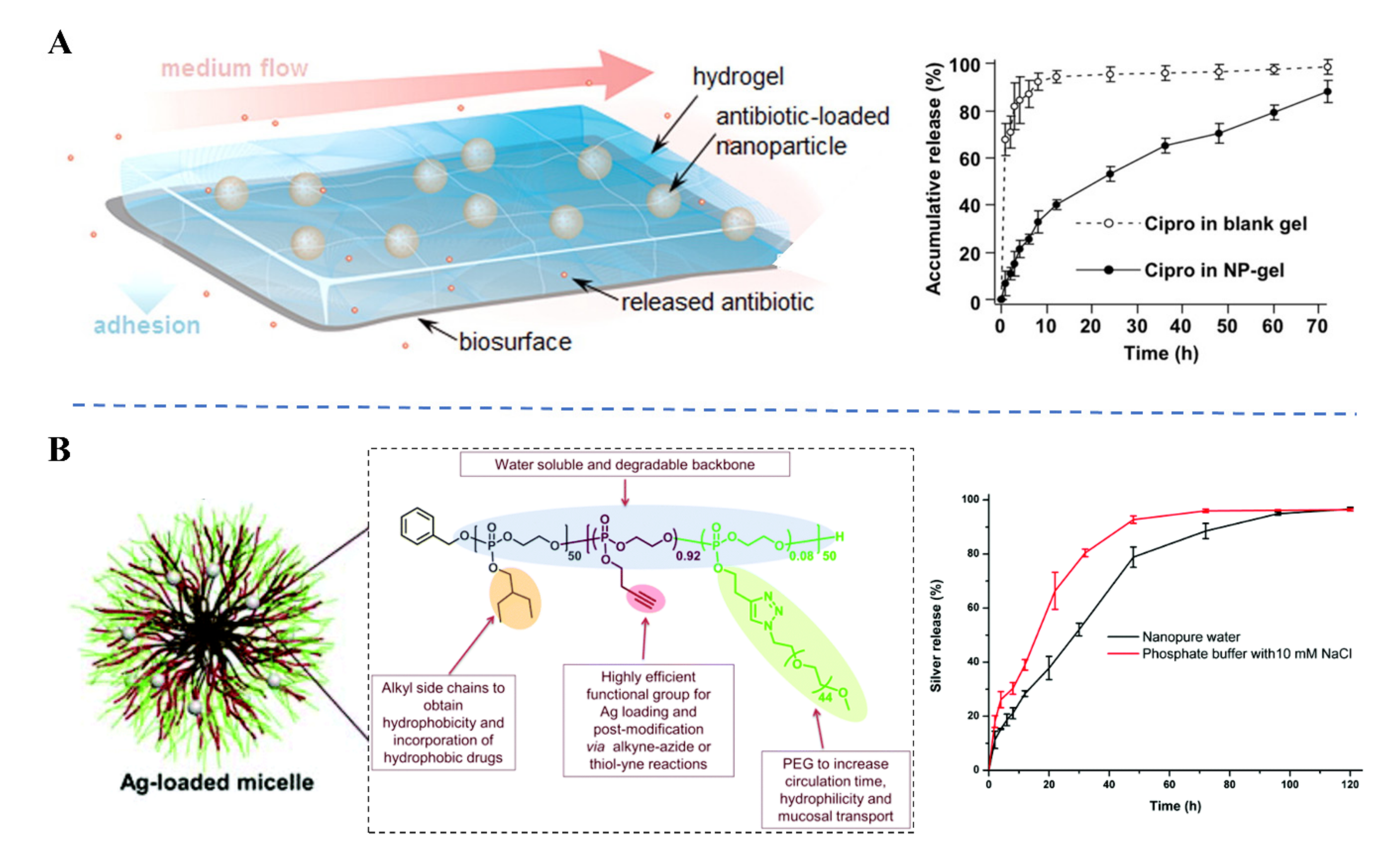
3. Controlled Antibiotic Delivery
3.1. Sustained Antibiotic Delivery
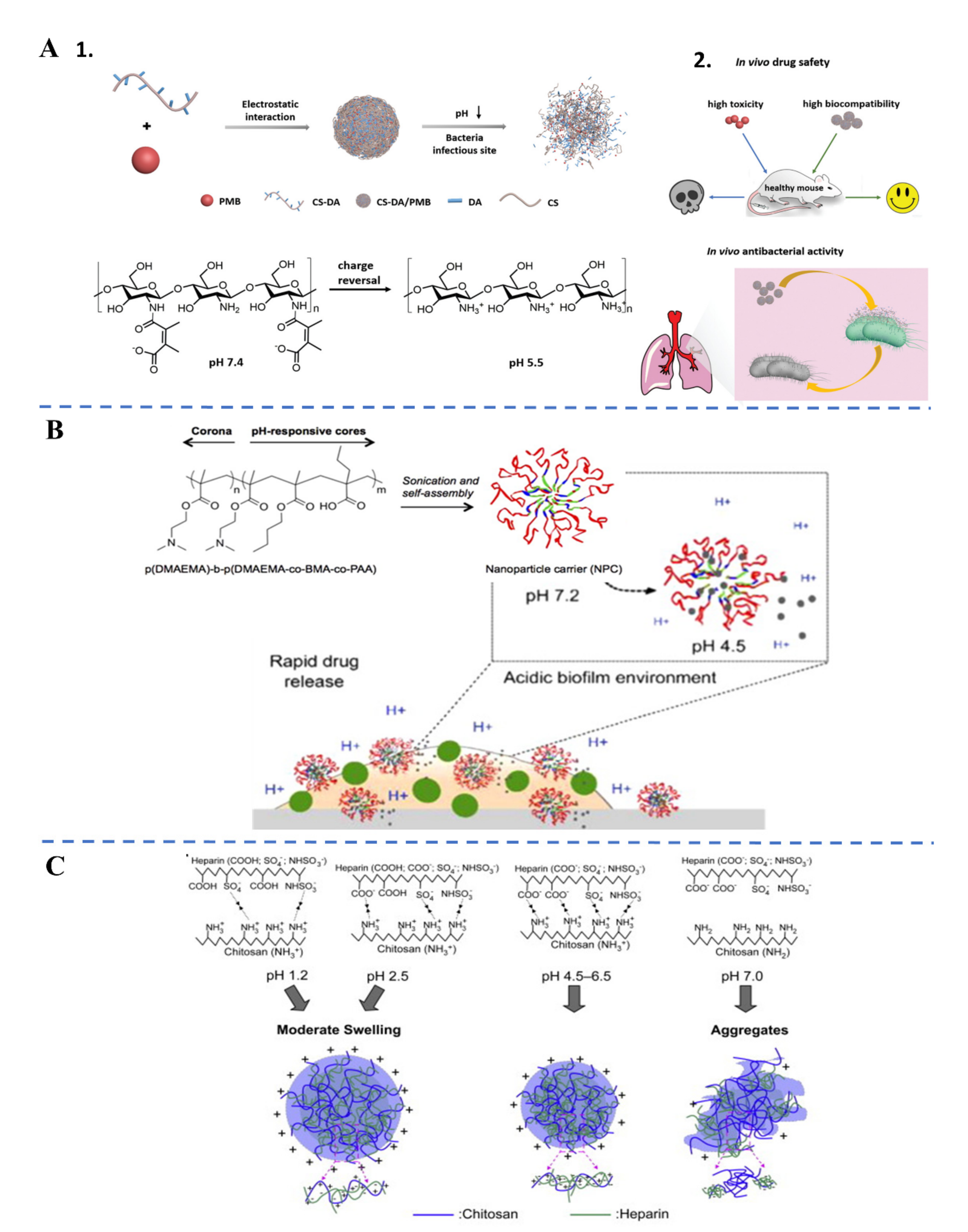
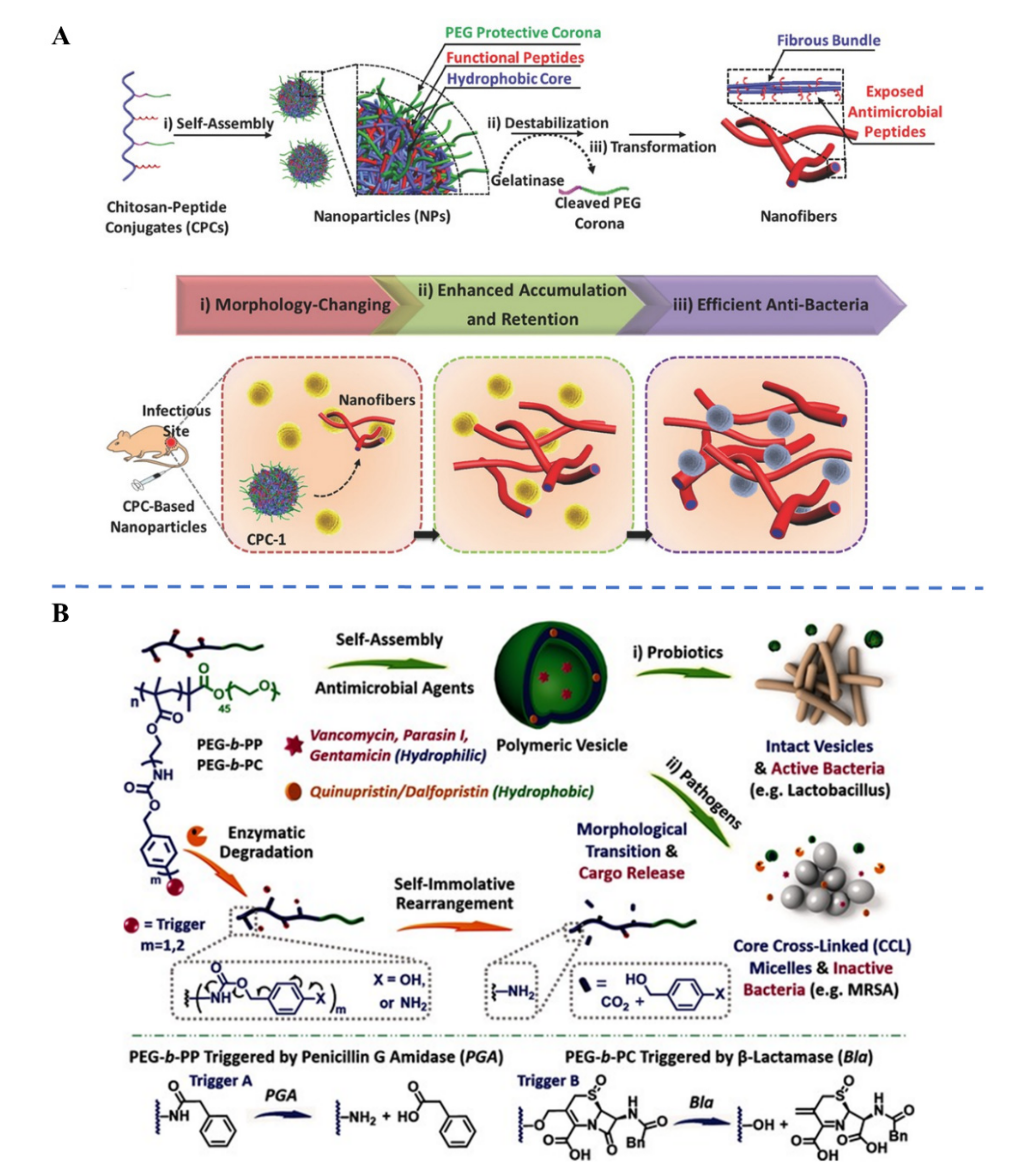
3.2. Endogenous Stimuli-Responsive Antibiotic Delivery
| Stimulus | Nanocarrier | Stimuli-Responsive Compound | Ref. | ||
|---|---|---|---|---|---|
| Endogenous stimuli | pH | Acid pH in infection site | Nanocomplex | 2,3-dimethyl maleic anhydride grafted chitoligosaccharide | [48] |
| Polymeric NPs | Poly(β-amino ester) | [62] | |||
| Acid pH in biofilm | Nanocomplex | 2,3-dimethyl maleic anhydride modified PEG | [63] | ||
| Micelle | Poly(2-(dimethylamino)ethyl methacrylate) | [19] | |||
| Acidic intracellular pH of the host cell | Polymeric NPs | Poly(diacetone acrylamide-hydrazone-isoniazid) | [51] | ||
| Neutral pH in H. Pylori site | Nanocomplex | Chitosan | [49] | ||
| Enzymes | Lipase: Phosphatase and phospholipase (S. aureus, E. coli, P. aeruginosa) | Micelle, NPs | Poly(ε-caprolactone) (PCL) | [54,56,59] | |
| Micelle | Poly (ß-amino ester) (ester bonds) PEG-DSPE (phosphoester bonds) | [55] | |||
| Nanogel | Polyphosphoester | [57] | |||
| Nanogel | PCL and polyphosphoester | [58] | |||
| Hyaluronidase | Nanocapsule, Hybrid NPs | Hyaluronic acid | [64,65,66] | ||
| Protease: Esterase, Proteinase K, P. aeruginosa’s elastase (LasB), Gelatinase | Polymeric NPs | Poly(L-lactide) | [67] | ||
| Nanocomplex | LasB responsive peptide | [68] | |||
| Peptide-grafted chitosan NPs | Gelatinase-cleavable peptide (GPLGVRGC) | [60] | |||
| β-lactamase and penicillin G amidase | Polymeric vesicles | Copolymers with enzyme-cleavable self-immolative side linkages | [61] | ||
| S. aureus nuclease | Silica nanocapsule | Engineered oligonucleotide (Gatekeeper with specific degradation by S. aureus nuclease) | [69] | ||
| α-toxin | Liposome-based nanoreactors | Liposome (Gatekeeper with toxin pore-formation activity) | [70] | ||
| Reactive oxygen species (ROS) | Polymeric NPs | Copolymer containing arylboronic ester moieties | [71,72] | ||
| Reduced glutathione (GSH) | Nanogel | Hydrogel with disulfide crosslinker (cystine dimethacrylate) | [73] | ||
| Micelle | Polyprodrug | [74] | |||
| Nitric oxide (NO) | Micelle | o-phenylenediamine moieties (NO-cleavable linker) | [75] | ||
| Exogenous stimuli | Light: Ultraviolet (UV), visible light, and near-infrared (NIR) | Dendrimer | Polymer–antibiotic conjugate via photocleavable ortho-nitrobenzyl | [76] | |
| Polymeric NPs | Polydopamine, Polypyrrole | [77,78] | |||
| Ultrasound | Polymeric NPs/MPs | Polymer matrix (alginate, PLGA) | [79] | ||
| Hybrid NPs | Complex Vancomycin-peptide target sequence (-D-Ala-D-Ala) | [80] | |||
| Magnetic field | Polymeric NPs/MPs | Iron oxide NPs | [81,82,83] | ||
3.3. Exogenous Stimuli-Responsive Antibiotic Delivery
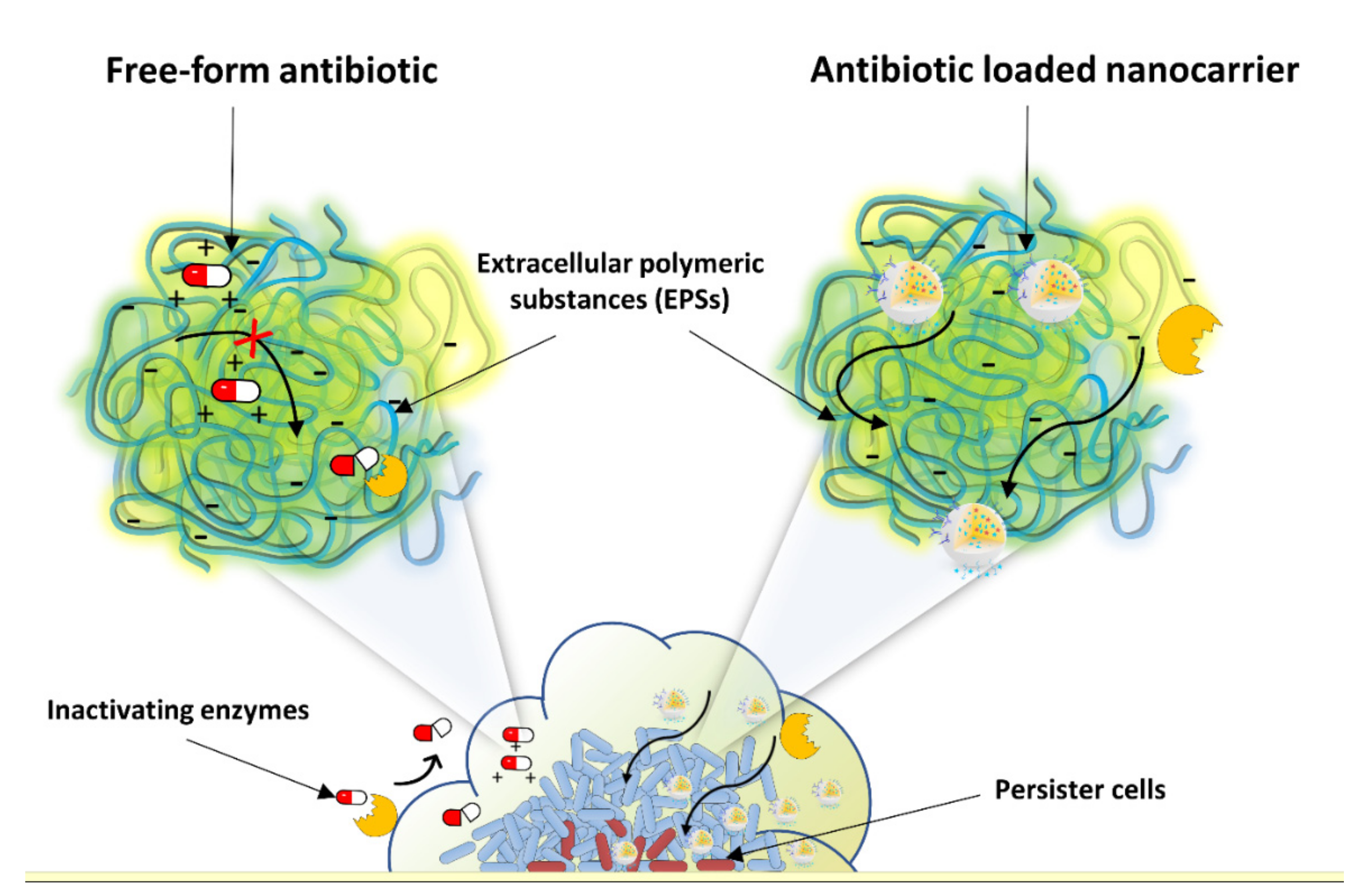
4. Targeted Delivery of Antibiotics to Sites of Infection
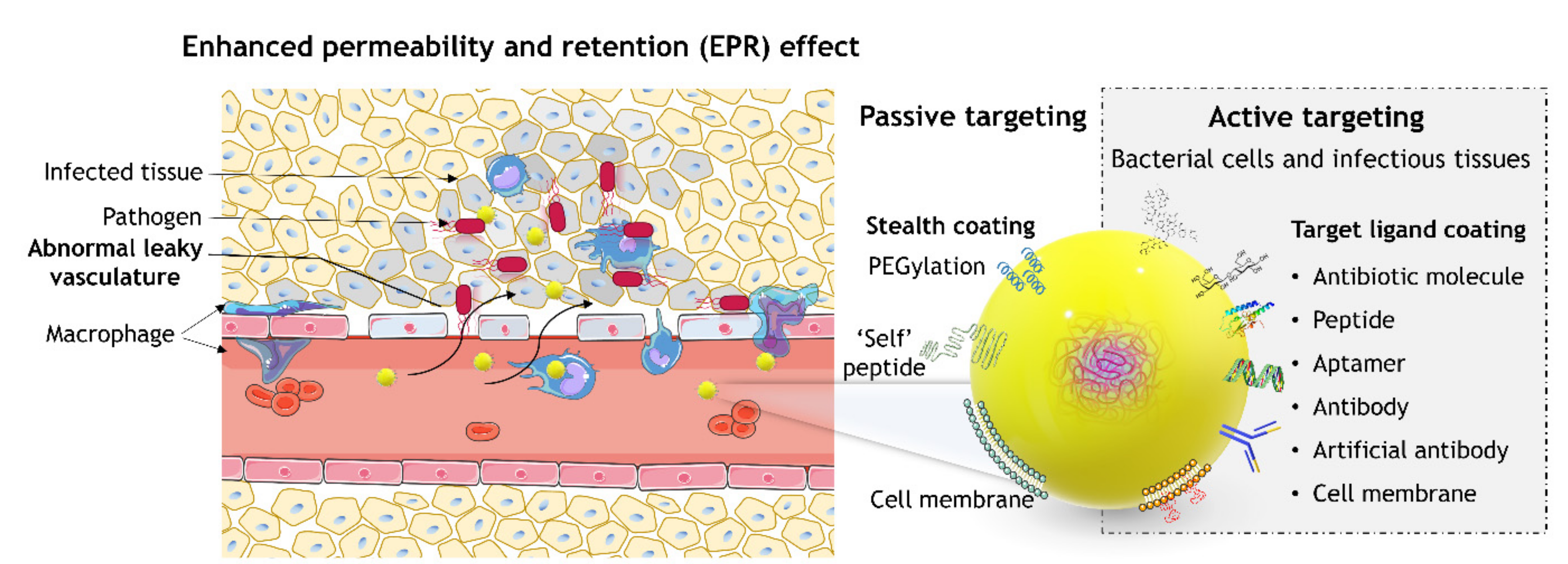
4.1. Passive Targeting: Enhanced Permeability and Retention Effect
4.2. Active Targeting
4.2.1. Active Targeting to Bacterial Cells
4.2.2. Active Targeting to Infectious Microenvironments
5. Codelivery Platforms for Combination Antibiotic Therapy
6. Discussions and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Ropponen, H.-K.; Richter, R.; Hirsch, A.K.H.; Lehr, C.-M. Mastering the Gram-negative bacterial barrier—Chemical approaches to increase bacterial bioavailability of antibiotics. Adv. Drug Deliv. Rev. 2021, 172, 339–360. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Birk, S.E.; Boisen, A.; Nielsen, L.H. Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 2021, 174, 30–52. [Google Scholar] [CrossRef] [PubMed]
- Abed, N.; Couvreur, P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int. J. Antimicrob. Agents 2014, 43, 485–496. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Hermsen, R.; Deris, J.B.; Hwa, T. On the rapidity of antibiotic resistance evolution facilitated by a concentration gradient. Proc. Natl. Acad. Sci. USA 2012, 109, 10775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.-H.; Bao, Y.; Yang, X.-Z.; Zhu, Y.-H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef] [PubMed]
- Tsume, Y.; Mudie, D.M.; Langguth, P.; Amidon, G.E.; Amidon, G.L. The Biopharmaceutics Classification System: Subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur. J. Pharm. Sci. 2014, 57, 152–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tehler, U.; Fagerberg, J.H.; Svensson, R.; Larhed, M.; Artursson, P.; Bergström, C.A.S. Optimizing Solubility and Permeability of a Biopharmaceutics Classification System (BCS) Class 4 Antibiotic Drug Using Lipophilic Fragments Disturbing the Crystal Lattice. J. Med. Chem. 2013, 56, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, C.; Kadam, R.S.; Chandler, J.W.; Hutcherson, S.L.; Kompella, U.B. Nanosized Dendritic Polyguanidilyated Translocators for Enhanced Solubility, Permeability, and Delivery of Gatifloxacin. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5804–5816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhang, C.; Huang, F.; Liu, J.; Zhang, Y.; Yang, C.; Ren, C.; Chu, L.; Liu, B.; Liu, J. Triclosan-based supramolecular hydrogels as nanoantibiotics for enhanced antibacterial activity. J. Control. Release 2020, 324, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Gheffar, C.; Le, H.; Jouenne, T.; Schaumann, A.; Corbière, A.; Vaudry, D.; LeCerf, D.; Karakasyan, C. Antibacterial Activity of Ciprofloxacin-Loaded Poly(lactic-co-glycolic acid)-Nanoparticles Against Staphylococcus aureus. Part. Part. Syst. Charact. 2021, 38, 2000253. [Google Scholar] [CrossRef]
- Kalita, S.; Devi, B.; Kandimalla, R.; Sharma, K.K.; Sharma, A.; Kalita, K.; Kataki, A.C.; Kotoky, J. Chloramphenicol encapsulated in poly-ε-caprolactone-pluronic composite: Nanoparticles for treatment of MRSA-infected burn wounds. Int. J. Nanomed. 2015, 10, 2971–2984. [Google Scholar]
- Masood, F.; Yasin, T.; Bukhari, H.; Mujahid, M. Characterization and application of roxithromycin loaded cyclodextrin based nanoparticles for treatment of multidrug resistant bacteria. Mater. Sci. Eng. C 2016, 61, 1–7. [Google Scholar] [CrossRef]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landis, R.F.; Gupta, A.; Lee, Y.-W.; Wang, L.-S.; Golba, B.; Couillaud, B.; Ridolfo, R.; Das, R.; Rotello, V.M. Cross-Linked Polymer-Stabilized Nanocomposites for the Treatment of Bacterial Biofilms. ACS Nano 2017, 11, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Rahbar Takrami, S.; Ranji, N.; Sadeghizadeh, M. Antibacterial effects of curcumin encapsulated in nanoparticles on clinical isolates of Pseudomonas aeruginosa through downregulation of efflux pumps. Mol. Biol. Rep. 2019, 46, 2395–2404. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Popat, A.; Blaskovich, M.; Falconer, J.R. Formulation technologies and advances for oral delivery of novel nitroimidazoles and antimicrobial peptides. J. Control. Release 2020, 324, 728–749. [Google Scholar] [CrossRef]
- Rishi, P.; Bhogal, A.; Arora, S.; Pandey, S.K.; Verma, I.; Kaur, I.P. Improved oral therapeutic potential of nanoencapsulated cryptdin formulation against Salmonella infection. Eur. J. Pharm. Sci. 2015, 72, 27–33. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Zhang, H.; Liu, G.; Jiang, Z.; Qiu, Q.; Yu, C.; Murthy, N.; Zhao, K.; Lovell, J.F.; et al. Antibiotic Cross-linked Micelles with Reduced Toxicity for Multidrug-Resistant Bacterial Sepsis Treatment. ACS Appl. Mater. Interfaces 2021, 13, 9630–9642. [Google Scholar] [CrossRef]
- Jani, S.; Ramirez, M.S.; Tolmasky, M.E. Silencing Antibiotic Resistance with Antisense Oligonucleotides. Biomedicines 2021, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- González-Paredes, A.; Sitia, L.; Ruyra, A.; Morris, C.J.; Wheeler, G.N.; McArthur, M.; Gasco, P. Solid lipid nanoparticles for the delivery of anti-microbial oligonucleotides. Eur. J. Pharm. Biopharm. 2019, 134, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Bikard, D.; Barrangou, R. Using CRISPR-Cas systems as antimicrobials. Curr. Opin. Microbiol. 2017, 37, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Edson, J.A.; Kwon, Y.J. RNAi for silencing drug resistance in microbes toward development of nanoantibiotics. J. Control. Release 2014, 189, 150–157. [Google Scholar] [CrossRef]
- Perche, F.; le Gall, T.; Montier, T.; Pichon, C.; Malinge, J.-M. Cardiolipin-Based Lipopolyplex Platform for the Delivery of Diverse Nucleic Acids into Gram-Negative Bacteria. Pharmaceuticals 2019, 12, 81. [Google Scholar] [CrossRef] [Green Version]
- Greenhalgh, K.; Turos, E. In vivo studies of polyacrylate nanoparticle emulsions for topical and systemic applications. Nanomedicine 2009, 5, 46–54. [Google Scholar] [CrossRef]
- Shaaban, M.I.; Shaker, M.A.; Mady, F.M. Imipenem/cilastatin encapsulated polymeric nanoparticles for destroying carbapenem-resistant bacterial isolates. J. Nanobiotechnol. 2017, 15, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
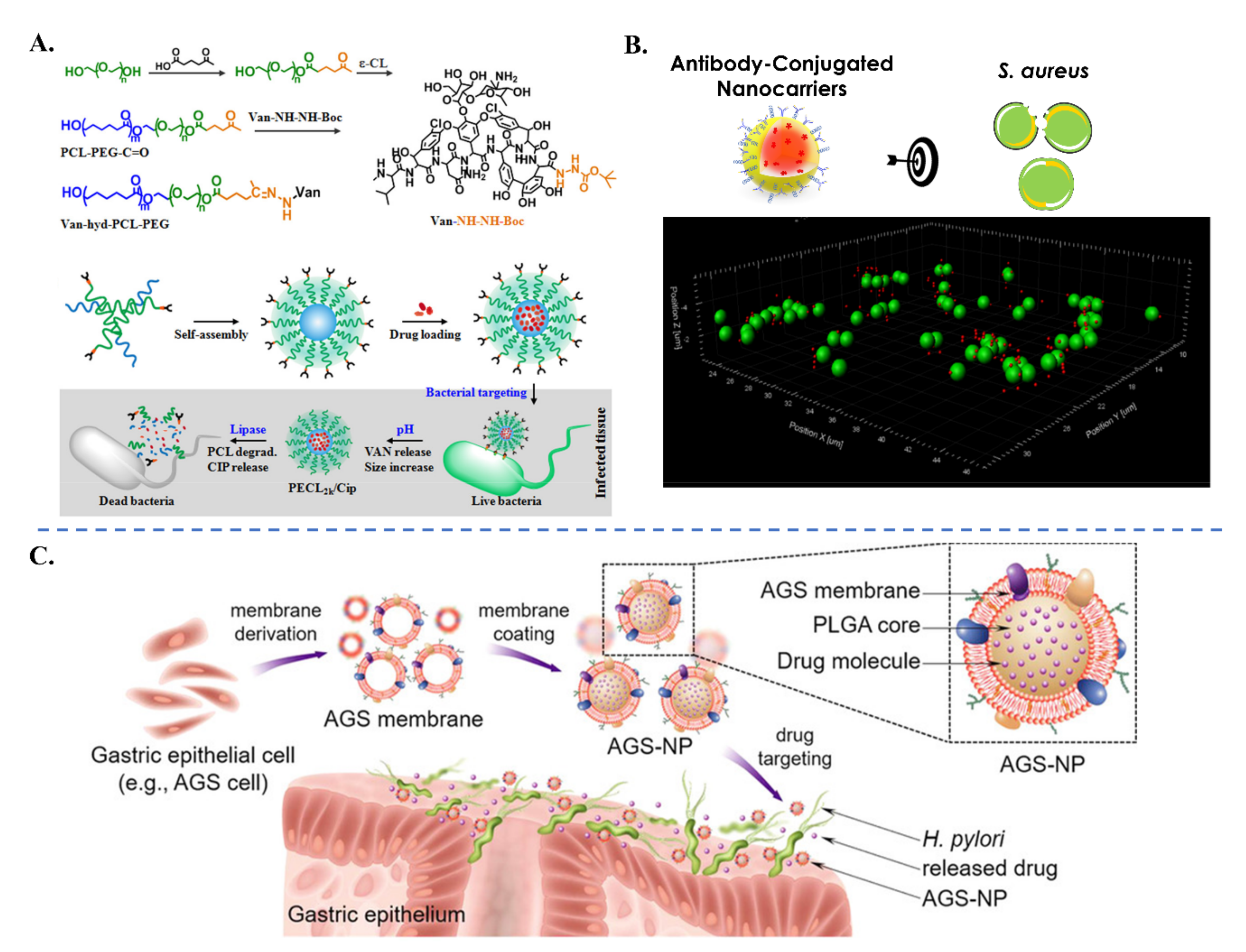
- Le, H.; Arnoult, C.; Dé, E.; Schapman, D.; Galas, L.; le Cerf, D.; Karakasyan, C. Antibody-Conjugated Nanocarriers for Targeted Antibiotic Delivery: Application in the Treatment of Bacterial Biofilms. Biomacromolecules 2021, 22, 1639–1653. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.-T.; Chen, C.-F.; Chu, I.M.; Li, Y.-M.; Hsu, W.-H.; Hsu, R.W.-W.; Chang, P.-J. Treatment of osteomyelitis with teicoplanin-encapsulated biodegradable thermosensitive hydrogel nanoparticles. Biomaterials 2010, 31, 5227–5236. [Google Scholar] [CrossRef]
- Yus, C.; Irusta, S.; Sebastian, V.; Arruebo, M. Controlling Particle Size and Release Kinetics in the Sustained Delivery of Oral Antibiotics Using pH-Independent Mucoadhesive Polymers. Mol. Pharm. 2020, 17, 3314–3327. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, J.; Chen, M.; Gong, H.; Thamphiwatana, S.; Eckmann, L.; Gao, W.; Zhang, L. A Bioadhesive Nanoparticle-Hydrogel Hybrid System for Localized Antimicrobial Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 18367–18374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillen, K.; Vandervoort, J.; van den Mooter, G.; Ludwig, A. Evaluation of ciprofloxacin-loaded Eudragit® RS100 or RL100/PLGA nanoparticles. Int. J. Pharm. 2006, 314, 72–82. [Google Scholar] [CrossRef]
- Zhang, F.; Smolen, J.A.; Zhang, S.; Li, R.; Shah, P.N.; Cho, S.; Wang, H.; Raymond, J.E.; Cannon, C.L.; Wooley, K.L. Degradable polyphosphoester-based silver-loaded nanoparticles as therapeutics for bacterial lung infections. Nanoscale 2015, 7, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, S.; Khuller, G.K. Lectin-functionalized poly (lactide-co-glycolide) nanoparticles as oral/aerosolized antitubercular drug carriers for treatment of tuberculosis. J. Antimicrob. Chemother. 2004, 54, 761–766. [Google Scholar] [CrossRef]
- Lueth, P.; Haughney, S.L.; Binnebose, A.M.; Mullis, A.S.; Peroutka-Bigus, N.; Narasimhan, B.; Bellaire, B.H. Nanotherapeutic provides dose sparing and improved antimicrobial activity against Brucella melitensis infections. J. Control. Release 2019, 294, 288–297. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Esin, S.; Gazzarri, M.; Batoni, G.; Chiellini, F. Preparation, physical-chemical and biological characterization of chitosan nanoparticles loaded with lysozyme. Int. J. Biol. Macromol. 2014, 67, 124–131. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Barcia-Macay, M.; Lemaire, S.; Tulkens, P.M. Cellular pharmacodynamics and pharmacokinetics of antibiotics: Current views and perspectives. Curr. Opin. Drug Discov. Dev. 2006, 9, 218–230. [Google Scholar]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maji, R.; Omolo, C.A.; Agrawal, N.; Maduray, K.; Hassan, D.; Mokhtar, C.; Mackhraj, I.; Govender, T. pH-Responsive Lipid-Dendrimer Hybrid Nanoparticles: An Approach To Target and Eliminate Intracellular Pathogens. Mol. Pharm. 2019, 16, 4594–4609. [Google Scholar] [CrossRef]
- Sonawane, S.J.; Kalhapure, R.S.; Jadhav, M.; Rambharose, S.; Mocktar, C.; Govender, T. AB2-type amphiphilic block copolymer containing a pH-cleavable hydrazone linkage for targeted antibiotic delivery. Int. J. Pharm. 2020, 575, 118948. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Gao, Y.; Liu, J.; Deng, Y.; Hu, D.; Jin, Q.; Ji, J. Polymyxin B-Polysaccharide Polyion Nanocomplex with Improved Biocompatibility and Unaffected Antibacterial Activity for Acute Lung Infection Management. Adv. Healthc. Mater. 2020, 9, 1901542. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chang, C.-H.; Wu, Y.-S.; Hsu, Y.-M.; Chiou, S.-F.; Chen, Y.-J. Development of pH-responsive chitosan/heparin nanoparticles for stomach-specific anti-Helicobacter pylori therapy. Biomaterials 2009, 30, 3332–3342. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.D.; Su, F.Y.; Chiu, D.Y.; Srinivasan, S.; Wilson, J.T.; Ratner, D.M.; Stayton, P.S.; Convertine, A.J. Dynamic intracellular delivery of antibiotics via pH-responsive polymersomes. Polym. Chem. 2015, 6, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunn, A.M.; Unnikrishnan, M.; Perrier, S. Dual pH-Responsive Macrophage-Targeted Isoniazid Glycoparticles for Intracellular Tuberculosis Therapy. Biomacromolecules 2021, 22, 3756–3768. [Google Scholar] [CrossRef]
- Arif, M.; Dong, Q.-J.; Raja, M.A.; Zeenat, S.; Chi, Z.; Liu, C.-G. Development of novel pH-sensitive thiolated chitosan/PMLA nanoparticles for amoxicillin delivery to treat Helicobacter pylori. Mater. Sci. Eng. C 2018, 83, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lin, Y.-H.; Yeh, C.-L.; Chen, Y.-C.; Chiou, S.-F.; Hsu, Y.-M.; Chen, Y.-S.; Wang, C.-C. Nanoparticles Incorporated in pH-Sensitive Hydrogels as Amoxicillin Delivery for Eradication of Helicobacter pylori. Biomacromolecules 2010, 11, 133–142. [Google Scholar] [CrossRef] [PubMed]
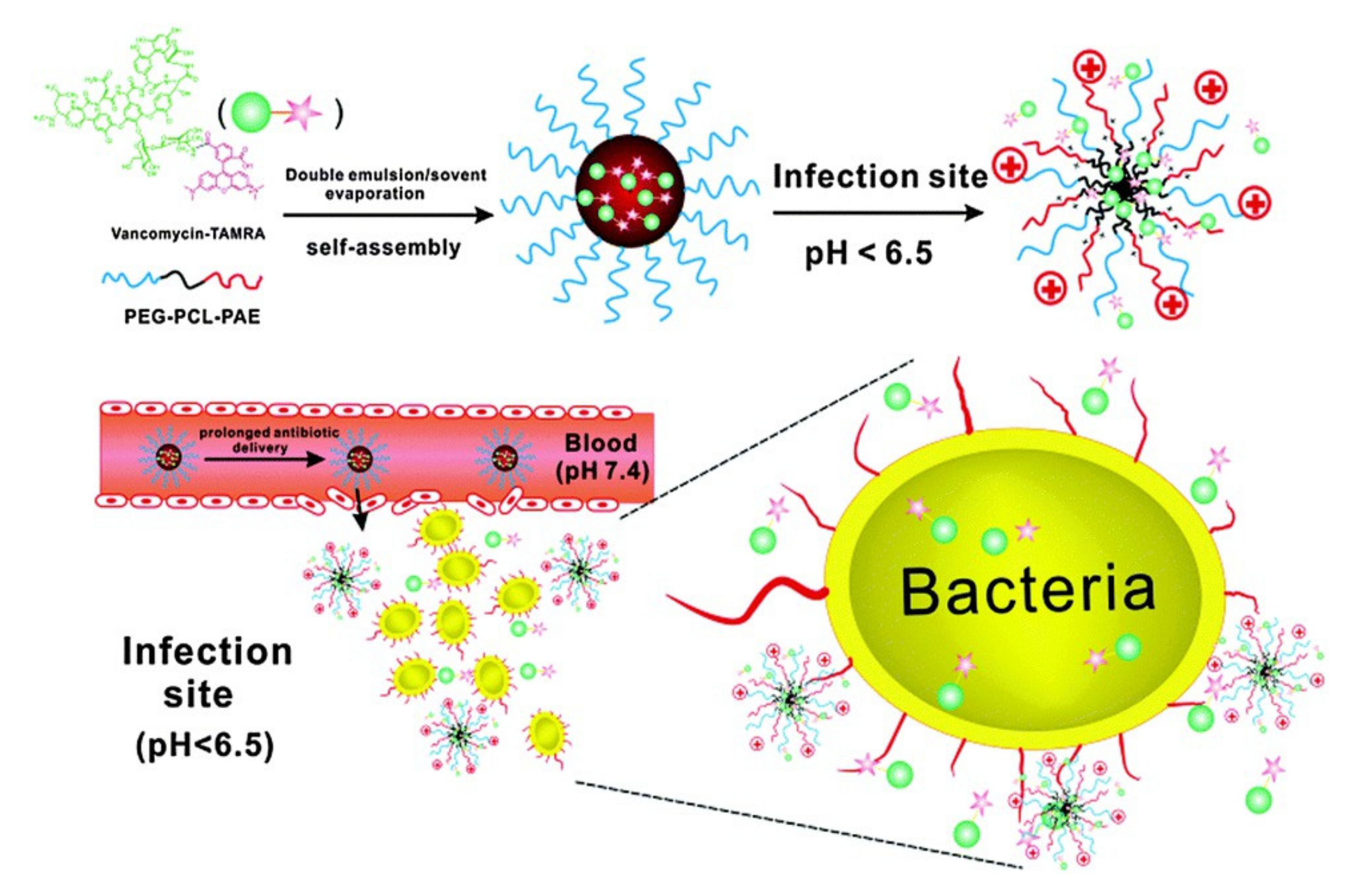
- Chen, M.; Xie, S.; Wei, J.; Song, X.; Ding, Z.; Li, X. Antibacterial Micelles with Vancomycin-Mediated Targeting and pH/Lipase-Triggered Release of Antibiotics. ACS Appl. Mater. Interfaces 2018, 10, 36814–36823. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gao, J.; Wang, Z. Bioresponsive Nanoparticles Targeted to Infectious Microenvironments for Sepsis Management. Adv. Mater. 2018, 30, 1803618. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, L.; Meng, F.; Qiao, Z.; Yao, Y.; Luo, J. Triclosan loaded polyurethane micelles with pH and lipase sensitive properties for antibacterial applications and treatment of biofilms. Mater. Sci. Eng. C 2018, 93, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.-H.; Li, Y.-J.; Bao, Y.; Yang, X.-Z.; Hu, B.; Wang, J. Bacteria-Responsive Multifunctional Nanogel for Targeted Antibiotic Delivery. Adv. Mater. 2012, 24, 6175–6180. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.-H.; Bao, Y.; Yang, X.-Z.; Wang, Y.-C.; Sun, B.; Wang, J. Lipase-Sensitive Polymeric Triple-Layered Nanogel for “On-Demand” Drug Delivery. J. Am. Chem. Soc. 2012, 134, 4355–4362. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ahmed, N.; Permana, A.D.; Rodgers, A.M.; Donnelly, R.F.; Rehman, A.U. Enhancement in Site-Specific Delivery of Carvacrol against Methicillin Resistant Staphylococcus aureus Induced Skin Infections Using Enzyme Responsive Nanoparticles: A Proof of Concept Study. Pharmaceutics 2019, 11, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, G.-B.; Zhang, D.; Liu, F.-H.; Qiao, Z.-Y.; Wang, H. An “On-Site Transformation” Strategy for Treatment of Bacterial Infection. Adv. Mater. 2017, 29, 1703461. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Wang, X.; Hu, J.; Liu, S. Enzyme-Responsive Polymeric Vesicles for Bacterial-Strain-Selective Delivery of Antimicrobial Agents. Angew. Chem. Int. Ed. 2016, 55, 1760–1764. [Google Scholar] [CrossRef]
- Chu, L.; Gao, H.; Cheng, T.; Zhang, Y.; Liu, J.; Huang, F.; Yang, C.; Shi, L.; Liu, J. A charge-adaptive nanosystem for prolonged and enhanced in vivo antibiotic delivery. Chem. Commun. 2016, 52, 6265–6268. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.; Chai, M.; Li, X.; Deng, Y.; Jin, Q.; Ji, J. Size and Charge Adaptive Clustered Nanoparticles Targeting the Biofilm Microenvironment for Chronic Lung Infection Management. ACS Nano 2020, 14, 5686–5699. [Google Scholar] [CrossRef] [PubMed]
- Baier, G.; Cavallaro, A.; Vasilev, K.; Mailänder, V.; Musyanovych, A.; Landfester, K. Enzyme Responsive Hyaluronic Acid Nanocapsules Containing Polyhexanide and Their Exposure to Bacteria to Prevent Infection. Biomacromolecules 2013, 14, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Long, Y.; Li, Q.-L.; Han, S.; Ma, J.; Yang, Y.-W.; Gao, H. Layer-by-Layer (LBL) Self-Assembled Biohybrid Nanomaterials for Efficient Antibacterial Applications. ACS Appl. Mater. Interfaces 2015, 7, 17255–17263. [Google Scholar] [CrossRef]
- Ji, H.; Dong, K.; Yan, Z.; Ding, C.; Chen, Z.; Ren, J.; Qu, X. Bacterial Hyaluronidase Self-Triggered Prodrug Release for Chemo-Photothermal Synergistic Treatment of Bacterial Infection. Small 2016, 12, 6200–6206. [Google Scholar] [CrossRef]
- Baier, G.; Cavallaro, A.; Friedemann, K.; Müller, B.; Glasser, G.; Vasilev, K.; Landfester, K. Enzymatic degradation of poly(l-lactide) nanoparticles followed by the release of octenidine and their bactericidal effects. Nanomedicine 2014, 10, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Insua, I.; Liamas, E.; Zhang, Z.; Peacock, A.F.; Krachler, A.M.; Fernandez-Trillo, F. Enzyme-responsive polyion complex (PIC) nanoparticles for the targeted delivery of antimicrobial polymers. Polym. Chem. 2016, 7, 2684–2690. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, F.J.; Hernandez, L.I.; Kavruk, M.; Arıca, Y.M.; Bayramoğlu, G.; Borsa, B.A.; Öktem, H.A.; Schäfer, T.; Özalp, V.C. NanoKeepers: Stimuli responsive nanocapsules for programmed specific targeting and drug delivery. Chem. Commun. 2014, 50, 9489–9492. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Z.; Wang, H.; Han, H. Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections. Nat. Commun. 2019, 10, 4464. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Yang, G.; Xia, L.; Yu, F.; Chen, C.; Zhang, L.; Cao, H. A pH/H2O2 dual triggered nanoplatform for enhanced photodynamic antibacterial efficiency. J. Mater. Chem. B 2021, 9, 5076–5082. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Q.; Feng, W.; Pu, W.; Ding, J.; Zhang, H.; Li, X.; Yang, B.; Dai, Q.; Cheng, L.; et al. Targeted delivery of antibiotics to the infected pulmonary tissues using ROS-responsive nanoparticles. J. Nanobiotechnol. 2019, 17, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, J.; Chen, W.; Angsantikul, P.; Spiekermann, K.A.; Fang, R.H.; Gao, W.; Zhang, L. Erythrocyte membrane-coated nanogel for combinatorial antivirulence and responsive antimicrobial delivery against Staphylococcus aureus infection. J. Control. Release 2017, 263, 185–191. [Google Scholar] [CrossRef]
- Cao, B.; Xiao, F.; Xing, D.; Hu, X. Polyprodrug Antimicrobials: Remarkable Membrane Damage and Concurrent Drug Release to Combat Antibiotic Resistance of Methicillin-Resistant Staphylococcus aureus. Small 2018, 14, 1802008. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xiao, Y.; Liu, Y.; Sun, F.; Qiu, Y.; Mu, H.; Duan, J. Hyaluronic acid-based levofloxacin nanomicelles for nitric oxide-triggered drug delivery to treat bacterial infections. Carbohydr. Polym. 2020, 229, 115479. [Google Scholar] [PubMed]
- Wong, P.T.; Tang, S.; Mukherjee, J.; Tang, K.; Gam, K.; Isham, D.; Murat, C.; Sun, R.; Baker, J.R.; Choi, S.K. Light-controlled active release of photocaged ciprofloxacin for lipopolysaccharide-targeted drug delivery using dendrimer conjugates. Chem. Commun. 2016, 52, 10357–10360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95. [Google Scholar]
- Chiang, W.-L.; Lin, T.-T.; Sureshbabu, R.; Chia, W.-T.; Hsiao, H.-C.; Liu, H.-Y.; Yang, C.-M.; Sung, H.-W. A rapid drug release system with a NIR light-activated molecular switch for dual-modality photothermal/antibiotic treatments of subcutaneous abscesses. J. Control. Release 2015, 199, 53–62. [Google Scholar] [CrossRef]
- Di, J.; Kim, J.; Hu, Q.; Jiang, X.; Gu, Z. Spatiotemporal drug delivery using laser-generated-focused ultrasound system. J. Control. Release 2015, 220, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Huo, S.; Zhao, P.; Shi, Z.; Zou, M.; Yang, X.; Warszawik, E.; Loznik, M.; Göstl, R.; Herrmann, A. Mechanochemical bond scission for the activation of drugs. Nat. Chem. 2021, 13, 131–139. [Google Scholar]
- Hua, X.; Tan, S.; Bandara, H.M.H.N.; Fu, Y.; Liu, S.; Smyth, H.D.C. Externally Controlled Triggered-Release of Drug from PLGA Micro and Nanoparticles. PLoS ONE 2014, 9, e114271. [Google Scholar] [CrossRef] [Green Version]
- Sirivisoot, S.; Harrison, B.S. Magnetically stimulated ciprofloxacin release from polymeric microspheres entrapping iron oxide nanoparticles. Int. J. Nanomed. 2015, 10, 4447–4458. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, A.; Harris, M.A.; LeVine, D.; Ghimire, M.; Jennings, J.A.; Morshed, B.I.; Haggard, W.O.; Bumgardner, J.D.; Mishra, S.R.; Fujiwara, T. Magnetic stimulus responsive vancomycin drug delivery system based on chitosan microbeads embedded with magnetic nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2169–2176. [Google Scholar] [CrossRef]
- Kumari, P.; Kulkarni, A.; Sharma, A.K.; Chakrapani, H. Visible-Light Controlled Release of a Fluoroquinolone Antibiotic for Antimicrobial Photopharmacology. ACS Omega 2018, 3, 2155–2160. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [Green Version]
- Boissenot, T.; Bordat, A.; Fattal, E.; Tsapis, N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. J. Control. Release 2016, 241, 144–163. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, J.; Liu, X.; Wang, R.; Xia, L. A Review of the Combination Therapy of Low Frequency Ultrasound with Antibiotics. BioMed Res. Int. 2017, 2017, 2317846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzopardi, E.A.; Ferguson, E.L.; Thomas, D.W. The enhanced permeability retention effect: A new paradigm for drug targeting in infection. J. Antimicrob. Chemother. 2013, 68, 257–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Bakker-Woudenberg, I.A.J.M.; Lokerse, A.F.; ten Kate, M.T.; Mouton, J.W.; Woodle, M.C.; Storm, G. Liposomes with Prolonged Blood Circulation and Selective Localization in Klebsiella pneumoniae-Infected Lung Tissue. J. Infect. Dis. 1993, 168, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.; Licciardi, M.; Mansueto, S.; Schillaci, D.; Giammona, G. Amoxicillin-loaded polyethylcyanoacrylate nanoparticles: Influence of PEG coating on the particle size, drug release rate and phagocytic uptake. Biomaterials 2001, 22, 2857–2865. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941. [Google Scholar] [CrossRef] [PubMed]
- Radovic-Moreno, A.F.; Lu, T.K.; Puscasu, V.A.; Yoon, C.J.; Langer, R.; Farokhzad, O.C. Surface Charge-Switching Polymeric Nanoparticles for Bacterial Cell Wall-Targeted Delivery of Antibiotics. ACS Nano 2012, 6, 4279–4287. [Google Scholar] [CrossRef] [Green Version]
- Kell, A.J.; Stewart, G.; Ryan, S.; Peytavi, R.; Boissinot, M.; Huletsky, A.; Bergeron, M.G.; Simard, B. Vancomycin-Modified Nanoparticles for Efficient Targeting and Preconcentration of Gram-Positive and Gram-Negative Bacteria. ACS Nano 2008, 2, 1777–1788. [Google Scholar] [CrossRef]
- Wong, P.T.; Tang, S.; Tang, K.; Coulter, A.; Mukherjee, J.; Gam, K.; Baker, J.R.; Choi, S.K. A lipopolysaccharide binding heteromultivalent dendrimer nanoplatform for Gram negative cell targeting. J. Mater. Chem. B 2015, 3, 1149–1156. [Google Scholar] [CrossRef]
- Jiang, H.; Xiong, M.; Bi, Q.; Wang, Y.; Li, C. Self-enhanced targeted delivery of a cell wall- and membrane-active antibiotics, daptomycin, against staphylococcal pneumonia. Acta Pharm. Sin. B 2016, 6, 319–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Han, X.; Yang, Y.; Qiao, H.; Yu, Z.; Liu, Y.; Wang, J.; Tang, T. Bacteria-Targeting Nanoparticles with Microenvironment-Responsive Antibiotic Release to Eliminate Intracellular Staphylococcus aureus and Associated Infection. ACS Appl. Mater. Interfaces 2018, 10, 14299–14311. [Google Scholar] [CrossRef]
- Eissa, A.M.; Abdulkarim, A.; Sharples, G.J.; Cameron, N.R. Glycosylated Nanoparticles as Efficient Antimicrobial Delivery Agents. Biomacromolecules 2016, 17, 2672–2679. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yu, C.; Yu, Y.; Wei, X.; Duan, X.; Dai, X.; Zhang, X. Bioinspired Heteromultivalent Ligand-Decorated Nanotherapeutic for Enhanced Photothermal and Photodynamic Therapy of Antibiotic-Resistant Bacterial Pneumonia. ACS Appl. Mater. Interfaces 2019, 11, 39648–39661. [Google Scholar] [CrossRef]
- Michaud, G.; Visini, R.; Bergmann, M.; Salerno, G.; Bosco, R.; Gillon, E.; Richichi, B.; Nativi, C.; Imberty, A.; Stocker, A.; et al. Overcoming antibiotic resistance in Pseudomonas aeruginosa biofilms using glycopeptide dendrimers. Chem. Sci. 2016, 7, 166–182. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Zhao, Y.; Wang, Y.; Zhao, M.; Yodsanit, N.; Xie, R.; Andes, D.; Gong, S. A Dual-Responsive Antibiotic-Loaded Nanoparticle Specifically Binds Pathogens and Overcomes Antimicrobial-Resistant Infections. Adv. Mater. 2021, 33, 2006772. [Google Scholar] [CrossRef]
- Kavruk, M.; Celikbicak, O.; Ozalp, V.C.; Borsa, B.A.; Hernandez, F.J.; Bayramoglu, G.; Salih, B.; Arica, M.Y. Antibiotic loaded nanocapsules functionalized with aptamer gates for targeted destruction of pathogens. Chem. Commun. 2015, 51, 8492–8495. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef]
- Pang, X.; Liu, X.; Cheng, Y.; Zhang, C.; Ren, E.; Liu, C.; Zhang, Y.; Zhu, J.; Chen, X.; Liu, G. Sono-Immunotherapeutic Nanocapturer to Combat Multidrug-Resistant Bacterial Infections. Adv. Mater. 2019, 31, 1902530. [Google Scholar] [CrossRef]
- Long, Y.; Li, Z.; Bi, Q.; Deng, C.; Chen, Z.; Bhattachayya, S.; Li, C. Novel polymeric nanoparticles targeting the lipopolysaccharides of Pseudomonas aeruginosa. Int. J. Pharm. 2016, 502, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-M.J.; Fang, R.H.; Wang, K.-C.; Luk, B.T.; Thamphiwatana, S.; Dehaini, D.; Nguyen, P.; Angsantikul, P.; Wen, C.H.; Kroll, A.V.; et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature 2015, 526, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Zhang, L.; Miron, R.J.; Liang, J.; Shi, M.; Mo, W.; Zheng, S.; Zhao, Y.; Zhang, Y. Pretreated Macrophage-Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Adv. Mater. 2018, 30, 1804023. [Google Scholar] [CrossRef]
- Angsantikul, P.; Thamphiwatana, S.; Zhang, Q.; Spiekermann, K.; Zhuang, J.; Fang, R.H.; Gao, W.; Obonyo, M.; Zhang, L. Coating Nanoparticles with Gastric Epithelial Cell Membrane for Targeted Antibiotic Delivery against Helicobacter pylori Infection. Adv. Ther. 2018, 1, 1800016. [Google Scholar] [CrossRef]
- Gao, J.; Wang, S.; Dong, X.; Leanse, L.G.; Dai, T.; Wang, Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun. Biol. 2020, 3, 680. [Google Scholar] [CrossRef]
- Gao, J.; Dong, X.; Su, Y.; Wang, Z. Human neutrophil membrane-derived nanovesicles as a drug delivery platform for improved therapy of infectious diseases. Acta Biomater. 2021, 123, 354–363. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.; Fan, B.; Wang, Y.; Mao, Z.; Wang, W.; Wu, J. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release 2020, 321, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Gao, J.; Wang, Z. Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection. ACS Nano 2015, 9, 11800–11811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunus Basha, R.; TS, S.K.; Doble, M. Dual delivery of tuberculosis drugs via cyclodextrin conjugated curdlan nanoparticles to infected macrophages. Carbohydr. Polym. 2019, 218, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Xu, L.; Yang, B.; Fan, F.; Yang, L. Kill the Real with the Fake: Eliminate Intracellular Staphylococcus aureus Using Nanoparticle Coated with Its Extracellular Vesicle Membrane as Active-Targeting Drug Carrier. ACS Infect. Dis. 2019, 5, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Menina, S.; Labouta, H.I.; Geyer, R.; Krause, T.; Gordon, S.; Dersch, P.; Lehr, C.-M. Invasin-functionalized liposome nanocarriers improve the intracellular delivery of anti-infective drugs. RSC Adv. 2016, 6, 41622–41629. [Google Scholar] [CrossRef] [Green Version]
- Labouta, H.I.; Menina, S.; Kochut, A.; Gordon, S.; Geyer, R.; Dersch, P.; Lehr, C.-M. Bacteriomimetic invasin-functionalized nanocarriers for intracellular delivery. J. Control. Release 2015, 220, 414–424. [Google Scholar] [CrossRef]
- Benchaala, I.; Mishra, M.K.; Wykes, S.M.; Hali, M.; Kannan, R.M.; Whittum-Hudson, J.A. Folate-functionalized dendrimers for targeting Chlamydia-infected tissues in a mouse model of reactive arthritis. Int. J. Pharm. 2014, 466, 258–265. [Google Scholar] [CrossRef]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Hiroshi Morisaki, J.; et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 2015, 527, 323. [Google Scholar] [CrossRef]
- Moretton, M.A.; Chiappetta, D.A.; Andrade, F.; das Neves, J.; Ferreira, D.; Sarmento, B.; Sosnik, A. Hydrolyzed Galactomannan-Modified Nanoparticles and Flower-Like Polymeric Micelles for the Active Targeting of Rifampicin to Macrophages. J. Biomed. Nanotechnol. 2013, 9, 1076–1087. [Google Scholar] [CrossRef]
- Brezden, A.; Mohamed, M.F.; Nepal, M.; Harwood, J.S.; Kuriakose, J.; Seleem, M.N.; Chmielewski, J. Dual Targeting of Intracellular Pathogenic Bacteria with a Cleavable Conjugate of Kanamycin and an Antibacterial Cell-Penetrating Peptide. J. Am. Chem. Soc. 2016, 138, 10945–10949. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Kandpal, H.; Gupta, H.P.; Singh, N.B.; Gupta, C.M. Tuftsin-bearing liposomes as rifampin vehicles in treatment of tuberculosis in mice. Antimicrob. Agents Chemother. 1994, 38, 588–593. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, S.; Hashida, M. Glycosylation-mediated targeting of carriers. J. Control. Release 2014, 190, 542–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zhang, Q.; Li, W.; Yuan, M.; Zhou, J.; Hua, L.; Chen, Y.; Ye, C.; Ma, Y. Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J. Control. Release 2020, 317, 1–22. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Yan, J.; Li, Y.; Wang, J.; Yang, Y.Y.; Yuan, P.; Ding, X. Bacterial Outer Membrane-Coated Mesoporous Silica Nanoparticles for Targeted Delivery of Antibiotic Rifampicin against Gram-Negative Bacterial Infection In Vivo. Adv. Funct. Mater. 2021, 31, 2103442. [Google Scholar] [CrossRef]
- Pandey, R.; Khuller, G.K. Oral nanoparticle-based antituberculosis drug delivery to the brain in an experimental model. J. Antimicrob. Chemother. 2006, 57, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Seleem, M.N.; Jain, N.; Pothayee, N.; Ranjan, A.; Riffle, J.S.; Sriranganathan, N. Targeting Brucella melitensis with polymeric nanoparticles containing streptomycin and doxycycline. FEMS Microbiol. Lett. 2009, 294, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Toti, U.S.; Guru, B.R.; Hali, M.; McPharlin, C.M.; Wykes, S.M.; Panyam, J.; Whittum-Hudson, J.A. Targeted delivery of antibiotics to intracellular chlamydial infections using PLGA nanoparticles. Biomaterials 2011, 32, 6606–6613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramteke, S.; Ganesh, N.; Bhattacharya, S.; Jain, N.K. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J. Drug Target. 2009, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Geilich, B.M.; van de Ven, A.L.; Singleton, G.L.; Sepúlveda, L.J.; Sridhar, S.; Webster, T.J. Silver nanoparticle-embedded polymersome nanocarriers for the treatment of antibiotic-resistant infections. Nanoscale 2015, 7, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-K.; Selvanayagam, R.; Ho, K.K.K.; Chen, R.; Kutty, S.K.; Rice, S.A.; Kumar, N.; Barraud, N.; Duong, H.T.T.; Boyer, C. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci. 2016, 7, 1016–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.K.; Kwon, K.; Ryu, J.S.; Lee, H.N.; Park, C.; Chung, H.J. Nonviral Genome Editing Based on a Polymer-Derivatized CRISPR Nanocomplex for Targeting Bacterial Pathogens and Antibiotic Resistance. Bioconjug. Chem. 2017, 28, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qiu, B.; Zhang, Z.; Xie, S.; Liu, Y.; Xia, T.; Li, X. Light-triggerable and pH/lipase-responsive release of antibiotics and β-lactamase inhibitors from host-guest self-assembled micelles to combat biofilms and resistant bacteria. Chem. Eng. J. 2021, 424, 130330. [Google Scholar] [CrossRef]
- Sun, H.; Yu, Y.; Zhang, Y.; Li, J.; Cheng, Y.; Huang, S.; Wang, W.; Zhang, X. Glycosylated Nanotherapeutics with β-Lactamase Reversible Competitive Inhibitory Activity Reinvigorates Antibiotics against Gram-Negative Bacteria. Biomacromolecules 2021, 22, 2834–2849. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Haselmann, G.M.; Wang, J.; Eder, D.; Schneider-Stickler, B. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr. Polym. 2018, 200, 35–42. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers. ACS Appl. Mater. Interfaces 2019, 11, 43902–43919. [Google Scholar] [CrossRef]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Smart active antibiotic nanocarriers with protease surface functionality can overcome biofilms of resistant bacteria. Mater. Chem. Front. 2021, 5, 961–972. [Google Scholar] [CrossRef]
- Patel, K.K.; Tripathi, M.; Pandey, N.; Agrawal, A.K.; Gade, S.; Anjum, M.M.; Tilak, R.; Singh, S. Alginate lyase immobilized chitosan nanoparticles of ciprofloxacin for the improved antimicrobial activity against the biofilm associated mucoid P. aeruginosa infection in cystic fibrosis. Int. J. Pharm. 2019, 563, 30–42. [Google Scholar] [CrossRef]
- Singh, N.; Romero, M.; Travanut, A.; Monteiro, P.F.; Jordana-Lluch, E.; Hardie, K.R.; Williams, P.; Alexander, M.R.; Alexander, C. Dual bioresponsive antibiotic and quorum sensing inhibitor combination nanoparticles for treatment of Pseudomonas aeruginosa biofilms in vitro and ex vivo. Biomater. Sci. 2019, 7, 4099–4111. [Google Scholar] [CrossRef] [Green Version]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2020, 22, 385. [Google Scholar] [CrossRef]
- Grabowski, N.; Hillaireau, H.; Vergnaud, J.; Santiago, L.A.; Kerdine-Romer, S.; Pallardy, M.; Tsapis, N.; Fattal, E. Toxicity of surface-modified PLGA nanoparticles toward lung alveolar epithelial cells. Int. J. Pharm. 2013, 454, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Leroux, J.-C. The journey of a drug-carrier in the body: An anatomo-physiological perspective. J. Control. Release 2012, 161, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Wyss, P.P.; Lamichhane, S.P.; Abed, A.; Vonwil, D.; Kretz, O.; Huber, T.B.; Sarem, M.; Shastri, V.P. Renal clearance of polymeric nanoparticles by mimicry of glycan surface of viruses. Biomaterials 2020, 230, 119643. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Wei, Y.; Quan, L.; Zhou, C.; Zhan, Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine 2018, 13, 1495–1512. [Google Scholar] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Leroux, J.-C. Editorial: Drug Delivery: Too Much Complexity, Not Enough Reproducibility? Angew. Chem. Int. Ed. 2017, 56, 15170–15171. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [Green Version]

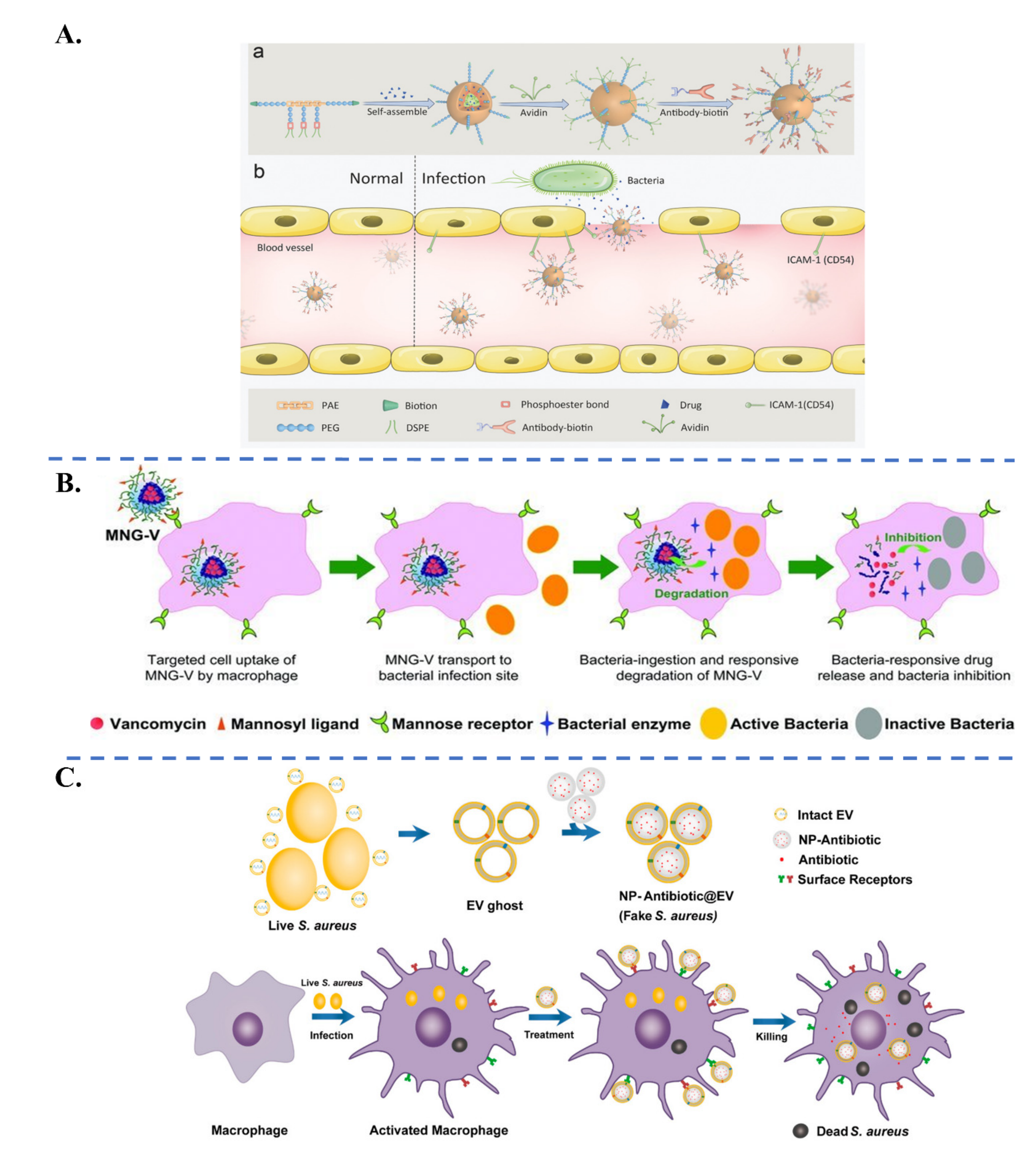
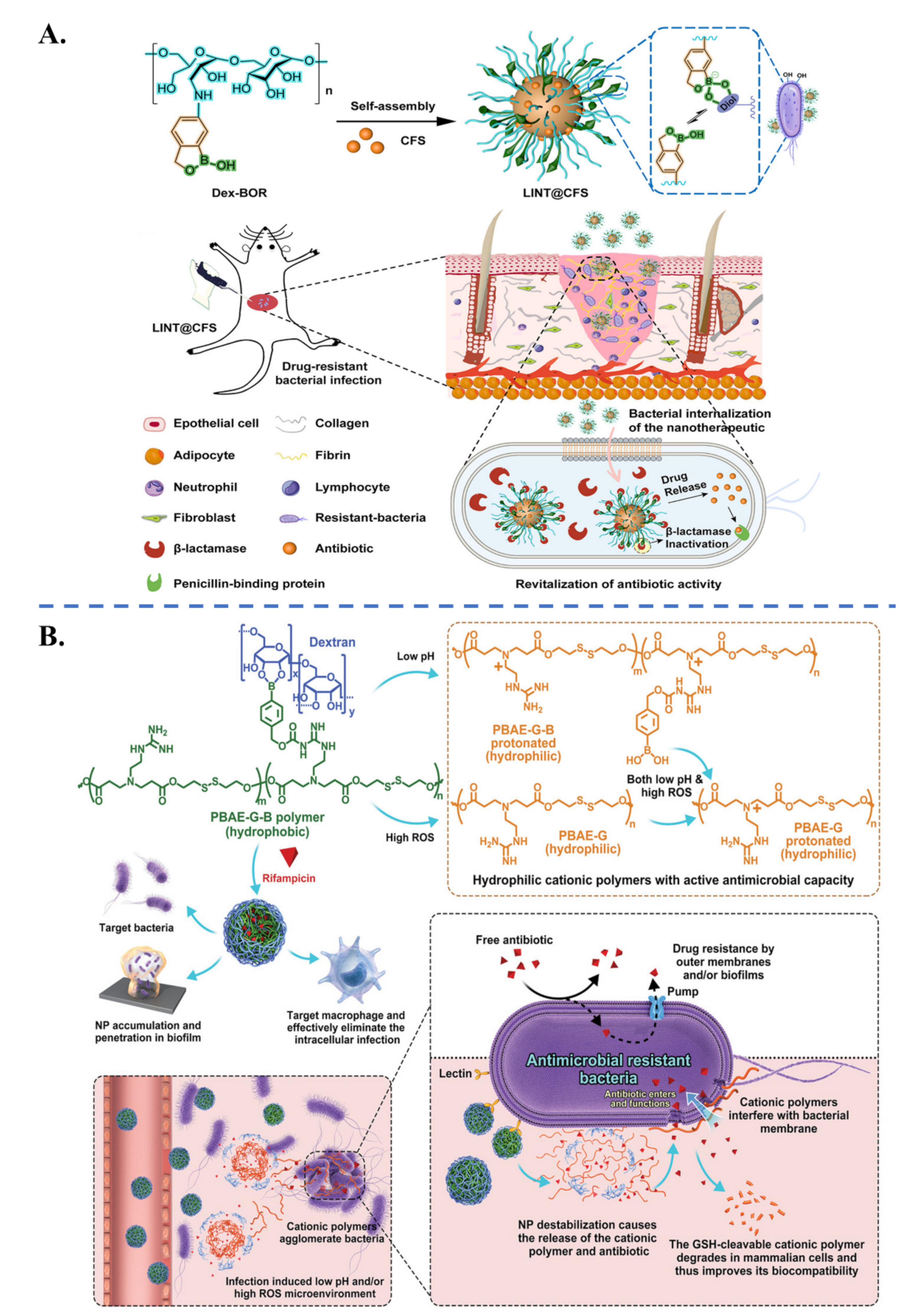
| Biological Parameters | Normal Tissues | Pathological Tissues |
|---|---|---|
| pH | 7.4 | 5–7.0 in inflammatory tissue 4.5–6.5 in endosomes and lysosomes Acid-pH gradients in biofilm (4.5–6.5) |
| Enzyme | Low expression | Overexpression (Lipase, Protease) Or specific enzyme expression (β-lactamase, elastase LasB) |
| Reduced glutathione (GSH) (Reducing environment) | Low level (2–20 μM) | High level in intracellular environment of macrophages and bacteria (2–10 mM) |
| Reactive oxygen species (ROS) | Low level | High level in inflammatory environment (Neutrophil production) High level in biofilm |
| Nitric oxide (NO) | Low level | High level (Proinflammatory mediator) |
| Specific Ligand | Target Receptor | Target | |
|---|---|---|---|
| Targeting of Bacterial Pathogens | Antibiotic molecules: Vancomycin [54,96] Polymyxin B [97], Daptomycin [98], Ubiquicidin UBI29–41 [99] | Antibiotic biding sites: D-Alanyl-D-alanine, Lipopolysaccharide | S. aureus, E. coli, P. aeruginosa |
| Carbohydrates: Glucopyranoside [100], Galactose [101], Fucose [102], Dextran [103] | Lectin: Concanavalin A, Lec A, Lec B | P. aeruginosa, E. coli, S. epidermidis, S. aureus | |
| Aptamer oligonucleotide SA20 hp [104], identified via SELEX procedure | Not determined | S. aureus | |
| Cyclic 9-amino-acid peptide CARG [105], identified via phage display in vivo | Not determined | S. aureus | |
| Antibodies: Anti protein A [33], Anti α-toxin antibody [106] | Protein A, α-toxin | S. aureus | |
| Artificial antibodies [107], identified via molecular imprinting | Lipopolysaccharides | P. aeruginosa | |
| Cell membranes: Platelet [108], Macrophage [109], Gastric epithelial cell [110] | Adhesin proteins Lipoproteins, RNA, Lipopolysaccharide | H. pylori, S. aureus, E. coli | |
| Targeting of Inflammatory Tissues | Neutrophil-membrane (integrin β2) [111,112] γ3 peptide (NNQKIVNLKEKVAQLEA) [113] Anti-ICAM-1 antibody [55] | Intercellular adhesion molecule-1 (ICAM-1) | Inflamed vasculature |
| Bovine serum albumin [114]) | Fcγ receptors on activated neutrophil | Inflamed tissues via neutrophil hitchhiking | |
| Erythrocyte membrane [73]) | Bacterial toxins | Immune cells in presence of bacterial toxins | |
| Carbohydrates: Mannose [57], Curdlan (a linear β-1,3 glucan) [115], Hyaluronic acid [75], Dextran [103] | Mannose receptor, Dectin-1 receptor, CD44 | Infected macrophages, Infection sites via macrophage hitchhiking | |
| S. aureus extracellular vesicles [116] | Toll-like receptors on infected macrophage (trained innate immunity) | S. aureus-infected macrophage | |
| Bacterial invasion proteins InvA497 [117,118] | β1-integrin receptors | Inflamed cells (caused by Yersinia, Salmonella species) | |
| Folic acid [119] | Folate receptors on infected cells | Chlamydia-infected tissues |
| Drug Combination | Nanocarrier | Co-Delivery Purpose | Targeted Bacteria | Ref. | |
|---|---|---|---|---|---|
| Antibiotic/Antibiotic Combinations | Rifampicin, Levofloxacin | Curdlan NPs | Simultaneous sustained release Targeted delivery | Intracellular M. smegmatis | [115] |
| Four first-line anti-TB drugs | PLGA NPs | Reduction in dosing frequency Improvements in patient compliance | Intracellular M. tuberculosis | [127] | |
| Streptomycin, Doxycycline | Polymeric Nanocomplex | Synergistic antimicrobial effect | Intracellular Brucella melitensis | [128] | |
| Rifampin, Azithromycin | PLGA NPs | Enhanced intracellular and intra-inclusion accumulation Sustained drug release | Intracellular C. trachomatis | [129] | |
| Amoxicillin, Clarithromycin, Omeprazole | Chitosan-glutamate NPs | Synergic effects Reduction of effective dose | H. pylori | [130] | |
| Colistin, Rifampicin | Micelle | Synergistic antimicrobial effect | E. coli, P. aeruginosa,A. baumannii | [25] | |
| Silver NPs, Ampicillin | Polymersome | Synergistic effect (at 1:0.64 ratio) Drug protection from hydrolysis by β-lactamase enzymes | Resistant E. coli | [131] | |
| Antibiotic/adjuvant Combinations | Gentamicin, Nitric oxide | Polymeric NPs | Synergistic effects of biofilm dispersal and enhanced bactericidal activity | P. aeruginosa biofilms | [132] |
| Free-Oxacillin, CRISPR-Cas9 | Polymeric Nanocomplex | Targeting antibiotic resistance (MecA) and therapeutic genome editing | MRSA | [133] | |
| Ampicillin, β-lactamase inhibitor | Micelle | Synchronous release of antibiotics and β-lactamase inhibitors for destruction of biofilms and restoration of the antibiotic activity to resistant bacteria | MRSA biofilms | [134] | |
| Cefoxitin, β-lactamase- inhibitors | Dextran NPs | Targeting co-delivery, overcoming the membrane barrier, and reversing acquired resistance | Resistant E. coli induced by sub-MIC of cefoxitin (AmpC) | [135] | |
| Oxacillin, Dnase I | Chitosan NPs | Destruction and eradication of biofilms | S. aureus biofilms | [136] | |
| Ciprofloxacin, Protease | Carbopol NPs, Shellac NPs | Destruction and eradication of biofilms | Biofilms | [137,138] | |
| Ciprofloxacin, Alginate lyase | Chitosan NPs | Destruction and eradication of biofilms | P. aeruginosa biofilm | [139] | |
| Ciprofloxacin, Quorum-sensing inhibitor (ACNQ) | Alginate NPs | Destruction and eradication of biofilms Concomitant drug release pH-responsive drug release | P. aeruginosa biofilm | [140] | |
| Imipenem, Cilastatin | PCL and PLGA NPs | Carbapenem protection by inhibiting the enzymatic degradation (renal dehydropeptidase) | K. pneumoniae, P. aeruginosa | [32] | |
| Triclosan, Micelle | Micelle | Synergistic antibacterial mechanisms: membrane damage, Increased antibiotic penetration, and Intra-bacterial GSH-responsive antibiotic release | MRSA | [74] | |
| Rifampicin, Cationic polymer | Dextran Nanocomplex | Synergistic activity Bacteria targeting Stimuli-controlled delivery (ROS, pH) | Biofilm, Intracellular infections | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, H.; Karakasyan, C.; Jouenne, T.; Le Cerf, D.; Dé, E. Application of Polymeric Nanocarriers for Enhancing the Bioavailability of Antibiotics at the Target Site and Overcoming Antimicrobial Resistance. Appl. Sci. 2021, 11, 10695. https://doi.org/10.3390/app112210695
Le H, Karakasyan C, Jouenne T, Le Cerf D, Dé E. Application of Polymeric Nanocarriers for Enhancing the Bioavailability of Antibiotics at the Target Site and Overcoming Antimicrobial Resistance. Applied Sciences. 2021; 11(22):10695. https://doi.org/10.3390/app112210695
Chicago/Turabian StyleLe, Hung, Carole Karakasyan, Thierry Jouenne, Didier Le Cerf, and Emmanuelle Dé. 2021. "Application of Polymeric Nanocarriers for Enhancing the Bioavailability of Antibiotics at the Target Site and Overcoming Antimicrobial Resistance" Applied Sciences 11, no. 22: 10695. https://doi.org/10.3390/app112210695
APA StyleLe, H., Karakasyan, C., Jouenne, T., Le Cerf, D., & Dé, E. (2021). Application of Polymeric Nanocarriers for Enhancing the Bioavailability of Antibiotics at the Target Site and Overcoming Antimicrobial Resistance. Applied Sciences, 11(22), 10695. https://doi.org/10.3390/app112210695






