Predicting Ethanol Steam Reforming Products of Au-Cu Supported over Nano-Shaped CeO2 Using the Johnsen Measure in PLS
Abstract
:1. Introduction
2. Material and Methods
2.1. Catalyst Data Set
2.2. Catalyst Characterization
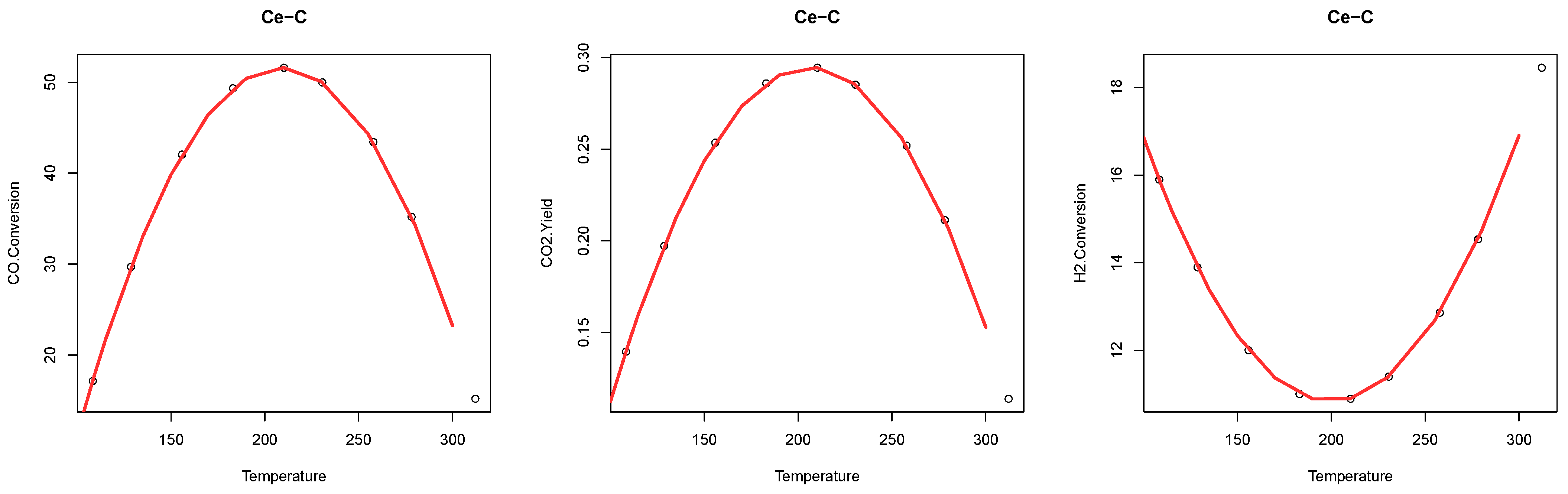
2.3. Interpolation of Ethanol Steam Reforming Products
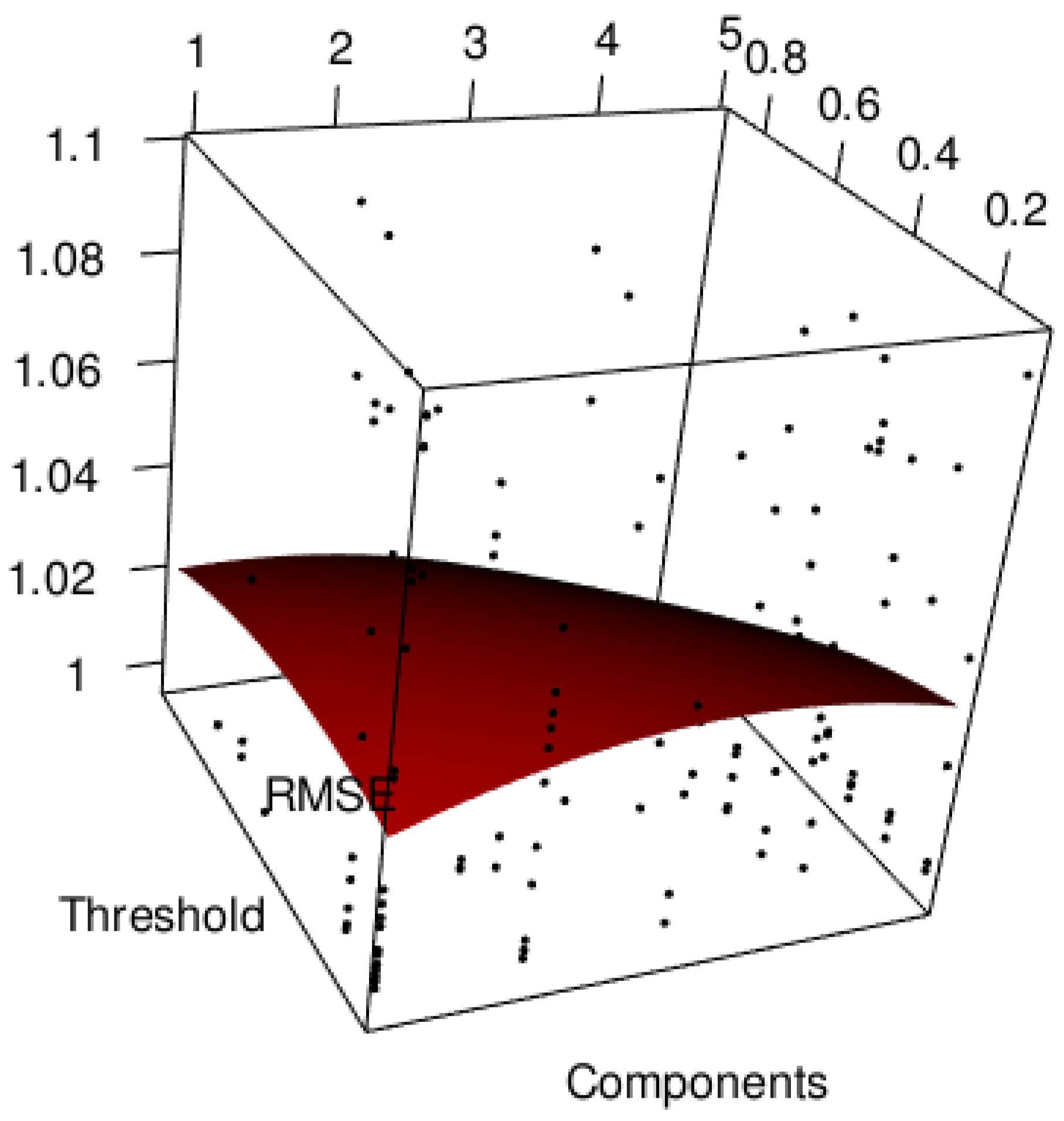
2.4. PLS Modeling of Ethanol Steam Reforming Products
2.5. Proposed Measure for Ethanol Steam Reforming Characterization
3. Simulation Study
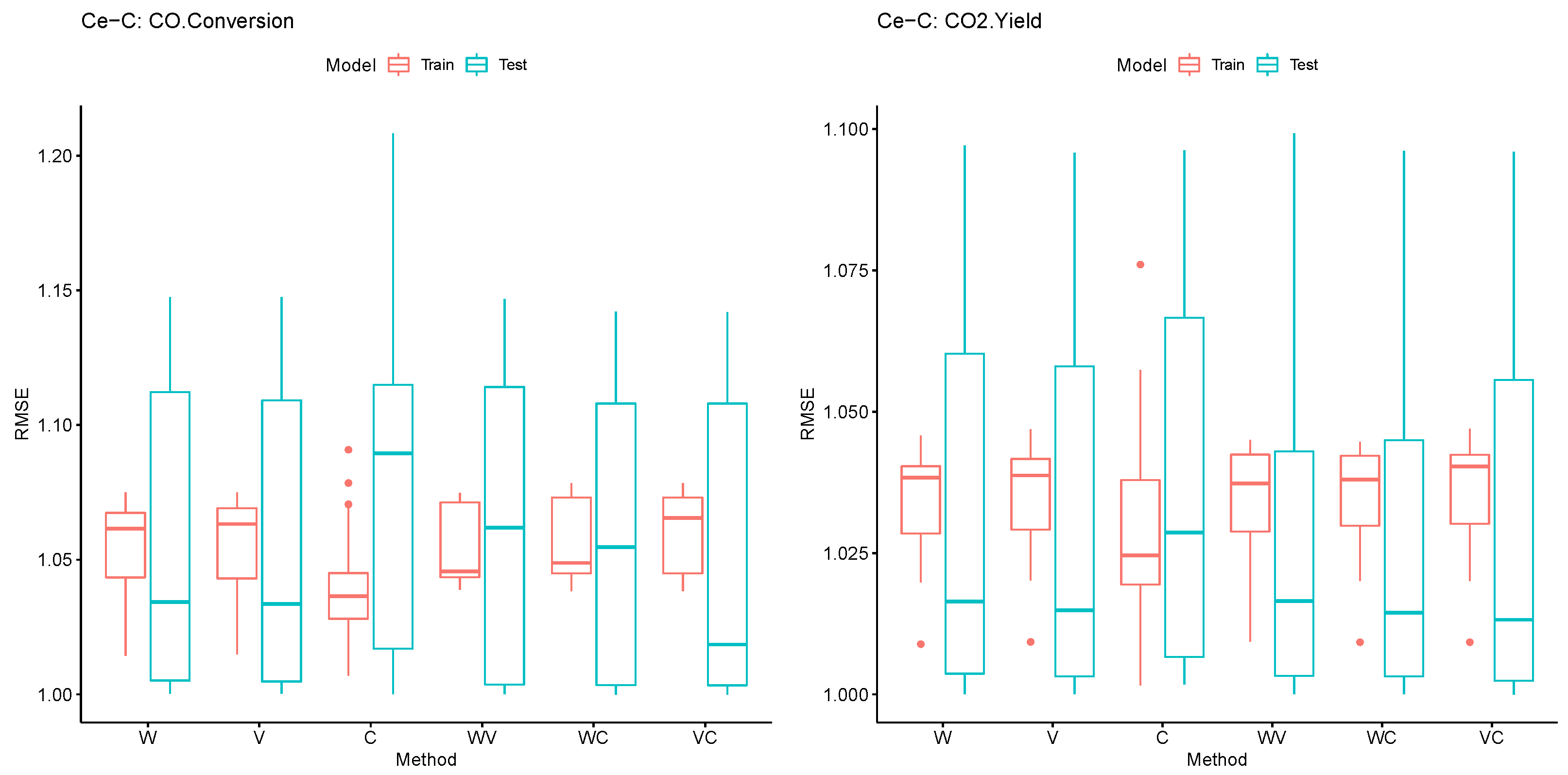
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appleby, A. Fuel cells and hydrogen fuel. Int. J. Hydrogen Energy 1994, 19, 175–180. [Google Scholar] [CrossRef]
- Cook, B. Introduction to fuel cells and hydrogen technology. Eng. Sci. Educ. J. 2002, 11, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Tromp, T.K.; Shia, R.L.; Allen, M.; Eiler, J.M.; Yung, Y.L. Potential environmental impact of a hydrogen economy on the stratosphere. Science 2003, 300, 1740–1742. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Kuzhaeva, A.; Dzhevaga, N.; Berlinskii, I. Modernization of catalyst systems for the processes of hydrocarbon conversion to synthesis gas. ARPN J. Eng. Appl. Sci. 2019, 14, 3535–3543. [Google Scholar]
- Ogo, S.; Sekine, Y. Recent progress in ethanol steam reforming using non-noble transition metal catalysts: A review. Fuel Process. Technol. 2020, 199, 106238. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, C.; Zong, L.; Lu, K.; Wang, X.; Cai, J. Hydrogen production from methanol steam reforming over TiO2 and CeO2 pillared clay supported Au catalysts. Appl. Sci. 2018, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Azizan, M.T.; Aqsha, A.; Ameen, M.; Syuhada, A.; Klaus, H.; Abidin, S.Z.; Sher, F. Catalytic reforming of oxygenated hydrocarbons for the hydrogen production: An outlook. Biomass Convers. Biorefin. 2020, 1–24. [Google Scholar] [CrossRef]
- Kim, C.; Choi, S.; Choi, M.J.; Lee, S.A.; Ahn, S.H.; Kim, S.Y.; Jang, H.W. Photoelectrochemical reduction of CO2 to syngas by reduced Ag catalysts on Si photocathodes. Appl. Sci. 2020, 10, 3487. [Google Scholar] [CrossRef]
- Ayastuy, J.; Fernández-Puertas, E.; González-Marcos, M.; Gutiérrez-Ortiz, M. Transition metal promoters in CuO/CeO2 catalysts for CO removal from hydrogen streams. Int. J. Hydrogen Energy 2012, 37, 7385–7397. [Google Scholar] [CrossRef]
- Si, R.; Raitano, J.; Yi, N.; Zhang, L.; Chan, S.W.; Flytzani-Stephanopoulos, M. Structure sensitivity of the low-temperature water-gas shift reaction on Cu–CeO2 catalysts. Catal. Today 2012, 180, 68–80. [Google Scholar]
- Qi, G.; Yang, R.T. Characterization and FTIR studies of MnOx-CeO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. J. Phys. Chem. B 2004, 108, 15738–15747. [Google Scholar] [CrossRef]
- Gonzalez-A, E.; Rangel, R.; Solís-Garcia, A.; Venezia, A.; Zepeda, T. FTIR investigation under reaction conditions during CO oxidation over Ru(x)-CeO2 catalysts. Mol. Catal. 2020, 493, 111086. [Google Scholar] [CrossRef]
- Milt, V.; Querini, C.; Miró, E.; Ulla, M. Abatement of diesel exhaust pollutants: NOx adsorption on Co, Ba, K/CeO2 catalysts. J. Catal. 2003, 220, 424–432. [Google Scholar] [CrossRef]
- Jobbagy, M.; Marino, F.; Schönbrod, B.; Baronetti, G.; Laborde, M. Synthesis of copper-promoted CeO2 catalysts. Chem. Mater. 2006, 18, 1945–1950. [Google Scholar] [CrossRef]
- Quiroz, J.; Giraudon, J.M.; Gervasini, A.; Dujardin, C.; Lancelot, C.; Trentesaux, M.; Lamonier, J.F. Total oxidation of formaldehyde over MnO x-CeO2 catalysts: The effect of acid treatment. Acs Catal. 2015, 5, 2260–2269. [Google Scholar]
- Martens, H.; Naes, T. Multivariate calibration. In Chemometrics; Springer: Berlin/Heidelberg, Germany, 1984; pp. 147–156. [Google Scholar]
- Mehmood, T.; Iqbal, M.; Hassan, R. Prediction of antibacterial activity in ionic liquids through FTIR spectroscopy with selection of wavenumber by PLS. Chemom. Intell. Lab. Syst. 2020, 206, 104124. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Bao, L.; Yang, J.; Damirin, A.; Zhang, J. The correlation between multiple variable factors and the autocatalytic properties of cerium oxide nanoparticles based on cell viability. New J. Chem. 2018, 42, 9975–9986. [Google Scholar]
- García Martínez, T.; Veses Roda, A.; López Sebastián, J.M.; Puértolas Lacambra, B.; Pérez-Ramírez, J.; Callén Romero, M. Determining bio-oil composition via chemometric tools based on infrared spectroscopy. Acs Sustain. Chem. Eng. 2017, 5, 8710–8719. [Google Scholar] [CrossRef]
- Tenenhaus, M. La Régression PLS: Théorie et Pratique; Editions Technip: Paris, France, 1998. [Google Scholar]
- Kvalheim, O.M.; Karstang, T.V. Interpretation of latent-variable regression models. Chemom. Intell. Lab. Syst. 1989, 7, 39–51. [Google Scholar] [CrossRef]
- Kvalheim, O.M. Interpretation of partial least squares regression models by means of target projection and selectivity ratio plots. J. Chemom. 2010, 24, 496–504. [Google Scholar]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A review of variable selection methods in partial least squares regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar]
- Mehmood, T.; Sæbø, S.; Liland, K.H. Comparison of variable selection methods in partial least squares regression. J. Chemom. 2020, 34, e3226. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.W. A heuristic method for estimating the relative weight of predictor variables in multiple regression. Multivar. Behav. Res. 2000, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M. The minimal transformation to orthonormality. Psychometrika 1966, 31, 61–66. [Google Scholar]
- Cifuentes, B.; Bustamante, F.; Araiza, D.G.; Diaz, G.; Cobo, M. Hydrogen purification of actual syngas streams for energy applications: Au-Cu supported over nano-shaped CeO2 as stable catalysts for the carbon monoxide removal. Appl. Catal. A Gen. 2020, 598, 117568. [Google Scholar] [CrossRef]
- Cifuentes, B.; Cobo, M.; Bustamante, F. Activity, selectivity and characterization data of nano-shaped CeO2 supports for carbon monoxide removal from an actual syngas stream. Mendeley Data 2019, V2. [Google Scholar] [CrossRef]
- Liland, K.H.; Almøy, T.; Mevik, B.H. Optimal choice of baseline correction for multivariate calibration of spectra. Appl. Spectrosc. 2010, 64, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
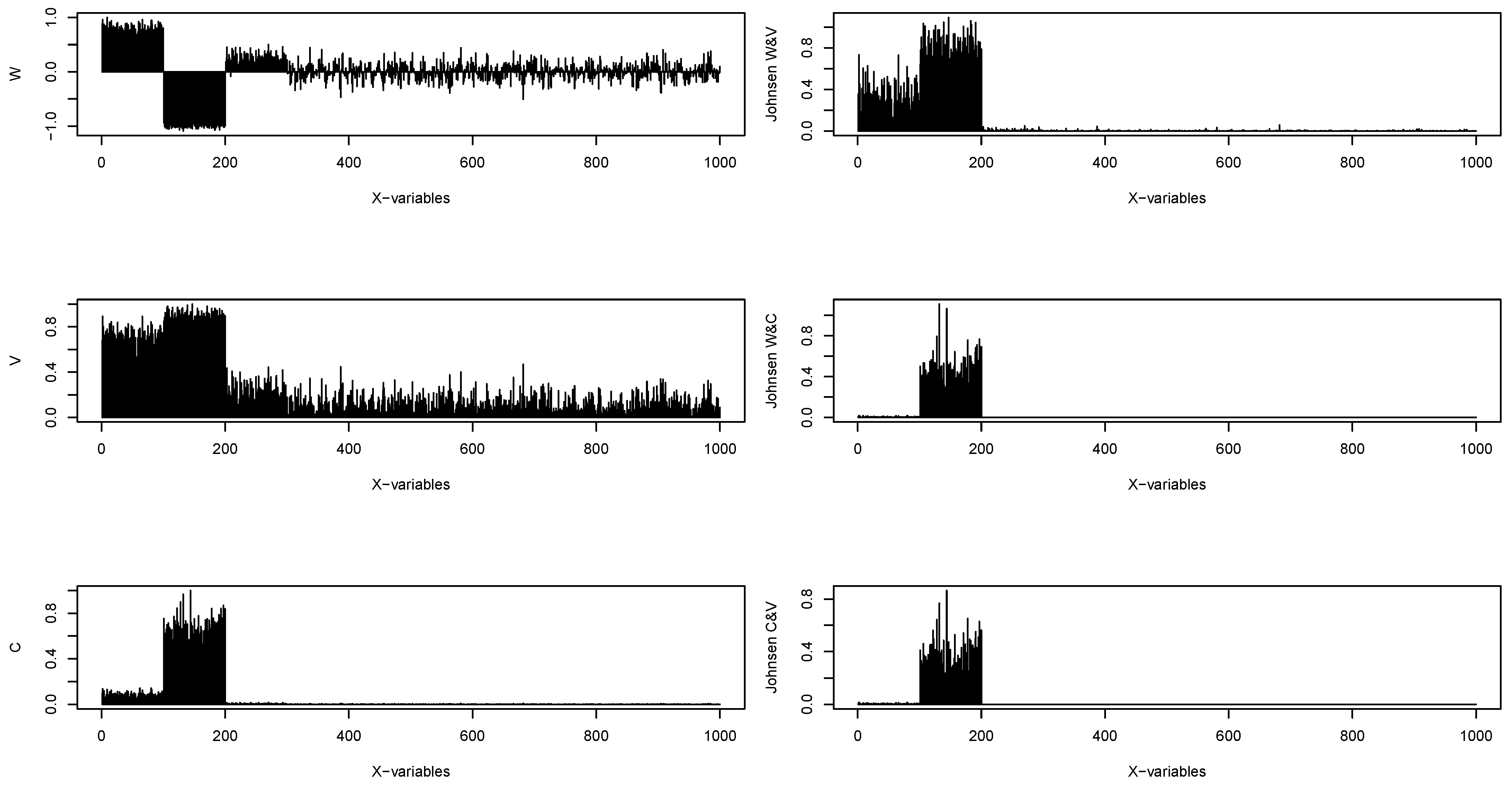
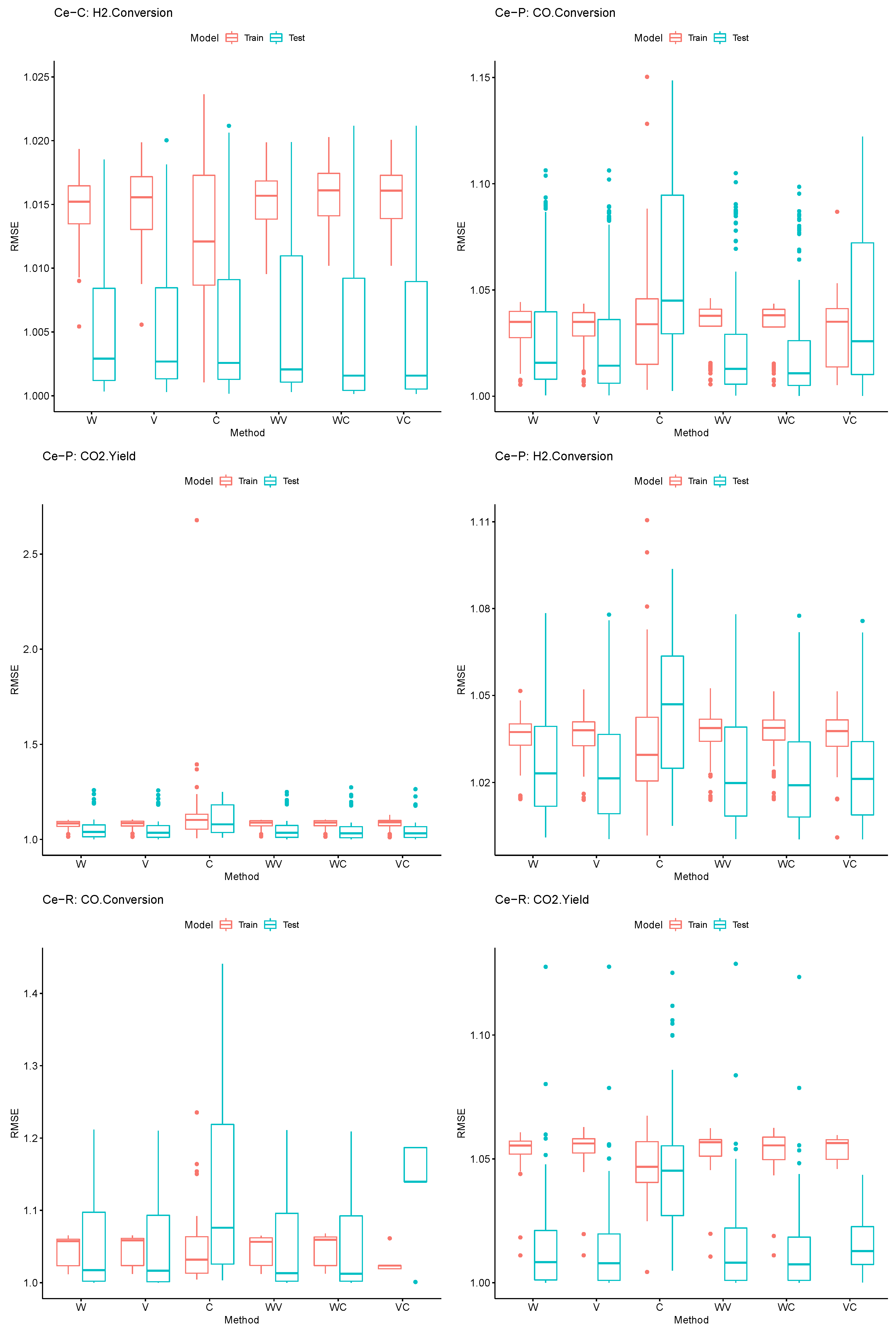
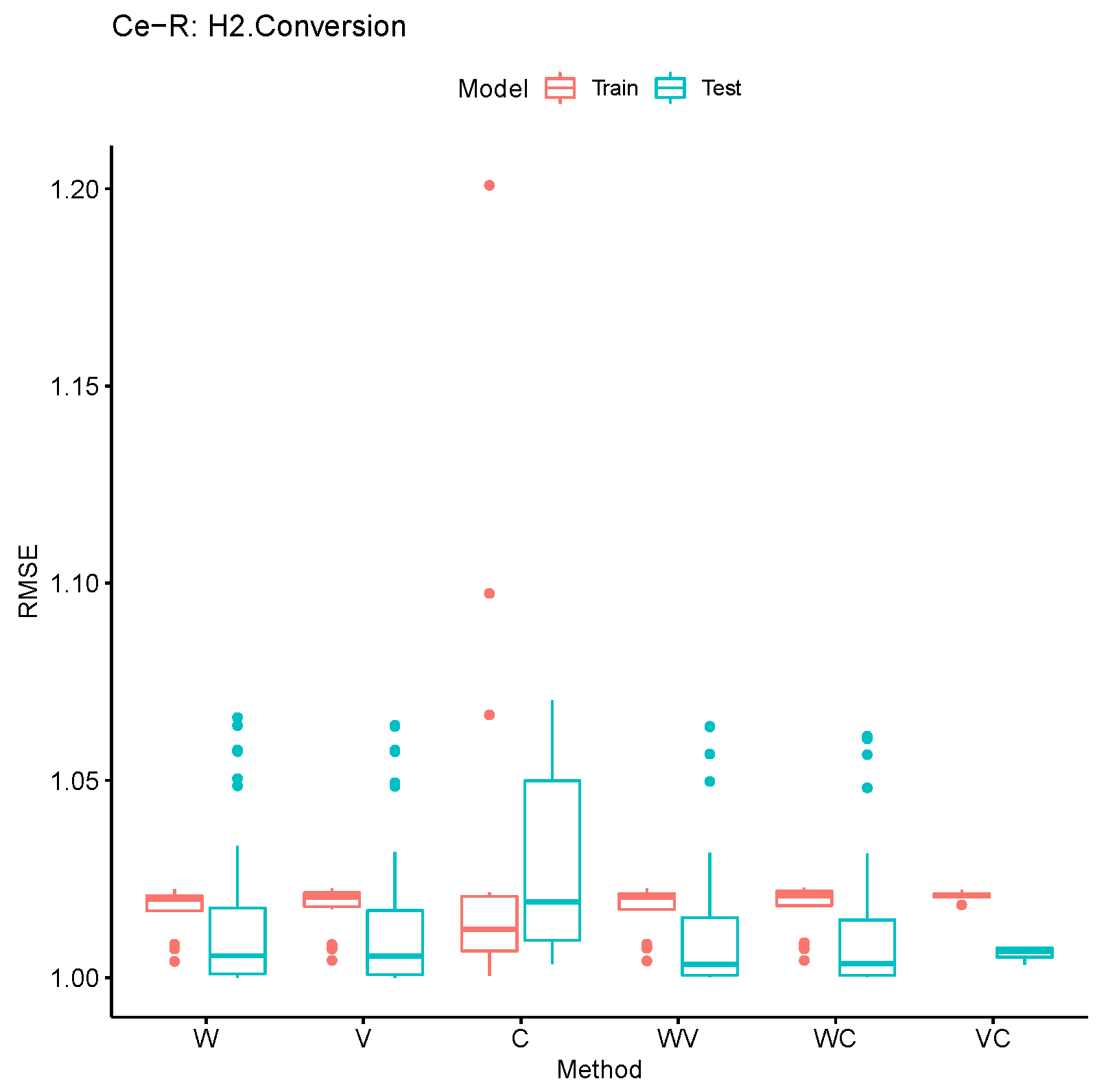
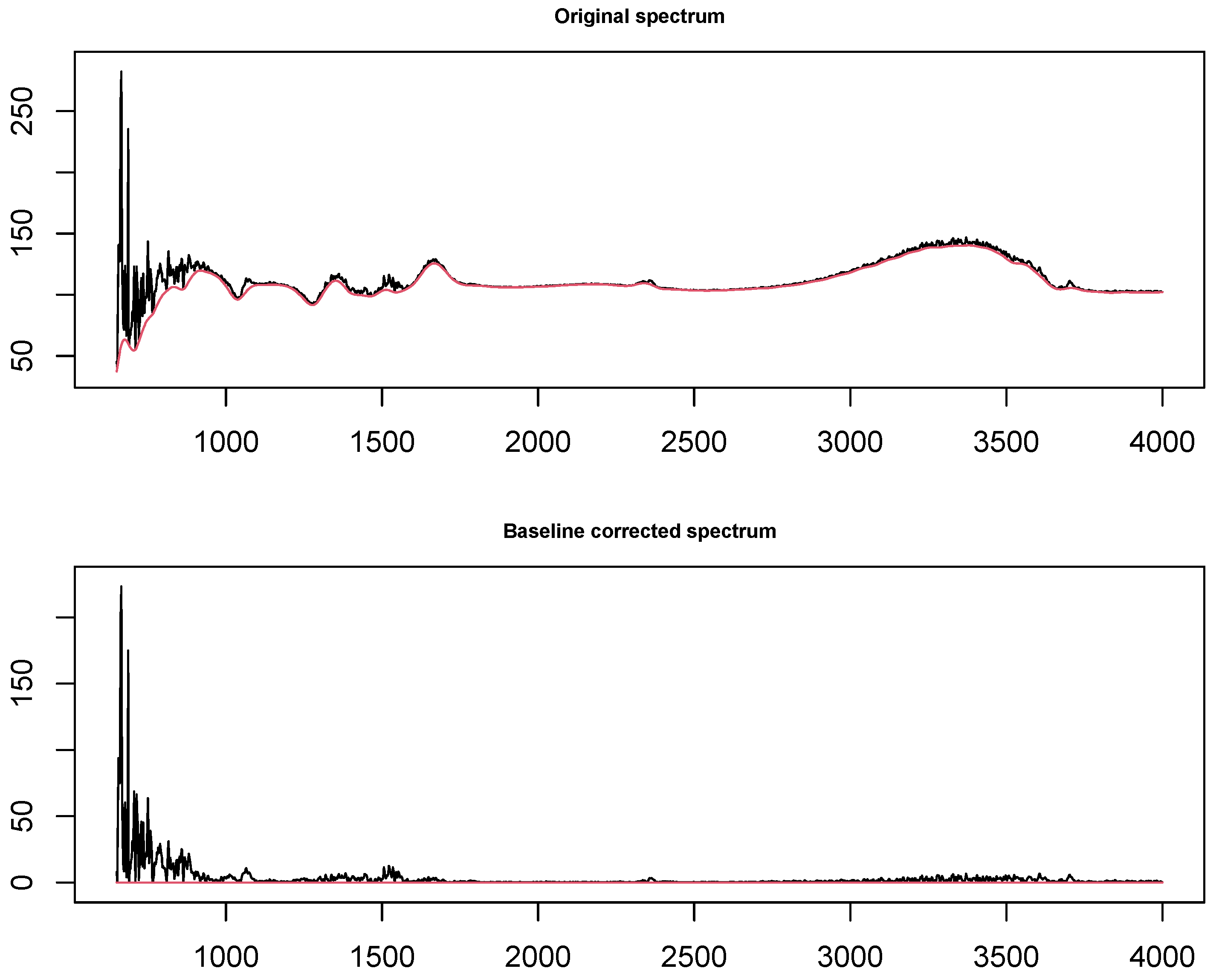
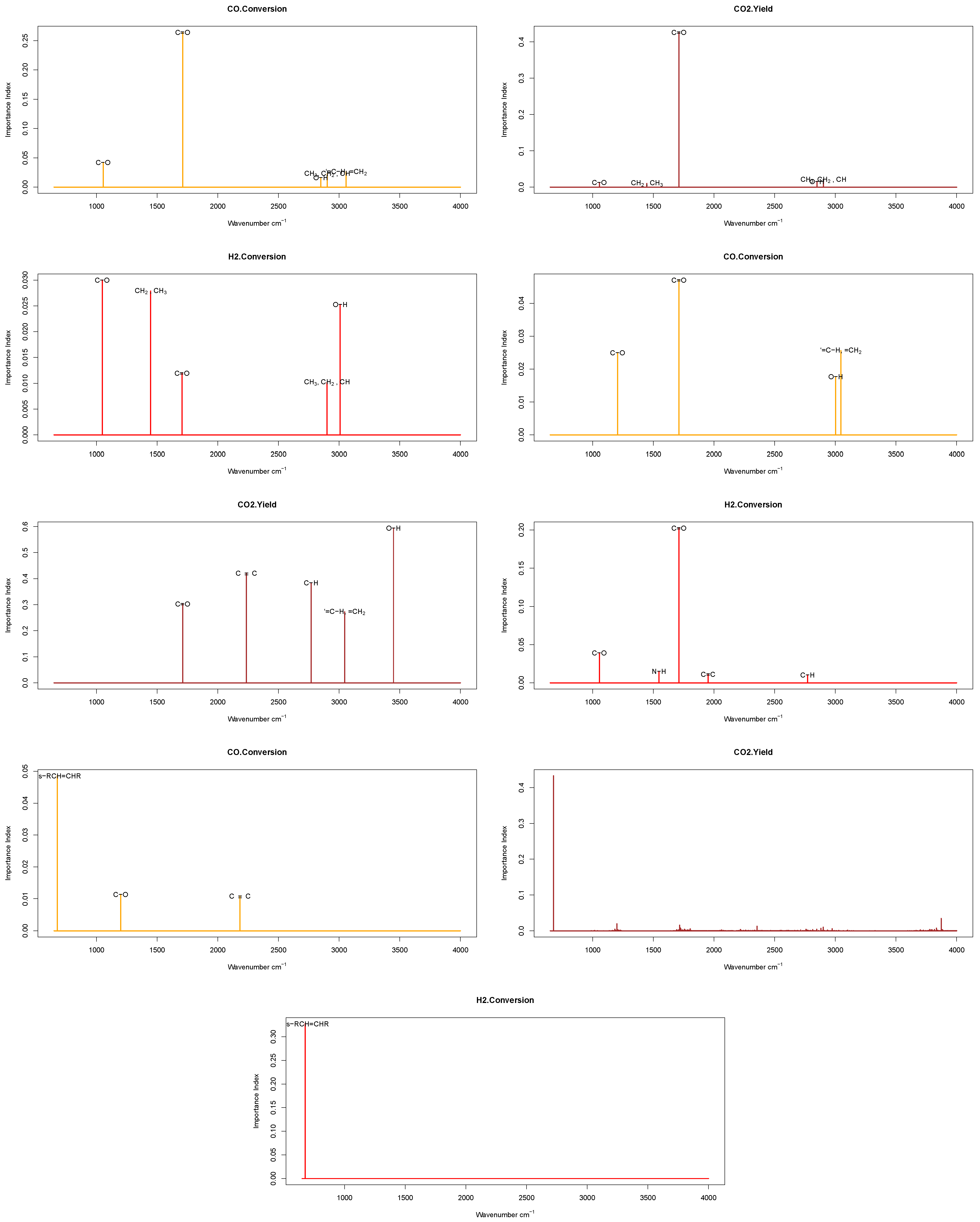
| p | Rohs | Method | Sensitivity (%) | Specificity(%) | Accuracy (%) |
|---|---|---|---|---|---|
| 100 | R1 | W | 0.86 | 0.50 | 0.79 |
| V | 0.81 | 0.90 | 0.83 | ||
| C | 0.99 | 0.65 | 0.92 | ||
| WV | 0.97 | 0.70 | 0.92 | ||
| WC | 0.99 | 0.65 | 0.92 | ||
| VC | 0.99 | 0.65 | 0.92 | ||
| R2 | W | 0.96 | 0.50 | 0.87 | |
| V | 0.95 | 1.00 | 0.96 | ||
| C | 1.00 | 1.00 | 1.00 | ||
| WV | 1.00 | 1.00 | 1.00 | ||
| WC | 1.00 | 1.00 | 1.00 | ||
| VC | 1.00 | 1.00 | 1.00 | ||
| 500 | R1 | W | 0.92 | 0.56 | 0.85 |
| V | 0.76 | 0.71 | 0.75 | ||
| C | 0.91 | 0.63 | 0.86 | ||
| WV | 0.96 | 0.52 | 0.87 | ||
| WC | 0.98 | 0.30 | 0.85 | ||
| VC | 0.98 | 0.30 | 0.85 | ||
| R2 | W | 0.98 | 0.50 | 0.88 | |
| V | 0.97 | 1.00 | 0.97 | ||
| C | 1.00 | 1.00 | 1.00 | ||
| WV | 1.00 | 1.00 | 1.00 | ||
| WC | 1.00 | 0.99 | 1.00 | ||
| VC | 1.00 | 1.00 | 1.00 | ||
| 1000 | R1 | W | 0.92 | 0.48 | 0.83 |
| V | 0.85 | 0.87 | 0.85 | ||
| C | 0.96 | 0.75 | 0.90 | ||
| WV | 0.97 | 0.77 | 0.92 | ||
| WC | 1.00 | 0.52 | 0.90 | ||
| VC | 1.00 | 0.52 | 0.90 | ||
| R2 | W | 0.90 | 0.81 | 0.88 | |
| V | 0.89 | 1.00 | 0.91 | ||
| C | 1.00 | 1.00 | 1.00 | ||
| WV | 1.00 | 1.00 | 1.00 | ||
| WC | 1.00 | 0.96 | 0.99 | ||
| VC | 1.00 | 1.00 | 1.00 |
| ESR Product | Morphology | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| CO Conversion (%) | Cubes | 15.22 | 51.61 | 37.09 | 13.81 |
| Polyhedral | 11.11 | 37.42 | 30.00 | 8.15 | |
| Rods | 6.56 | 34.31 | 25.65 | 8.87 | |
| CO yield (%) | Cubes | 0.11 | 0.29 | 0.24 | 0.07 |
| Polyhedral | 0.02 | 0.32 | 0.24 | 0.10 | |
| Rods | 0.05 | 0.25 | 0.19 | 0.06 | |
| H conversion (%) | Cubes | 10.90 | 18.45 | 13.44 | 2.54 |
| Polyhedral | 7.90 | 17.20 | 10.63 | 3.15 | |
| Rods | 6.26 | 13.33 | 11.18 | 2.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, C.; Tahir, M.; Mehmood, T. Predicting Ethanol Steam Reforming Products of Au-Cu Supported over Nano-Shaped CeO2 Using the Johnsen Measure in PLS. Appl. Sci. 2021, 11, 10402. https://doi.org/10.3390/app112110402
Zhi C, Tahir M, Mehmood T. Predicting Ethanol Steam Reforming Products of Au-Cu Supported over Nano-Shaped CeO2 Using the Johnsen Measure in PLS. Applied Sciences. 2021; 11(21):10402. https://doi.org/10.3390/app112110402
Chicago/Turabian StyleZhi, Chen, Muhammad Tahir, and Tahir Mehmood. 2021. "Predicting Ethanol Steam Reforming Products of Au-Cu Supported over Nano-Shaped CeO2 Using the Johnsen Measure in PLS" Applied Sciences 11, no. 21: 10402. https://doi.org/10.3390/app112110402
APA StyleZhi, C., Tahir, M., & Mehmood, T. (2021). Predicting Ethanol Steam Reforming Products of Au-Cu Supported over Nano-Shaped CeO2 Using the Johnsen Measure in PLS. Applied Sciences, 11(21), 10402. https://doi.org/10.3390/app112110402






