Featured Application
This work focuses on selective CO2 capture from gases in the presence of H2S. Examples of fields of application are natural gas, biogas and refinery gas treatment, and coal gasification.
Abstract
Removing CO2 from natural gas or biogas in the presence of H2S is technically challenging and expensive as it often requires separation of both acid gases from the gas, typically using an aqueous amine solution, followed by separation of CO2 from H2S and conversion of H2S into solid S. In this work, the proof of concept of electrochemical, instead of thermal, regeneration of an aqueous amine solution is developed. This invention might be a very promising technology and has several advantages. It has H2S versus CO2 selectivity of 100%, can directly convert H2S into S and H2, and is economically competitive with CO2 desorption energy around 100 kJmol−1 and H2S conversion around 200 kJmol−1. If renewable energy is used for electrochemical regeneration, CO2 emissions due to the CO2 capture process can be significantly reduced.
1. Introduction
Climate change due to the rapid accumulation of greenhouse gases in the atmosphere since the industrial revolution represents a major challenge for our society. The main anthropogenic greenhouse gas is CO2, which is predominantly produced from the massive burning of fossil fuels and, to lesser extent, biomass. CO2 is also naturally present, for example, in natural gas. Indeed, a significant part of the world’s natural gas reserves are sour, which means they contain CO2 and/or H2S. The CO2 associated with un- or underdeveloped sour gas fields worldwide has been estimated to total 15 Gt CO2, which is quite substantial [1]. Even before climate change became a major societal issue, scientists had developed technologies to remove CO2 and H2S from high-pressure natural gas so that the gas would meet international commercial and safety standards. Thus, several separation techniques exist or are under development to remove acid gases, such as absorption, adsorption, membranes, cryogenic separation, etc. [2,3,4,5,6,7]. The same technologies can be applied to remove CO2 from low-pressure flue gas, remove acid gases from biogas, purify syngas, etc. The concept developed in this paper will be applied to natural gas treatment as an example. For acid gas removal from natural gas, the most mature and widespread technology today is absorption by aqueous amine solutions [6,7].
Several types of amines have been studied. This work focuses on primary or secondary amines that react with CO2 via electrophilic addition (Lewis base) followed by deprotonation to form carbamate AmCOO−. The reaction mechanism is as follows [8]:
Electrophilic addition (formation of zwitterion):
Followed by deprotonation:
The reaction with H2S is a classical acid–base reaction:
For natural gas, the removal of CO2 and H2S with aqueous alkanolamines takes place in two stages in an acid gas removal unit (AGRU). First, the acid gases are chemically absorbed by the solvent in an absorption column. This process is spontaneous and exothermic. The sweet gas, depleted in CO2 and H2S, can be sent to a pipeline or to a liquefaction unit. The solvent enriched in CO2 and H2S is subsequently sent to a solvent regeneration column, also called a stripper, in which the acid gases are desorbed and separated from the amine solution. This process is endothermic, which means that energy needs to be supplied. Therefore, the amine solution is heated up to ~400 K in a reboiler. In addition, water vapor is generated, which helps the acid gases migrate from liquid to gas phase. The regeneration column is operated near atmospheric pressure [7].
Once the acid gases are separated from the natural gas, the H2S must be further separated because it is extremely toxic. The acid gases are therefore sent to a sulfur removal unit (SRU) to convert H2S into solid sulfur. There exist many H2S conversion processes. In this paper, as an example, the Claus process will be described, which is a standard process for large gas treatment plants [9,10]. This unit is divided into two stages: a thermal oxidation stage, followed by a catalytic stage. During the thermal step, H2S is brought into contact with air at a temperature between 1400 and 1600 K. One-third of the H2S undergoes partial oxidation and is converted into SO2:
The products of the thermal step are then cooled to ~400 K. During the catalytic step, the remaining H2S and SO2 react at temperatures between 460 and 650 K and in the presence of a catalyst, which can be an aluminum or titanium oxide, to form solid sulfur in its S8 form:
Downstream, the Claus unit is a tail gas treatment unit (TGTU), which removes the remaining sulfur compounds.
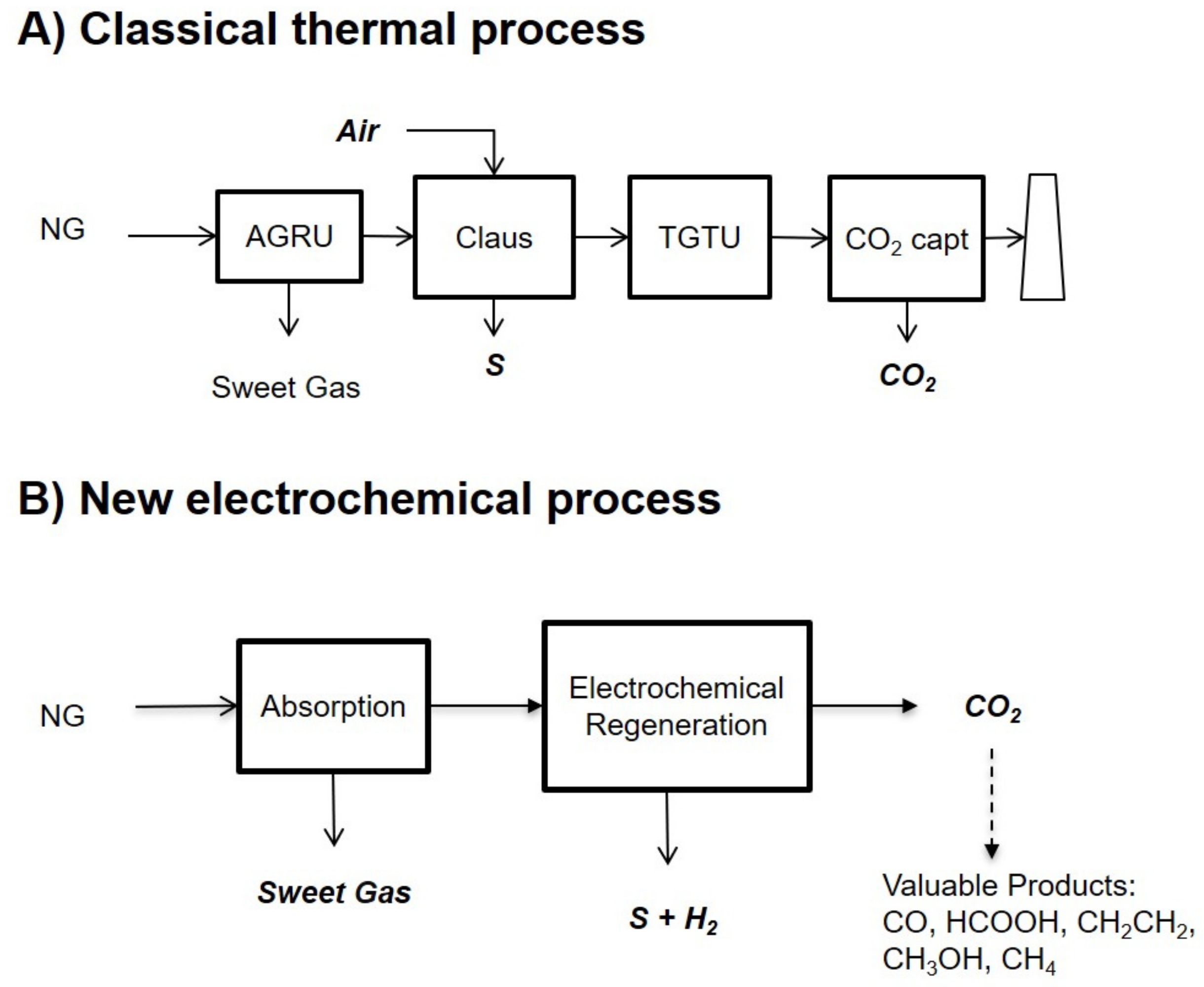
Despite being the most competitive process today, there are some significant drawbacks to the acid gas removal process with aqueous amines, namely the solvent regeneration step consumes a lot of energy (if thermal integration is not possible); the high regeneration temperatures tend to degrade the amine, resulting in performance and solvent losses; and there are corrosion issues. Because there is currently no solvent that is ultra-selective with respect to H2S at high pressure, the CO2 is simultaneously captured. Therefore, in the H2S conversion process, the CO2 also goes through the Claus units, resulting in larger units and higher energy consumption. Moreover, if one wants to capture this CO2 for storage or reuse (today, the CO2 is most often sent to the atmosphere), one needs to add a second AGRU downstream the Claus unit. This is shown in Figure 1A.
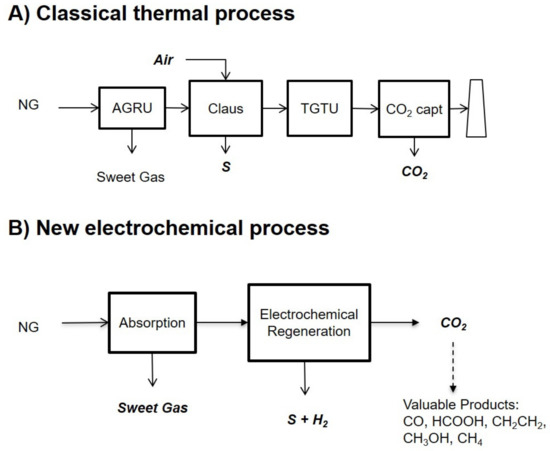
Figure 1.
(A) A classical thermal process to capture CO2 from natural gas (NG) when H2S is also present. First, both gases are removed in an AGRU (containing both absorption and thermal regeneration). In a Claus unit, the H2S is converted into solid sulfur, and the remaining sulfur compounds are removed in a TGTU unit. A second AGRU removes the CO2 before incineration of the remaining gases. (B) The electrochemical-based process proposed in this work involves capturing CO2 from gas in the presence of H2S. Both gases are first absorbed with an aqueous amine solvent. The regeneration is electrochemical, not thermal, with simultaneous conversion of H2S in solid S and H2 and separation of CO2.
The team of Hatton et al. [11,12,13,14,15,16,17,18,19,20] at MIT developed a post-combustion CO2 capture scheme with an electrochemistry mediated aqueous amine regeneration (EMAR). Here, instead of thermally regenerating the solvent to capture CO2, electrochemistry is used. In the electrochemical cell, the CO2 is detached from the carbamate over a copper anode. An amine–Cu complex is formed, and the amine is regenerated over the cathode. The Cu- and CO2-rich stream is sent from the anode to a flash tank to separate the CO2 from the Cu-rich stream, which is recycled to the cathode. The advantage of this early-stage technology is that the regeneration does not require heating and steam, thereby potentially reducing degradation of the amine. Moreover, it might take place at higher pressure, thus avoiding repressurization of the CO2 for reuse or storage. The main challenges are the overpotential intrinsic to electrochemical systems, efficiency losses due to ion migration, and a stable cyclic operation. The feasibility of the technology has been demonstrated with a continuous bench scale EMAR unit.
In this paper, we present a theoretical process adapted from the work of Hatton et al. to capture CO2 from gas when H2S is also present. The technology is also suitable for CO2 removal from gases in the absence of H2S. The key idea of this work is to regenerate the solvent in an electrochemical cell, namely, to desorb CO2 and H2S so that the solvent can be recycled back to the absorption column and CO2 and H2S can be separated simultaneously. This is shown in Figure 1B. Moreover, the H2S is converted into H2 and solid S during this process. Hence, the electrochemical regeneration cell replaces both the thermal regeneration column of the first AGRU as well as the Claus unit, the TGTU unit, the second AGRU, and the incineration unit.
2. Materials and Methods
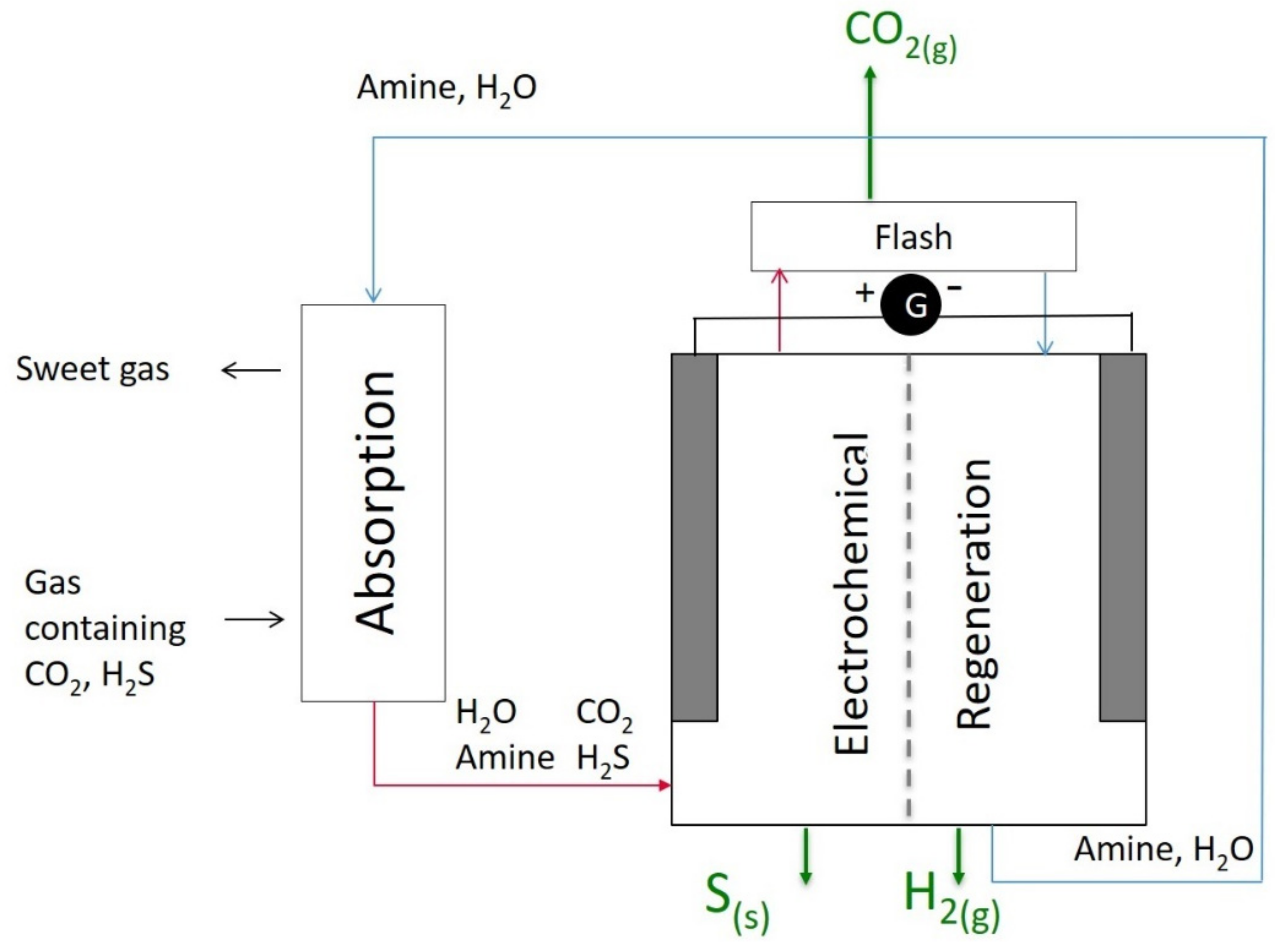
A schematic diagram of the electrochemistry-based process is shown in Figure 2. The gas containing CO2 and H2S is first sent to a classical absorption column. The lean aqueous amine chemically absorbs CO2 and H2S. As a result, two streams leave the column, namely the sweetened gas and the solvent loaded with acid gas. The acid-gas-rich solvent is sent to the electrochemical regeneration cell. During the regeneration stage, the solvent is regenerated, and the acid gases are separated. The lean solvent is then recycled to the absorption column. H2S is converted into solid sulfur and gaseous hydrogen via oxidation at the anode, while CO2 is released due to the preferential complexation of the amine with the metal ions. The detailed mechanisms are described in the next section.
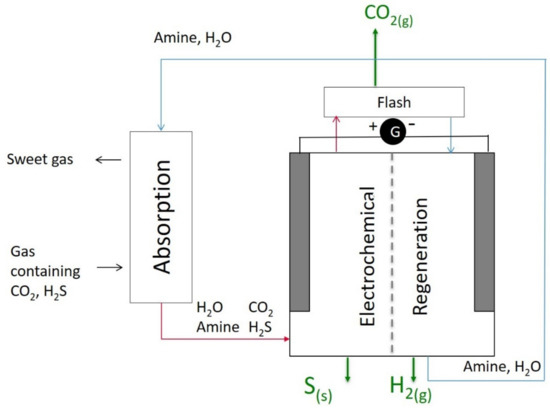
Figure 2.
A schematic diagram of the overall acid gas removal, separation, and conversion process consisting of an absorption column and an electrochemical solvent regeneration cell.
3. Results and Discussion
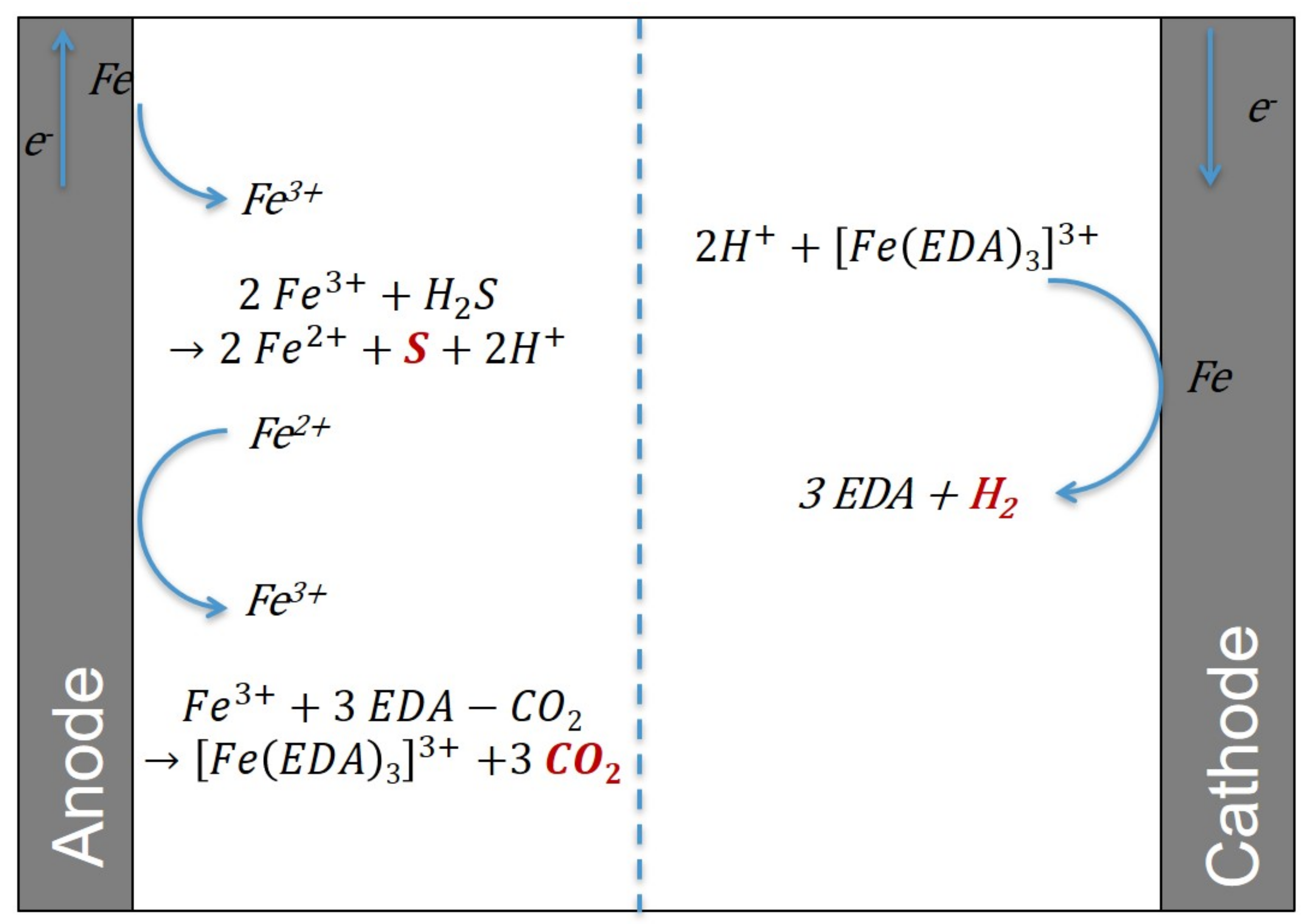
First, the method to extract CO2 will be described, followed by the mechanism to extract H2S. Because CO2 is removed in its gaseous phase and H2S as solid sulfur, the H2S versus CO2 selectivity should be excellent. A detailed diagram with the chemical reactions taking place in the electrochemical cell is shown in Figure 3.
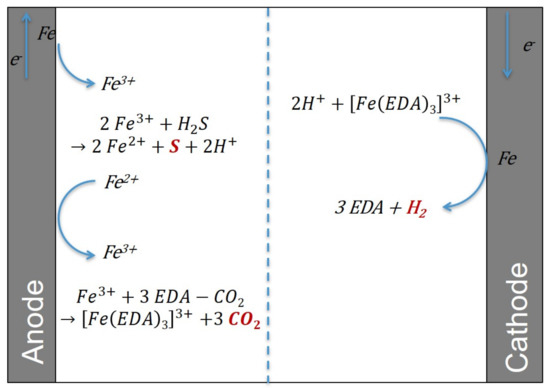
Figure 3.
A detailed diagram of the chemical reactions taking place in the electrochemical cell.
3.1. CO2 Mechanism
In this work, primary or secondary amines are considered to absorb CO2. The amine will act as a nucleophile and bind the CO2 via an electrophilic addition. The principle of electrochemical regeneration is to put this amine–CO2 bond in competition with an amine–metal cation bond. One must therefore find the metal cation that interacts more strongly with the amine than with the CO2. Metal cation was chosen so that the amine–metal cation complexation would be thermodynamically favored with respect to the amine–CO2 reaction. For the metal complex to be thermodynamically favored over the amine–CO2 bond, the constant for the formation of the metal complex must satisfy the following equation:
where β represents the global formation constant of the metal complex, nligand is the number of ligands complexing the metallic cation, and is the equilibrium constant of the amine–CO2 reaction.
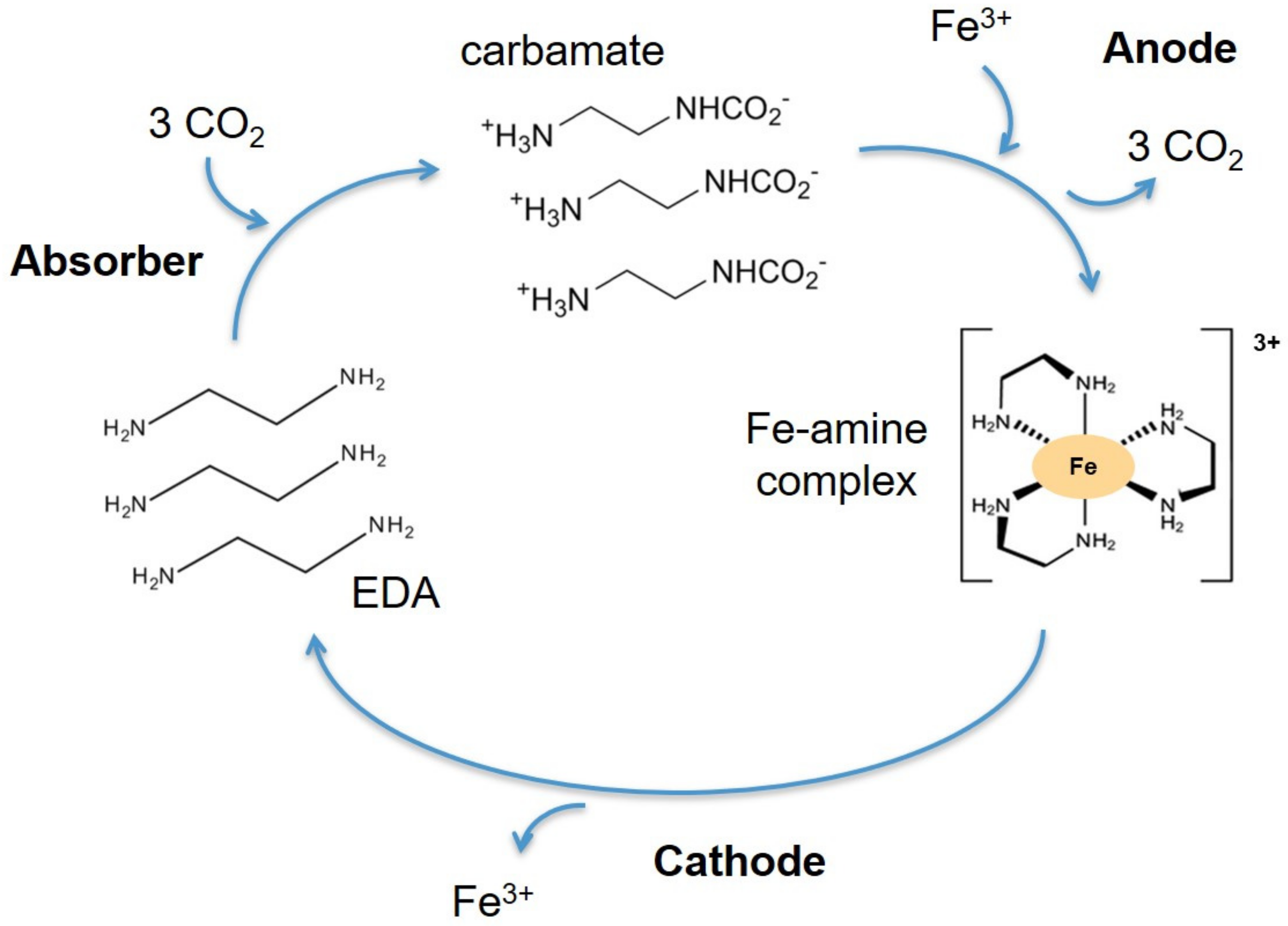
The amine selected in this work is ethylenediamine (EDA), and the metal cation is Fe3+. This amine was chosen for its chelating properties. Iron forms octahedral complexes. This means that it forms six coordination bonds with its ligands. Ethylenediamine can form coordination bonds with its lone pairs on its two amine functions. Because ethylenediamine has two lone pairs, it is a bidentate ligand. Thus, to form its octahedron, an Fe3+ cation binds with three molecules of ethylenediamine. The absorption cycle of CO2 is illustrated in Figure 4.
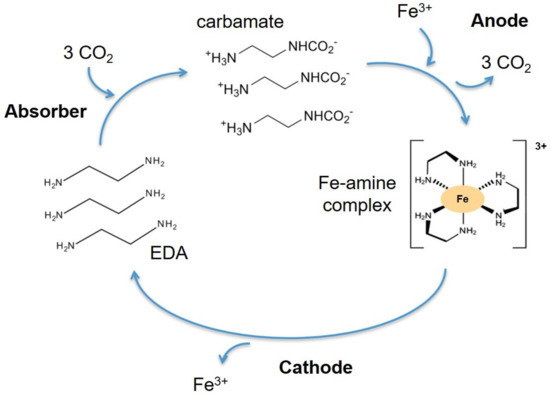
Figure 4.
The absorption cycle of CO2.
The amine solution, rich in absorbed gases, is sent to the anode compartment of an electrochemical cell (electrolyzer, see Figure 2), and a potential difference (voltage) is imposed on the electrodes to carry out redox reactions. The electrolyzer consists of two electrodes. The first is an anode where the oxidation reaction takes place; this is the positive pole of the electrolyzer. The second is a cathode where the reduction reaction occurs; this is the negative pole. If several redox reactions can happen at the anode and/or at the cathode, then at the anode, the oxidation reacting at lower potential will first and foremost occur and, conversely, at the cathode, the reduction reacting at higher potential will first and foremost take place. Here, the anode is iron, and it is oxidized to form Fe3+ ions. These will compete with the CO2 to interact with the amine. They complex with the amine and therefore release CO2. This solution is then sent to a flash drum to shift the equilibrium and extract the CO2 from the liquid phase to the vapor phase. Finally, this solution rich in metal cations and poor in CO2 is sent to the cathode compartment of the electrochemical cell. Fe3+ ions will be reduced to elemental iron with an iron cathode. Finally, this regenerated solution can be recycled and sent to the absorption column for a new cycle. The leads to the following overall reaction equation:
3.2. H2S Mechanism
H2S is absorbed by the amine solution and reacts only via acid–base reactions with amine and water. Therefore, H2S is present in the solution as H2S, HS− and S2− (but mainly as HS−). The aim is to extract H2S as solid S(s). To do so, an oxidizer is needed that is capable of oxidizing S(-II) to S(0). An oxidant that is thermodynamically capable of oxidizing H2S to S(s) must have an equilibrium potential greater than the one of S/H2S. However, we wanted to generate the oxidant at the anode of the electrolyzer. As previously explained, the oxidation taking place at lower potential will happen first on the anode. From a thermodynamic point of view, the oxidation of H2S will have to take place first on the anode, which would passivate the electrode. Consequently, to avoid the direct oxidation of H2S on the electrode, it is necessary that the oxidation of H2S is kinetically disadvantaged, i.e., that the oxidation of H2S must have an overvoltage allowing the oxidation of our oxidant before that of H2S. The couple Fe3+/Fe2+ was chosen to fulfill this role:
With
In practice, and as shown in Figure 3, the amine solution rich in sulfur elements arrives in the anode compartment of the electrochemical cell. There, the iron anode is oxidized to Fe3+. The cation Fe3+ oxidizes H2S to solid S, which precipitates. Finally, the Fe2+ ions will be reduced to solid iron in the cathode compartment of the electrochemical cell.
3.3. Reasons for Selecting the Fe3+/Fe2+ Couple
3.3.1. The Advantages of the Fe3+/Fe2+ Couple
This couple is already used to reduce H2S to sulfur in the industry. Only the ligand changes. In the literature, EDTA (ethylenediaminetetraacetic acid) [21], NTA (nitrilotriacetic acid) [22,23,24], and HEDTA (hydroxyethyl ethylenediaminetriacetic acid) [23,24] have been typically used as ligand. Here, we used EDA. has an equilibrium potential greater than that of S/H2S if one considers the free ions, and therefore it can oxidize H2S to S. The equilibrium potential is the potential at which the oxidation and reduction reaction take place at the same rate. This potential is calculated using the Nernst equation [25]:
If one considers the noncomplexed species: .
If one considers the potential between and :
and [19]. The latter value is only a gross estimate by Stern [19] and worth checking. With those values, .
The equilibrium potential between S/H2S is
(S/H2S) = Ctot is the total concentration of absorbed H2S. If one takes Ctot = 10−1 molL−1, one obtains −0.52 V/SHE.
The pH = 12 of the solution was obtained as follows:
The amine solution consists of H2S and EDA. Ethylenediamine (EDA) has two pKas: pKa1 = 9.7; pKa2 = 7. The amine is a weak base, and thus the pH of the solution can be calculated from pH = ½ (pKA + pKe + logCam) = 12. Table 1 shows the composition of the solution.

Table 1.
Composition of the solution.
With the value , the Fe3+ ions are not able to oxidize H2S because Eeq(/Eeq(S/H2S) (see Equation (14)). It should be stressed that the value is only an estimate [19]. The reaction is possible if 21.7, which is only a difference of 25 with respect to the estimate of 35. Hence, it is necessary to measure to confirm that the reaction is not possible.
The Fe3+ ion has a great affinity with amine functions. It has an overall formation constant with EDA of ~, so it will be able to regenerate the amine. The equilibrium constant for EDA–CO2 is ~, so the inequality between β and KCO2 is largely satisfied. Another advantage of iron is that it is inexpensive and nontoxic.
3.3.2. The Disadvantages of the Fe3+/Fe2+ Couple
At low pH, iron is no longer soluble in water. The products of the solubility of Fe3+ and Fe2+ are Fe(OH)3 and Fe(OH)2, respectively, with corresponding pKs3 = 37.2 and pKs2 = 15.1, respectively. If one calculates the pH corresponding to the precipitation limit for the two salts with a total iron concentration of 1 molL−1, one obtains for the Fe(OH)3 salt:
And for the Fe(OH)2 salt:
If one takes into account the stabilization of the ions by the ethylenediamine complexes, the pH corresponding to the precipitation limit will increase slightly. If the iron ions and the ethylenediamine are present in stoichiometric proportions, one has and . The pH of the precipitation limit is pH = 4.5. With the same hypotheses, one obtains for Fe(+II): and and a precipitation limit of pH = 7.9. Therefore, even with the complexation of the Fe2+ or Fe3+ ions with the amine, the pH at which precipitation occurs remains lower than the pH of the solution.
There exist many options to further increase the solubility of the Fe3+ and Fe2+ ions in the solution. The pH of the solution can be lowered. One could consider going down to 10, but if the pH is lower than the pKa of the amine (pKa of EDA is 9.96), then the predominant species will no longer be EDA but EDAH+, which will significantly reduce the absorption of the acid gases. The temperature could be increased, but this has a limited impact on the solubility, as illustrated in Table 2.

Table 2.
Temperature dependence of the water solubility of Fe2+ and Fe3+ ions.
A second ligand can be added to complex the residual iron ions. To be beneficial, this ligand must have similar or even better complexing constants than ethylenediamine. Finally, the amine/iron proportions can be changed so that iron becomes the limiting reagent of the complexation reaction. To change the concentration of free iron ions, it suffices that the amine is slightly in excess. In the following example with amine in excess of 0.1 molL−1, we managed to drastically increase the precipitation pH to 10.6 for Fe3+ and to 10 for Fe2+:
The extent of the reaction is calculated in Table 3 for Fe3+ complexation:

Table 3.
Extent of the reaction for Fe3+ complexation.
It is thus possible to find a system in which the ions are stable and do not precipitate in the solution.
3.4. Basic Technoeconomical Study
3.4.1. Comparison between Electrochemical and Thermal Regeneration with a Model Case from the Middle East
The comparative study of energy costs between a typical large natural gas treatment facility in the Middle East with and without electrochemical regeneration served to assess the industrial interest of this new technology. Electrochemical regeneration replaced the desorption column of the first AGRU as well as the entire downstream sulfur removal unit (Claus + TGTU) (see Figure 1). Fuel and steam consumption were evaluated for units replaced by electrochemical regeneration and were compared with the energy consumption of the latter. The unit chosen to compare these two systems was the energy contained in the methane required to produce steam or electricity.
Base Case AGRU/SRU
To be conservative, a gas treatment facility with optimal thermal integration was considered (lower energy consumption of the thermal reference case). The burners in the thermal part of the Claus unit produce 126 MW. They consume 12,600 kghr−1 of fuel gas at 5 bar(g) with a lower heating value (LHV) of 45 MJkg−1 with an efficiency of 80%. Combining these figures gives 126 MW. In addition, the SRU has a surplus of low and high-pressure steam. The SRU supplies 45 thr−1 of high-pressure steam at 41 bar(g) and 685 K. Under these conditions, 1 thr−1 is equivalent to 0.5 MW. The SRU therefore produces 22.5 MW. The SRU supplies 46 thr−1 of low-pressure steam at 5.5 bar(g) and 440 K. Under these conditions, 1 thr−1 is equivalent to 0.6 MW. The SRU therefore produces 27.6 MW. The AGRU consumes 177 thr−1 of low-pressure steam in the reboiler of the regenerator, which is equivalent to 106.2 MW. Therefore, the AGRU–SRU consumes 182.1 MW. This is summarized in Table 4.

Table 4.
Energy consumption of thermal SRU–AGRU.
Two model case configurations were studied, namely the base case without CO2 capture and the case with CO2 capture at the SRU outlet with a standard aqueous amine. CO2 capture for storage or reuse increases the energy consumption by 75% (one needs an entire extra AGRU unit with thermal regeneration). This is summarized in Table 5.

Table 5.
Comparison between the two base cases and electrochemical regeneration.
Electrochemical Regeneration
Electrochemical energy is defined by the following equation: , where n is the number of electrons exchanged, F is the Faraday constant, and is the voltage across the electrochemical cell. One electron is consumed to regenerate CO2, while two electrons are consumed to oxidize H2S to S. The applied voltage is estimated at 1 V, which gives an electrical energy of 100 kJmol−1 of CO2 and 200 kJmol−1 of H2S. To be able to compare with a typical installation in the Middle East, these molar energies were multiplied by the molar flow rate of H2S and CO2 of the model case. , the flow rate of H2S and , the flow rate of CO2, were 450 and 316 mols−1, respectively. With an energy consumption of 122 MW was obtained.
Methane Equivalent
To finalize the comparison between the two approaches, it is necessary to compare the ways of producing this energy. Thermal regeneration uses water vapor to heat the amine solution and burns fuel gas to heat the inlet gases to the Claus unit, whereas electrochemical regeneration uses electricity. They are different sources of energy, and to be able to compare them, we can look at the energy released by the combustion of methane before it is transformed into electricity or water vapor. The transformation of methane into water vapor can reach an efficiency of 85%; however, the transformation of methane into electricity only has an efficiency of around 60%. In methane equivalent, the thermal SRU and AGRU units consume 214 MW of methane without CO2 recovery and 375 MW with CO2 recovery (typical design recovery is 90%), while the electrochemical unit, which recovers 100% of the CO2, consumes 203 MW of methane. This is shown in Table 5.
3.4.2. Industrial Interest and Major Challenges of the Technology
The proposed electrochemical regeneration (with CO2 capture) consumes the same amount of energy as the classical thermal process without CO2 capture. However, compared with the thermal process with CO2 recovery, the electrochemical process is clearly attractive from an energy point of view. In addition, this technology has the advantage of converting H2S directly into H2 and solid S in the same unit as the regeneration of the solvent. This technology might therefore be more compact. In addition, instead of burning methane to regenerate the solvent, the use of renewable electricity can be considered for the electrochemical regeneration, which will reduce the associated CO2 emissions. This technology might be 100% selective between H2S and CO2 and allow for a recovery of 100% of the CO2. Because electrochemical regeneration takes place at lower temperature, the amine will be less degraded, resulting in less solvent losses as well as less potential emissions of harmful compounds. Finally, in the thermal process, the regeneration takes place at atmospheric pressure. The electrochemical process can be operated at higher pressures, partially avoiding repressurization of the CO2 for storage or reuse.
There are, however, many questions and challenges related to the new electrochemical process. One of the major constraints is to find a couple amine–metal suitable for not only the electrochemical regeneration process but also attractive for the absorption process. The CO2 absorption properties of aqueous EDA have been studied, and EDA seems comparable to MEA (monoethanolamine) [26,27,28]. More work is definitely required, not only on the CO2 but also on the H2S absorption properties because the treated gas often needs to respect stringent specifications (typical 4 ppm H2S), which are challenging to meet with primary or secondary amines. Another challenge is that side reactions might occur on the electrodes, resulting in more compounds (e.g., sulfur compounds, CO, etc.). Moreover, the practical removal of the products (gaseous H2, gaseous CO2, and solid sulfur) raises questions. Another important point is the quality of the sulfur, which should be comparable to the high purity obtained with the Claus unit. The potential precipitation of iron is also a potential challenge.
3.5. Future Research Directions
At this point, we lack some data to conclude on the theoretical suitability of electrochemical solvent regeneration in the presence of H2S. First of all, β, the global formation constant of the complex, is not known, and therefore the equilibrium potential of the Fe3+/Fe2+ couple complexed by EDA is undetermined. An electrochemical experiment is thus required to measure the equilibrium potential of the pair / and to extract the global formation constant of the complex. In addition, it is necessary to know the overvoltage of the H2S/S couple on an iron electrode. These experiments are not very complicated, and the data can be obtained rapidly in an electrochemistry lab.
If the measured values meet the conditions mentioned above (Equations (6), (14) and (15)), the system can, in theory, regenerate the amine solution in the presence of H2S. The next step would then be the construction of an electrochemical cell to experimentally verify the feasibility of the process.
4. Conclusions
In this work, the proof of concept of an AGRU unit consisting of an absorption column and an electrochemical cell for solvent regeneration is presented. The invention allows to selectively capture CO2 from a gas that also contains H2S. Simultaneously, the H2S is converted into solid sulfur and H2. This significantly reduces the overall energy of CO2 capture compared to a traditional thermal process.
Author Contributions
Conceptualization, F.d.M. and C.B.; methodology, F.d.M. and C.B.; investigation, F.d.M., C.B. and B.P.; writing—original draft preparation, F.d.M. and C.B.; writing—review and editing, F.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CCUS (Carbon Capture Utilization and Sequestration) R&D program of TotalEnergies S.E.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank Jean-Pierre Dath and Philip Llewellyn for the constructive discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burgers, W.F.J.; Northrop, P.S.; Kheshgi, H.S.; Valencia, J.A. Worldwide development potential for sour gas. Energy Procedia 2011, 4, 2178–2184. [Google Scholar] [CrossRef] [Green Version]
- Okoro, O.V.; Sun, Z. Desulphurization of biogas: A systematic qualitative and economic-based quantitative review of alternative strategies. Chemengineering 2019, 3, 76. [Google Scholar] [CrossRef] [Green Version]
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of hydrogen sulfide from various industrial gases: A review of the most promising adsorbing materials. Catalysts 2020, 10, 521. [Google Scholar] [CrossRef]
- Orlov, A.A.; Marcou, G.; Horvath, D.; Cabodevilla, A.E.; Varnek, A.; de Meyer, F. Computer-aided design of new physical solvents for hydrogen sulfide absorption. Ind. Eng. Chem. Res. 2021, 60, 8588–8596. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; Hallett, J.P.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar]
- Rochelle, G. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R. Gas Purification, 5th ed.; Gulf Professional Publishing: Houston, TX, USA, 1997. [Google Scholar]
- Versteeg, G.F.; van Swaaij, W.P.M. On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions –I. Primary and secondary amines. Chem. Eng. Sci. 1988, 43, 573–585. [Google Scholar] [CrossRef] [Green Version]
- Eow, J.S. Recovery of sulfur from sour acid gas: A review of the technology. Environ. Prog. Sustain. Energy 2004, 21, 143–162. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen sulfide capture: From absorption in polar liquids to oxide, zeolite, and metal-organic framework adsorbents and membranes. Chem. Rev. 2017, 117, 9755–9803. [Google Scholar] [CrossRef]
- Stern, M.C.; Simeon, F.; Herzog, H.; Hatton, T.A. Post-combustion carbon dioxide capture using electrochemically mediated amine regeneration. Energy Environ. Sci. 2013, 6, 2505–2517. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, A.O.; Stern, M.C.; Herzog, H.; Hatton, T.A. Energetics of electrochemically mediated amine regeneration. Energy Procedia 2014, 63, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Shaw, R.A.; Hatton, T.A. Electrochemical CO2 capture thermodynamics. Int. J. Greenh. Gas Control 2020, 95, 102878. [Google Scholar] [CrossRef]
- Rahimi, M.; Diederichsen, K.M.; Ozbek, N.; Wang, M.; Choi, W.; Hatton, T.A. An electrochemically mediated amine regeneration process with a mixed absorbent for post-combustion CO2 capture. Environ. Sci. Technol. 2020, 54, 8999–9007. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, H.Z.; Diederichsen, K.M.; Van Voorhis, T.; Hatton, T.A. Electrochemically mediated carbon dioxide separation with quinone chemistry in salt-concentrated aqueous media. Nat. Commun. 2020, 11, 2278. [Google Scholar] [CrossRef]
- Wang, M.; Rahini, M.; Kumar, A.; Hariharan, S.; Choi, W.; Hatton, T.A. Flue gas CO2 capture via electrochemically mediated amine regeneration: System design and performance. Appl. Energy 2019, 255, 113879. [Google Scholar] [CrossRef]
- Wang, M.; Hatton, T.A. Flue gas CO2 capture via electrochemically mediated amine regeneration: Desorption unit design and analysis. Ind. Eng. Chem. Res. 2020, 59, 10120–10129. [Google Scholar] [CrossRef]
- Voskian, S.; Brown, P.; Halliday, C.; Rajczykowski, K.; Hatton, T.A. Amine-based ionic liquid for CO2 capture and electrochemical or thermal regeneration. Sustain. Chem. Eng. 2020, 8, 8356–8361. [Google Scholar] [CrossRef]
- Stern, M.C. Electrochemically-Mediated Amine Regeneration for Carbon Dioxide Separations. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2013. [Google Scholar]
- Hatton, T.A. Electrochemically-Mediated Sorbent Regeneration in CO2 Scrubbing Process. Final Report DOE Project DE-FE0026489; U.S. Department of Energy: Washington, DC, USA, 2021. [Google Scholar]
- Iliuta, I.; Larachi, F. Concept of bifunctional redox iron-chelate process for H2S removal in pulp and paper atmospheric emissions. ChemEngSci 2013, 58, 5305–5314. [Google Scholar] [CrossRef]
- Neumann, D.W.; Lynn, S. Oxidative absorption of H2S and O2 by iron chelate solutions. AIChE J. 1984, 30, 62–69. [Google Scholar] [CrossRef]
- Wubbs, H.J.; Beenackers, A.A.C.M. Kinetics of H2S absorption into aqueous ferric solutions of EDTA and HEDTA. AIChE J. 1994, 40, 433–444. [Google Scholar] [CrossRef]
- Demminck, J.F.; Beenackers, A.A.C.M. Gas desulfurization with ferric chelates of EDTA and HEDTA: New model for the oxidative absorption of hydrogen sulfide. Ind. Eng. Chem. Res. 1998, 37, 1444–1453. [Google Scholar] [CrossRef]
- Lefrou, C.; Fabry, P.; Poignet, J.-C. Electrochemistry, the Basics with Examples; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Zhou, S.; Chen, X.; Nguyen, T.; Voice, A.K.; Rochelle, G.T. Aqueous ethylenediamine for CO2 capture. ChemSusChem 2010, 3, 913–918. [Google Scholar] [CrossRef]
- Weiland, R.H.; Trass, O. Absorption of carbon dioxide in ethylenediamine solutions i. absorption kinetics and equilibrium. Can. J. Chem. Eng. 1971, 49, 767–772. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H.; Butt, M.A. Absorption of carbon dioxide into aqueous solutions of ethylenediamine: Effect of interfacial turbulence. Chem. Eng. J. 1977, 13, 213–217. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).