Design of a Self-Expanding Stent Mechanism Enacted by Fluid Pressure Difference
Abstract
:1. Introduction
2. Basic Principle
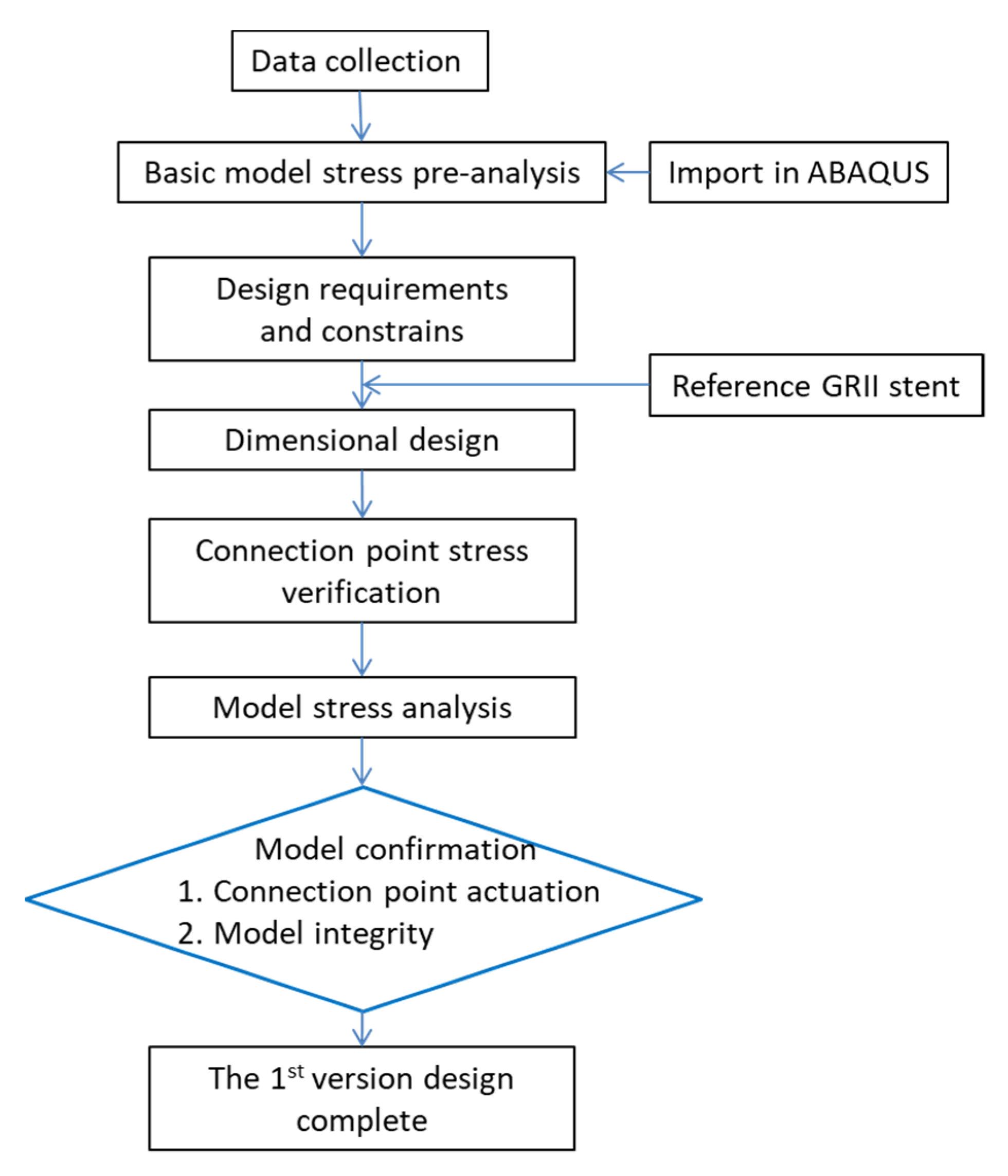
3. Conceptual Design
3.1. Design Requirements and Constraints
- The stent must withstand different fluid pressures, exerted in different places on the model.
- Fasteners or latches are needed in order to lock the intrinsic elastic force of the stent.
- Stress positions on the stent can be controlled by changes the cross-section area.
- The stent must retain its circular shape after expansion to maintain smooth flow through the vessel.
- The stent should have an expansion ratio of 2–3 times.
- Variation in axial length during or after expansion should be avoided.
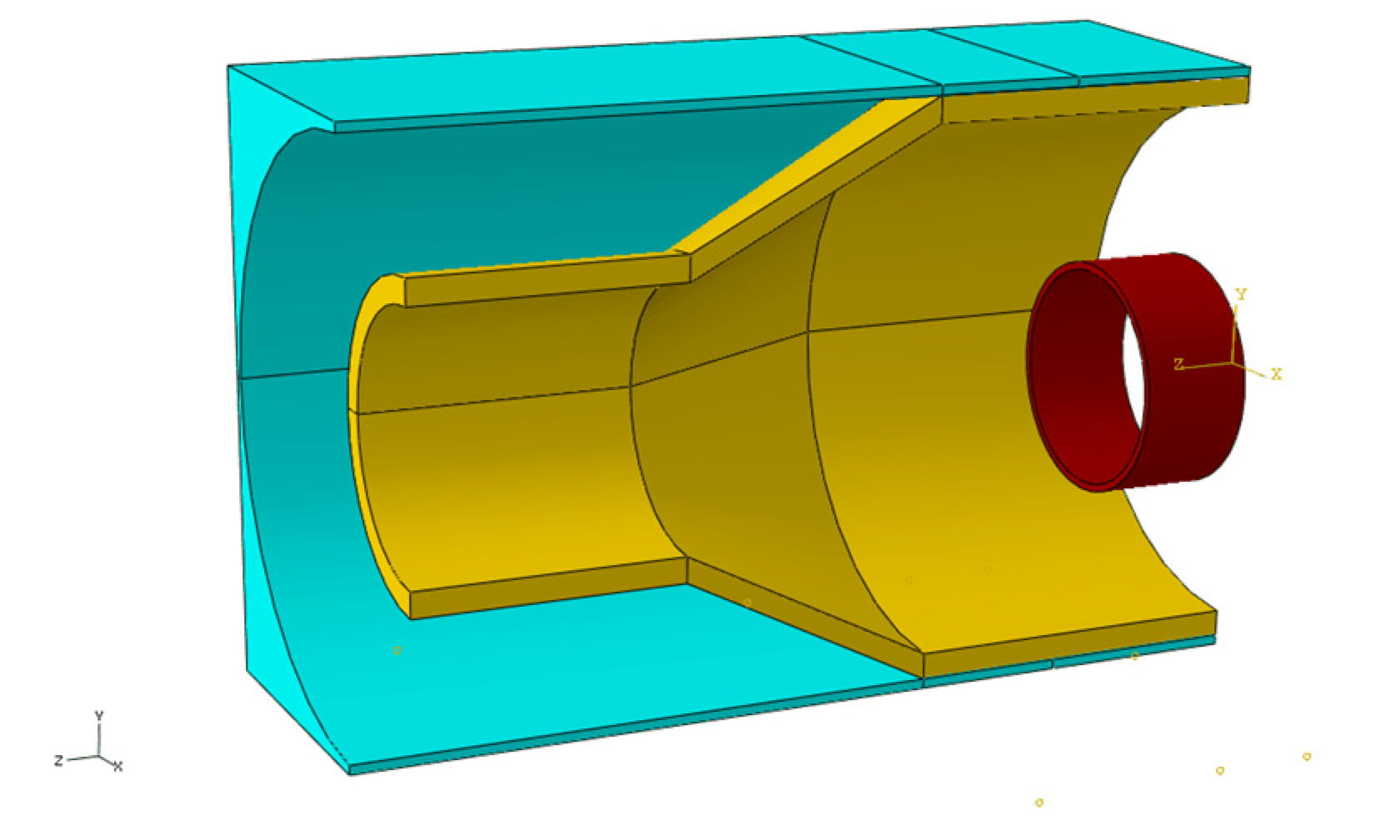
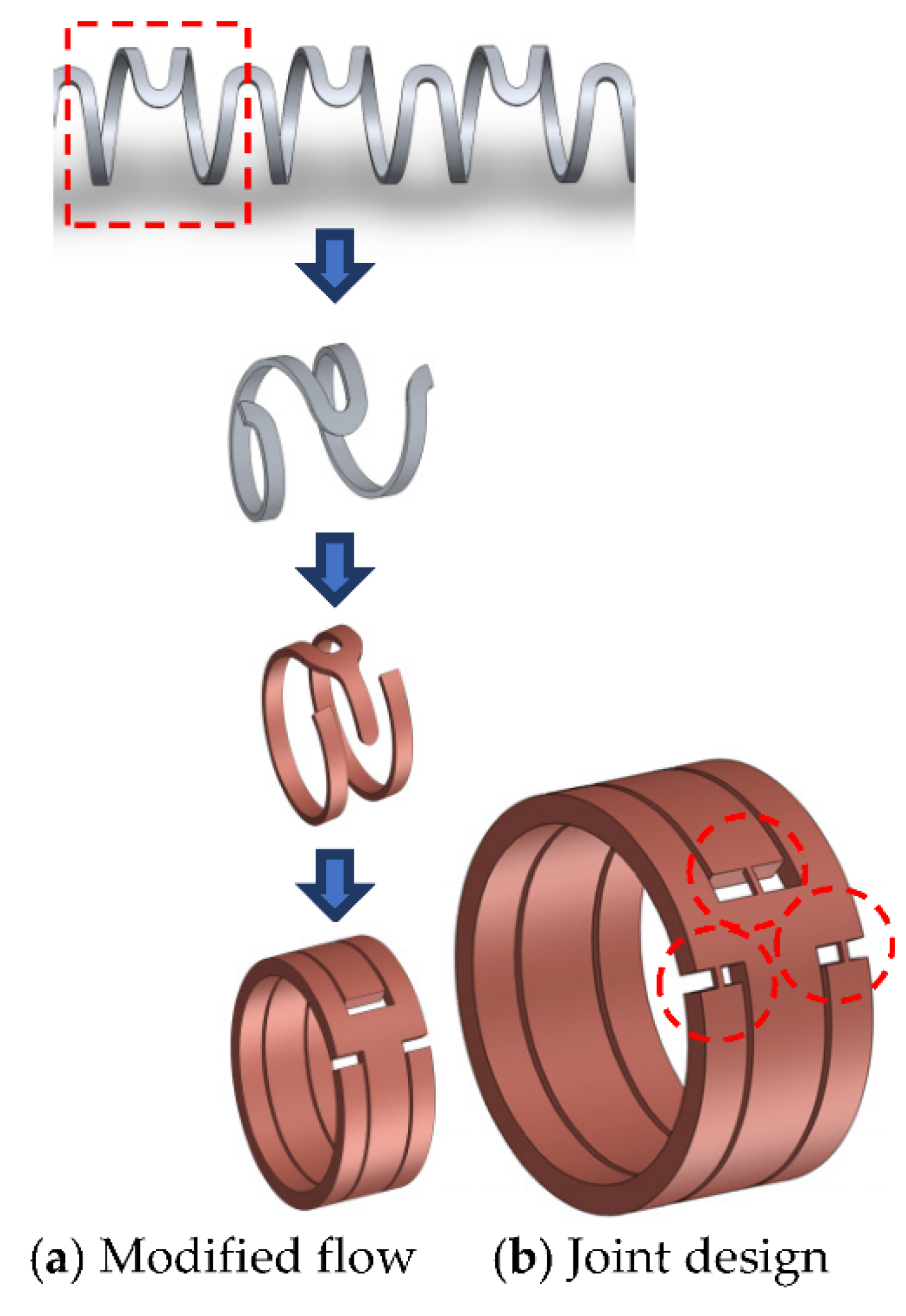
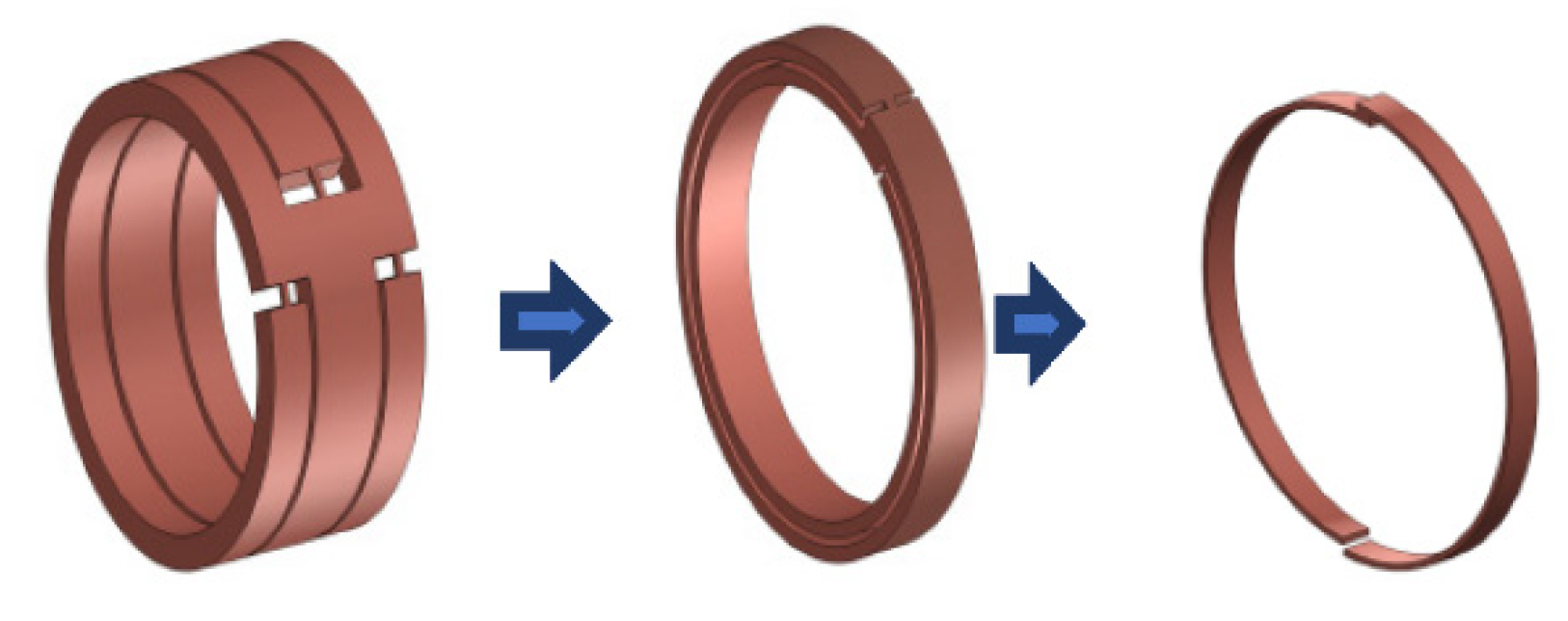
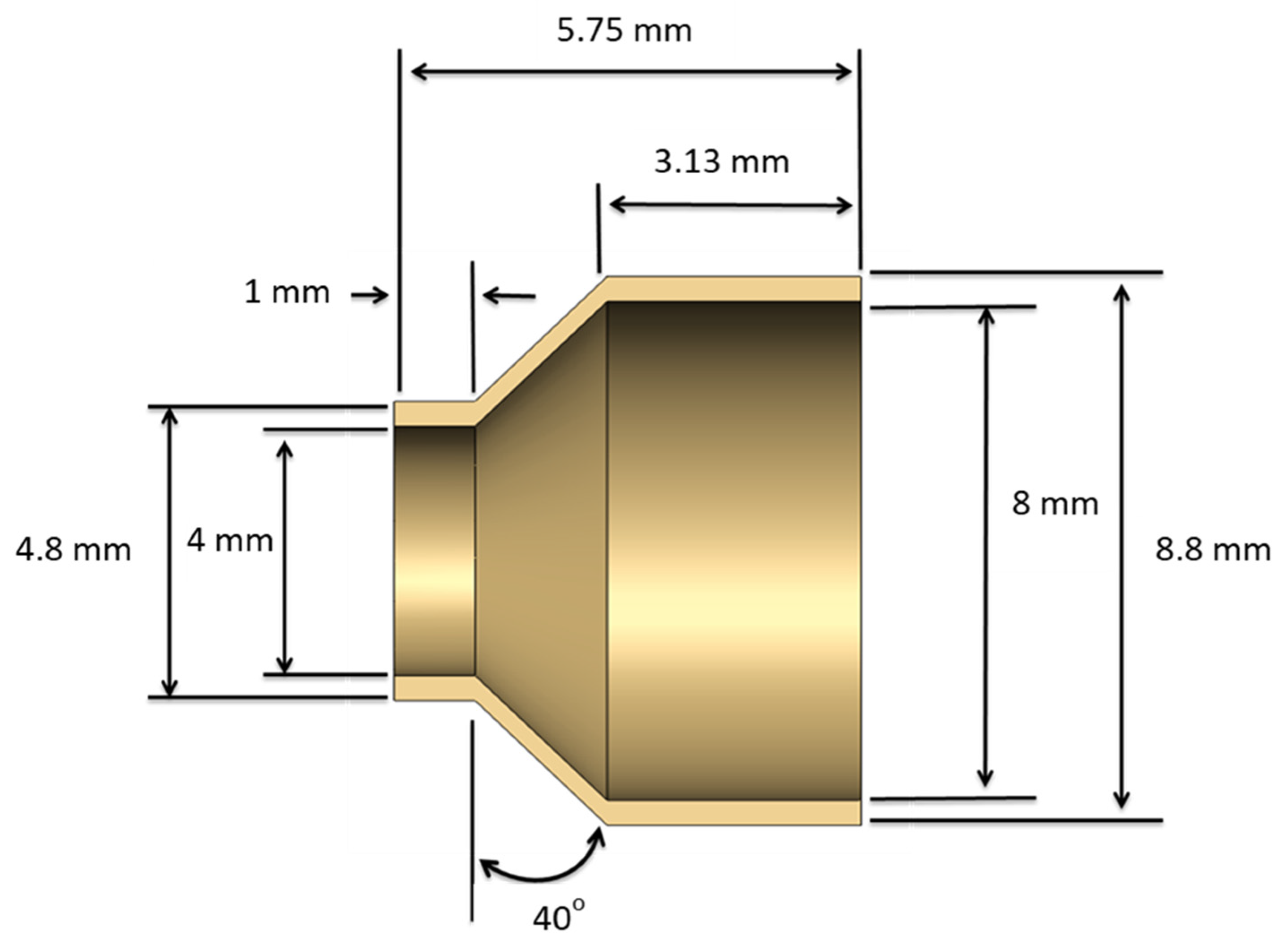
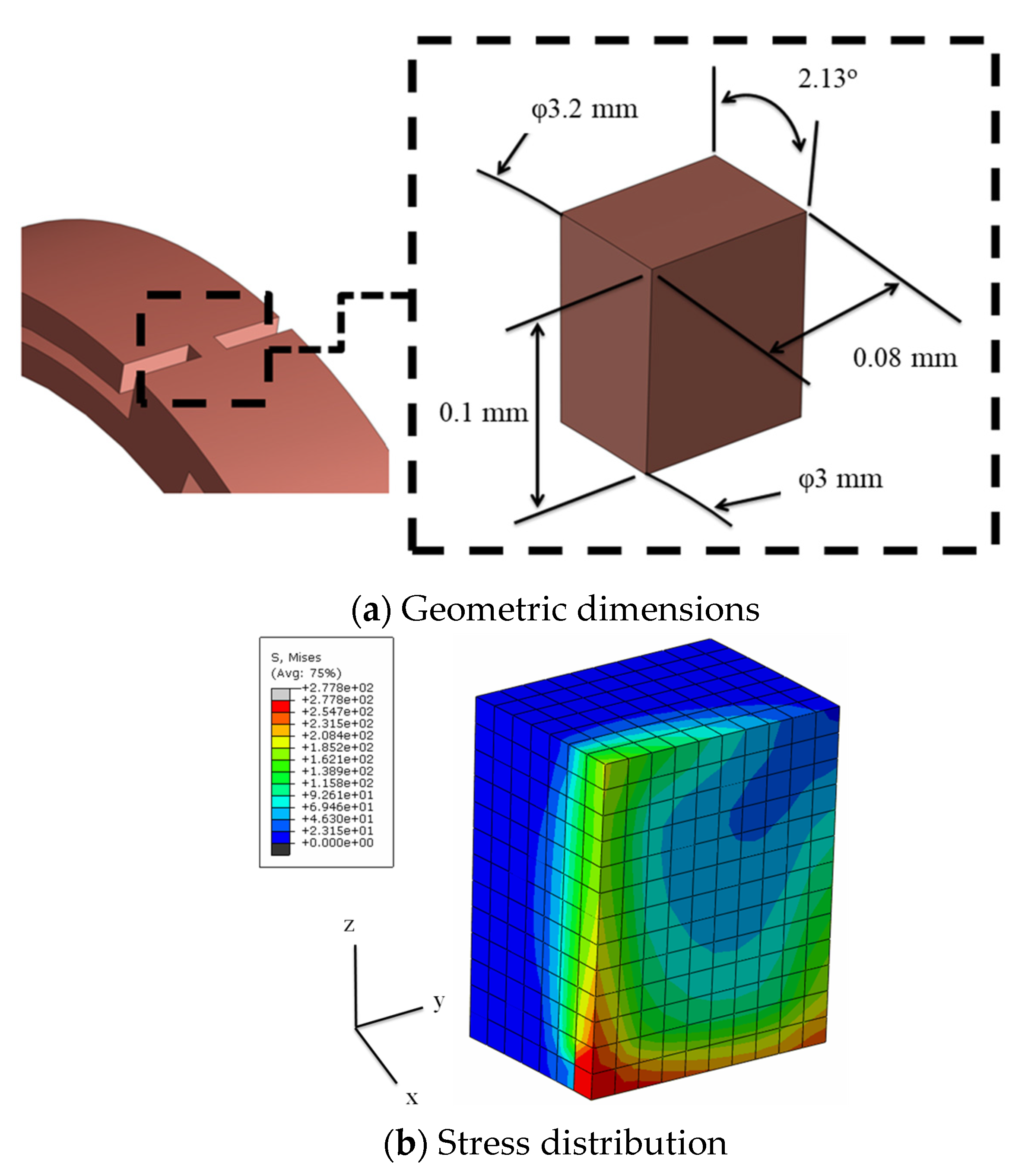
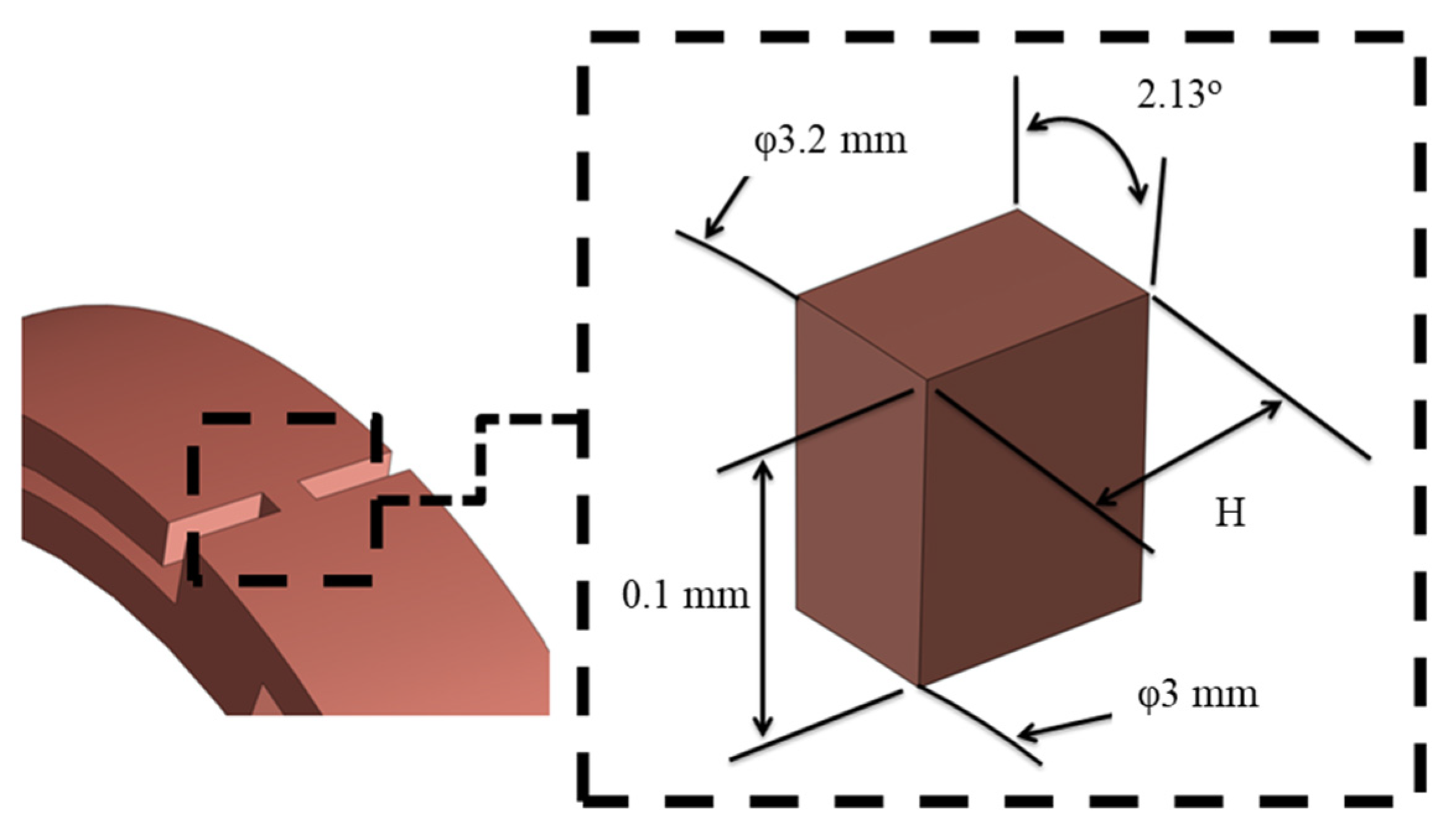
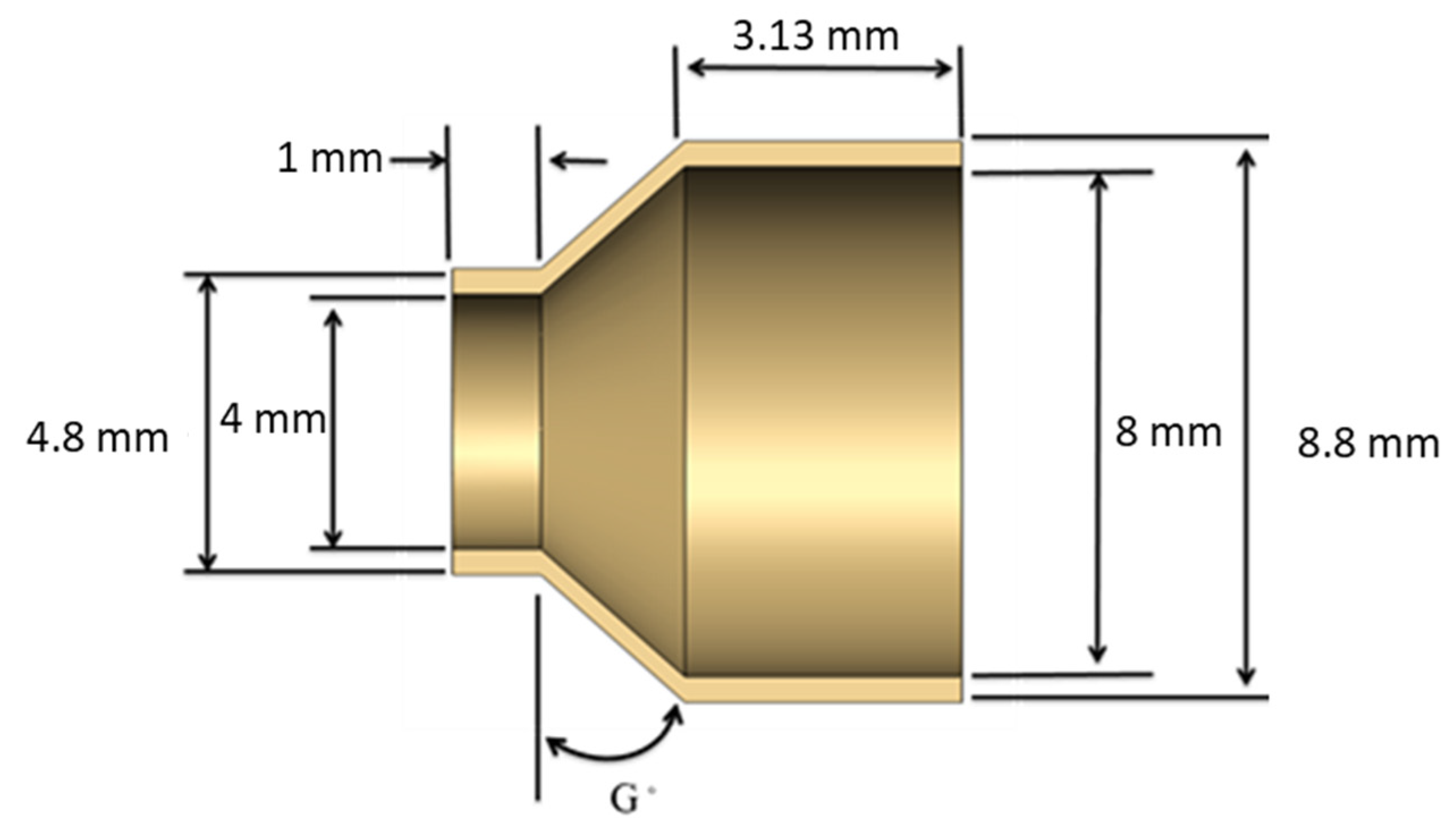
3.2. Creation of a Geometric Model
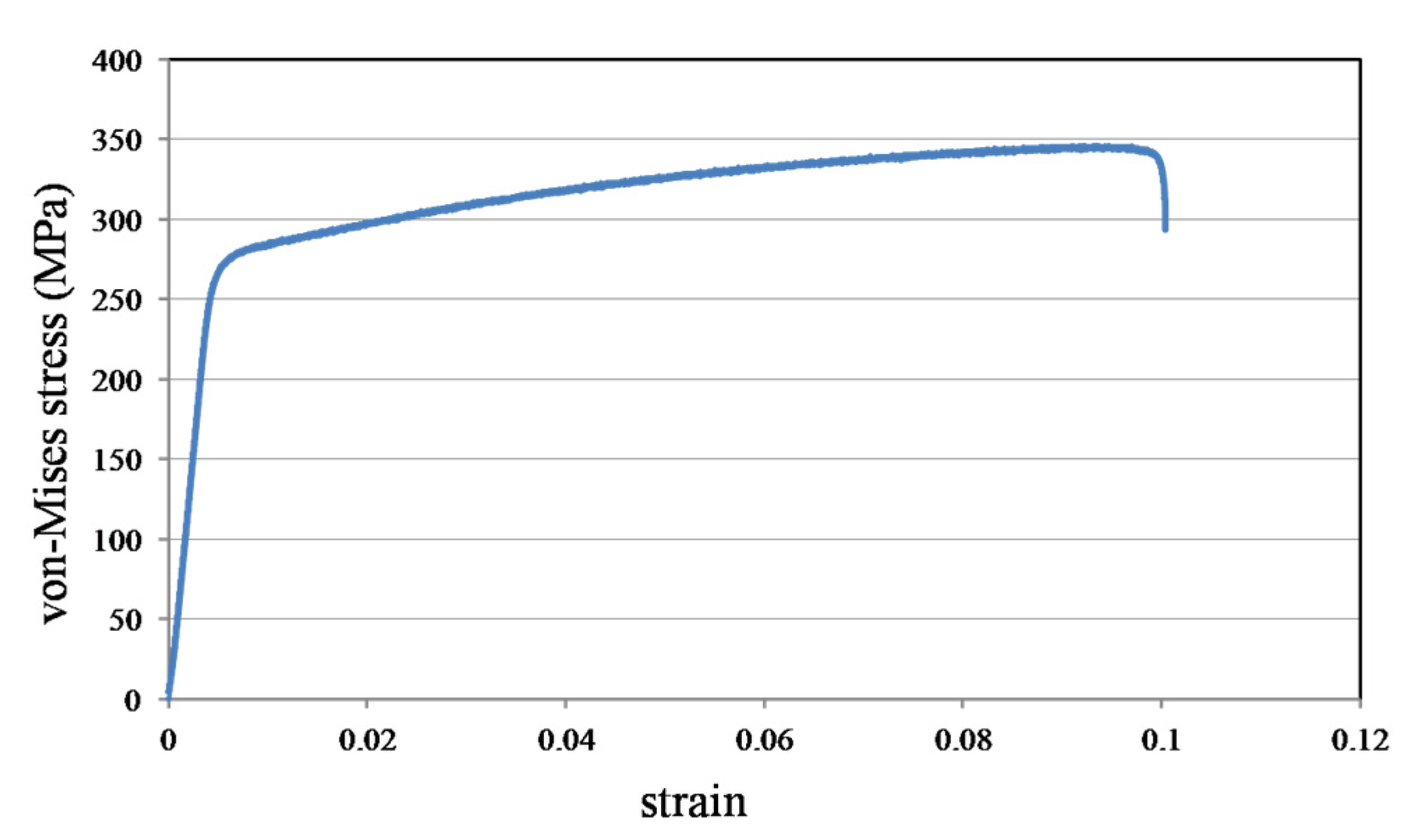
3.3. Material Properties
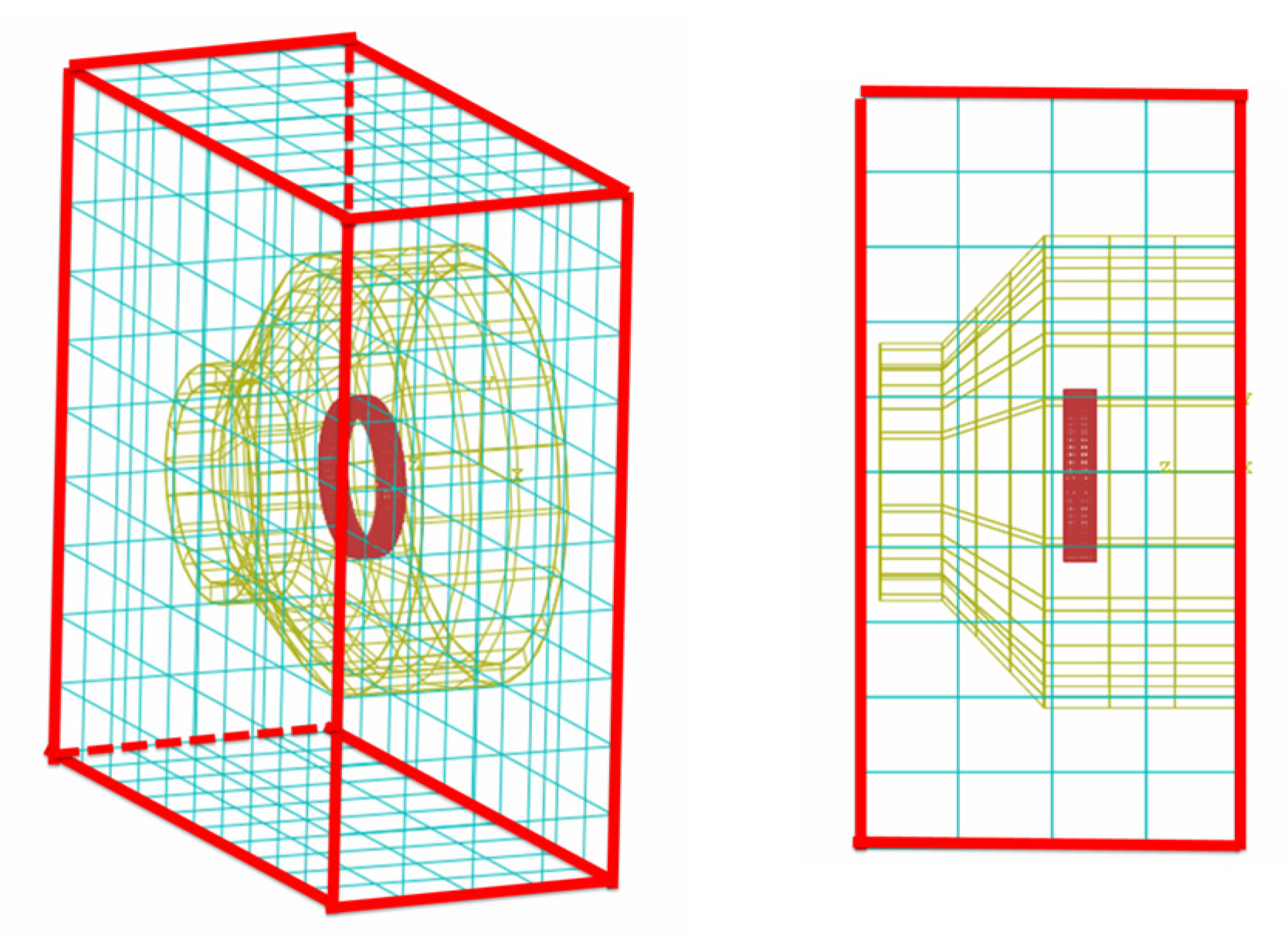
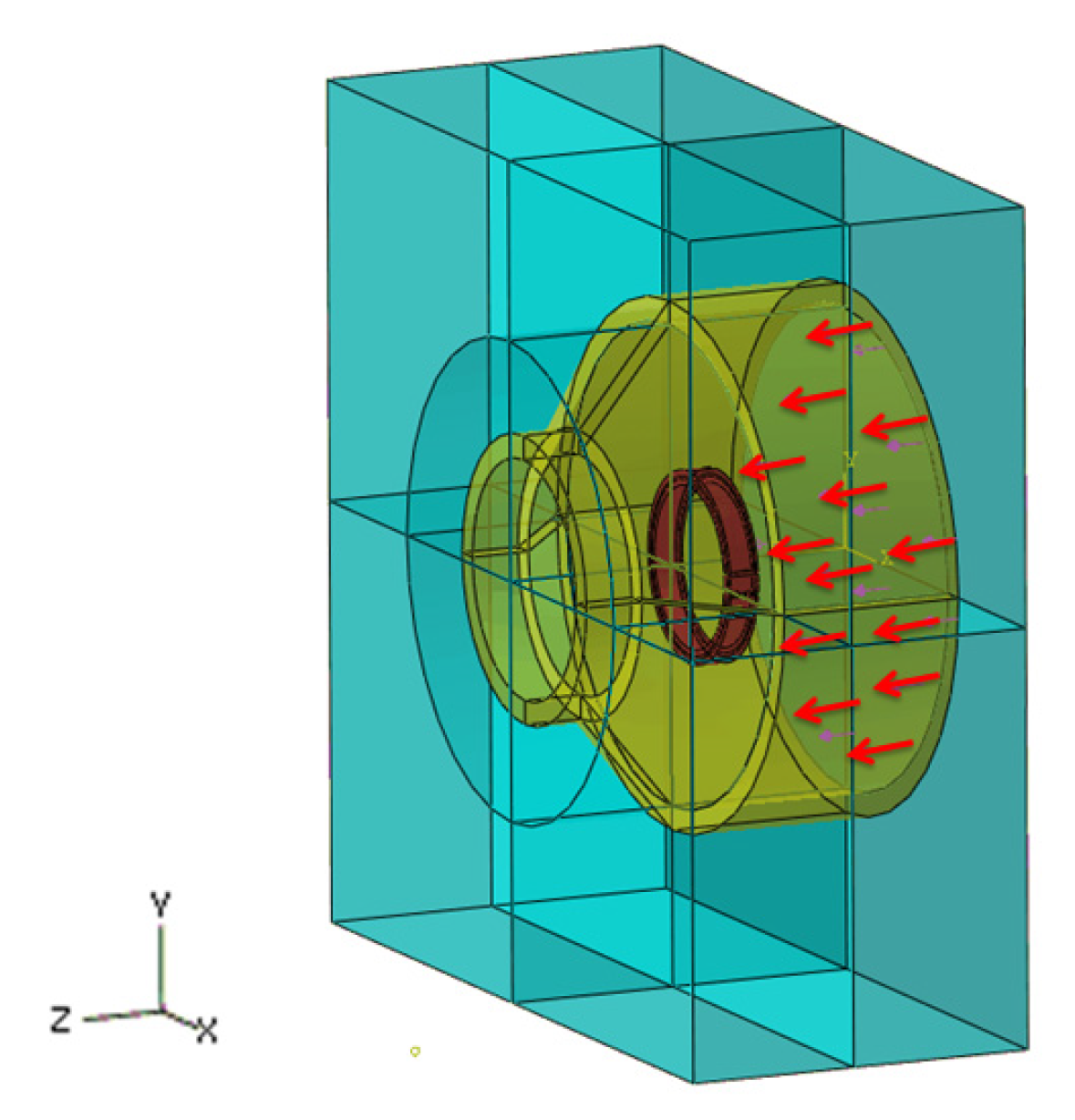
3.4. Boundary Condition for the Simulation
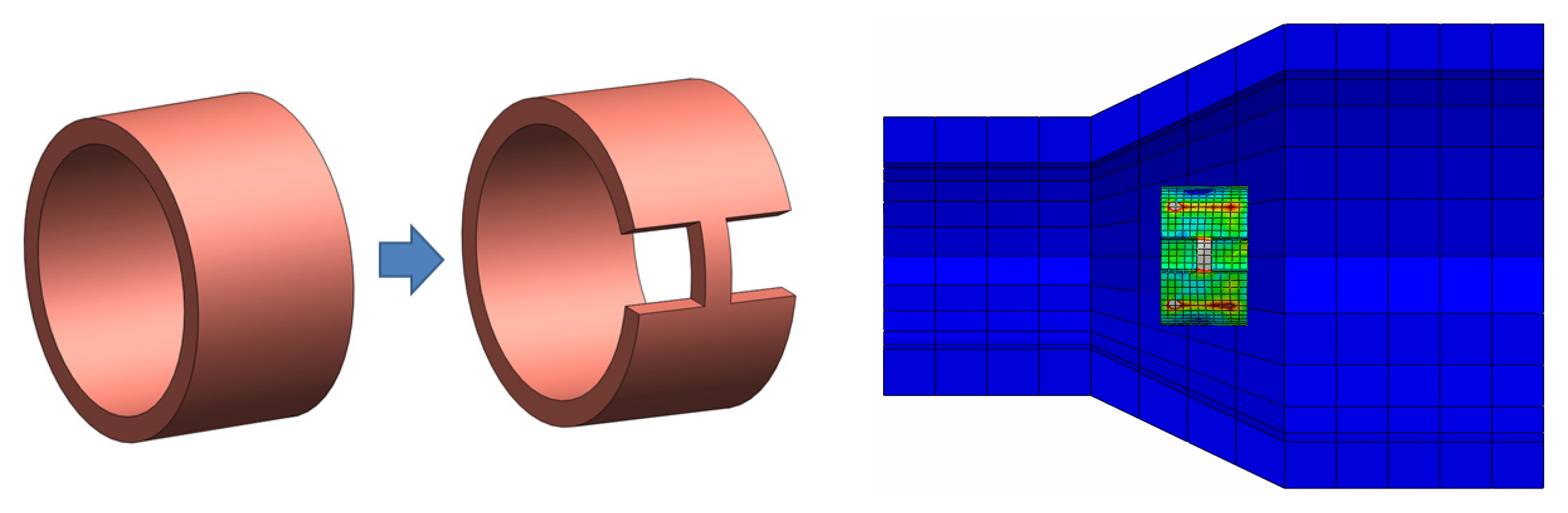
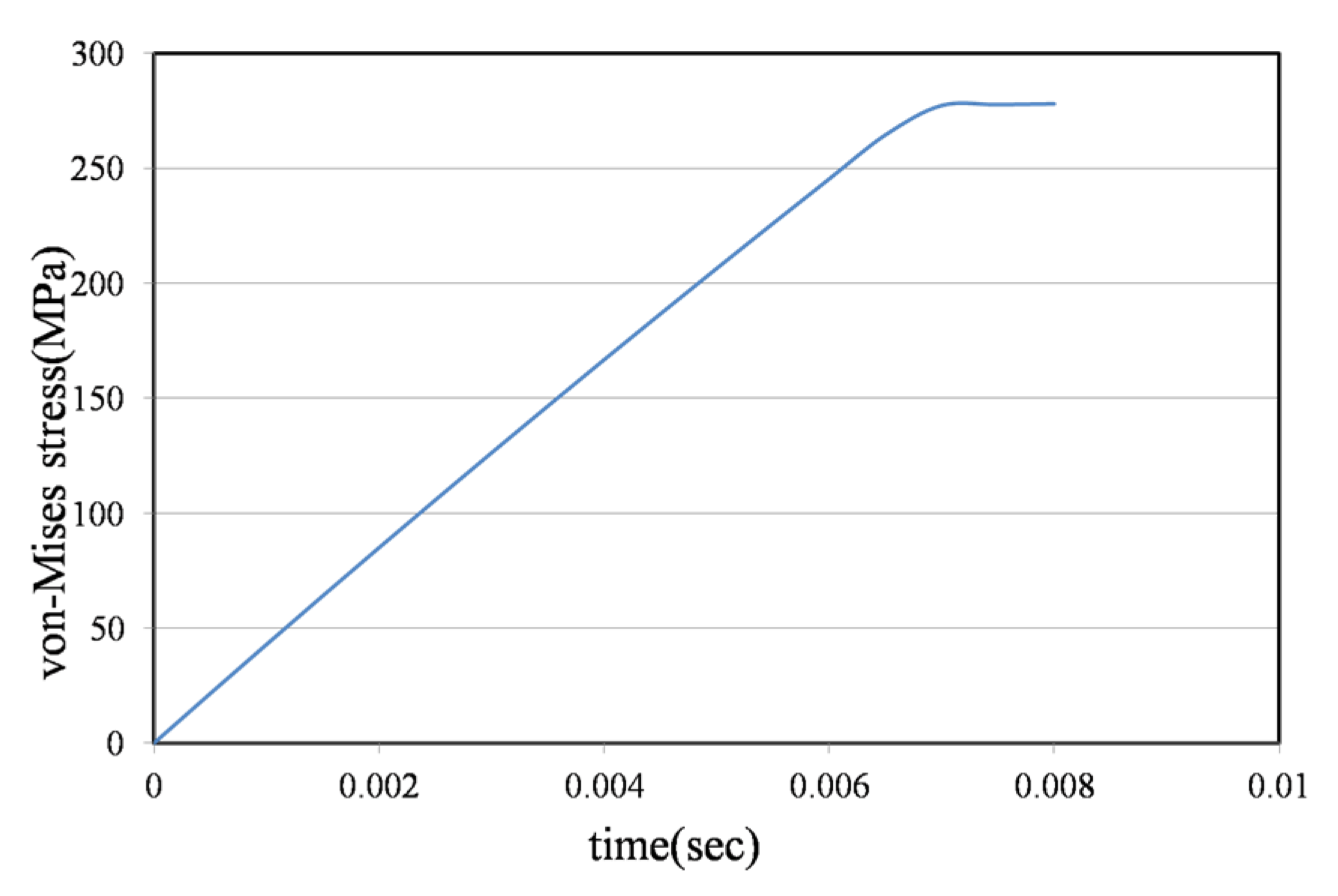
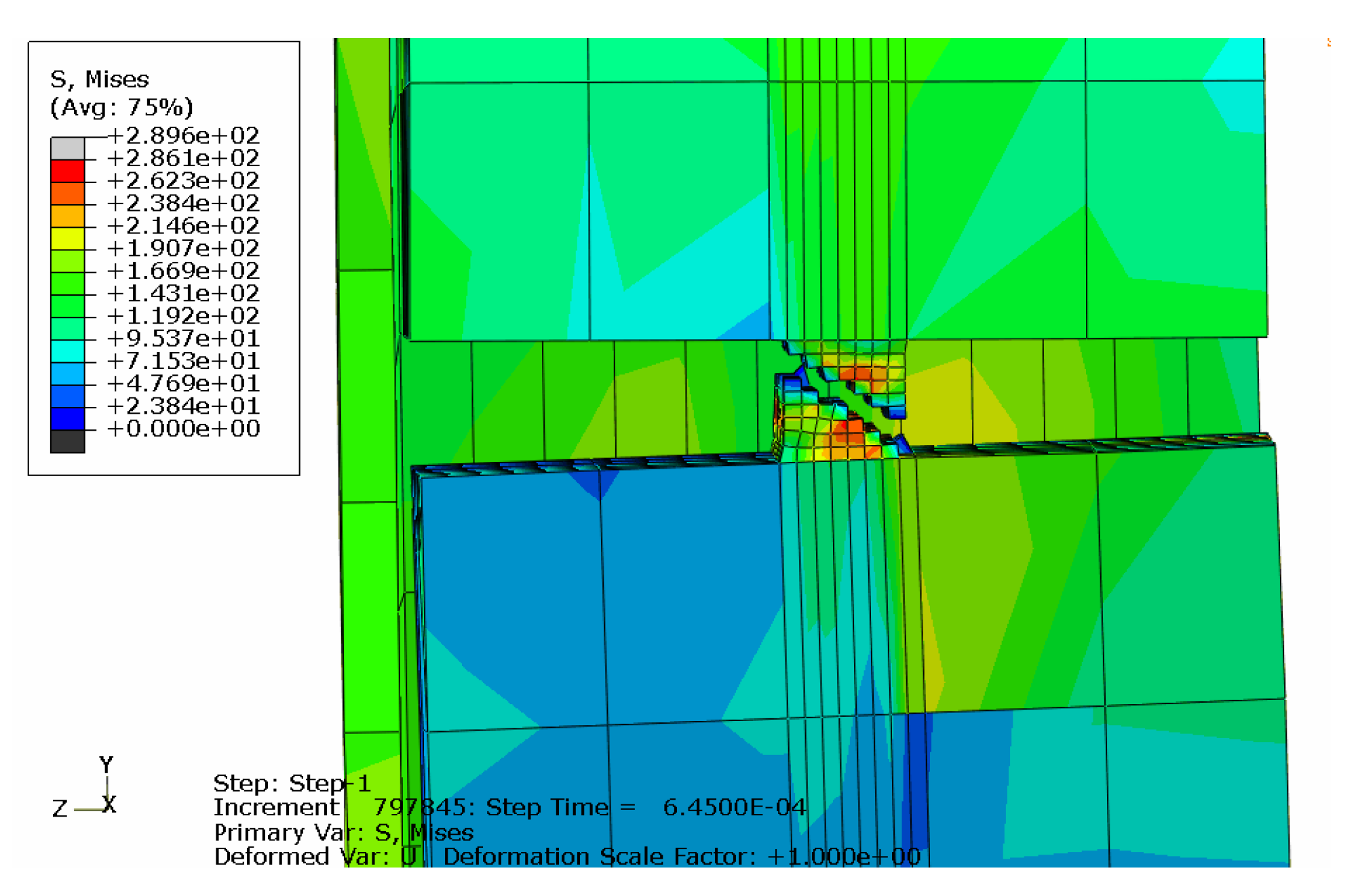
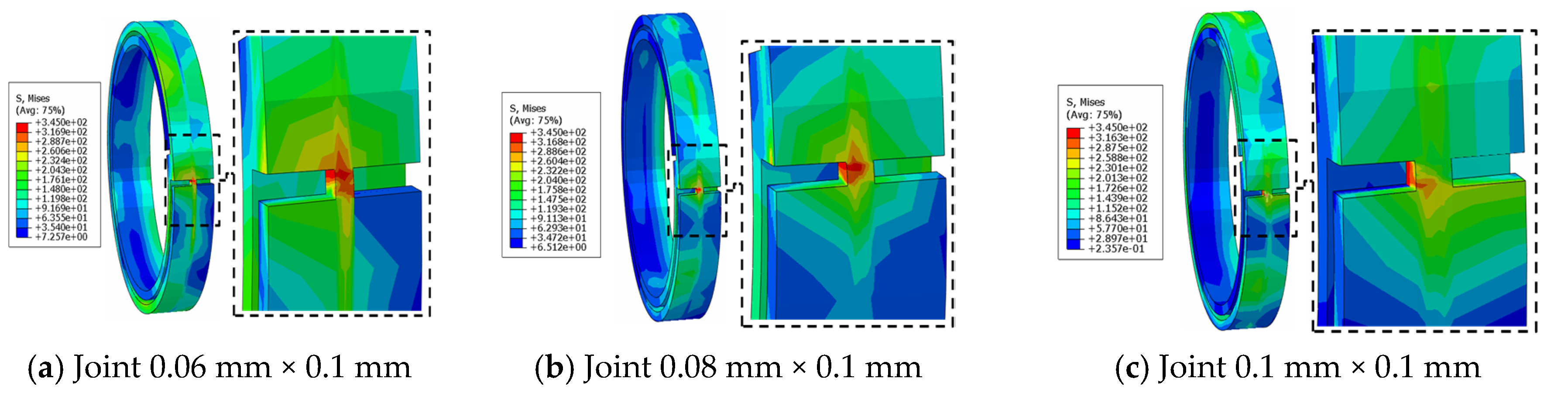
3.5. Joint Model Verification
4. Analysis Results
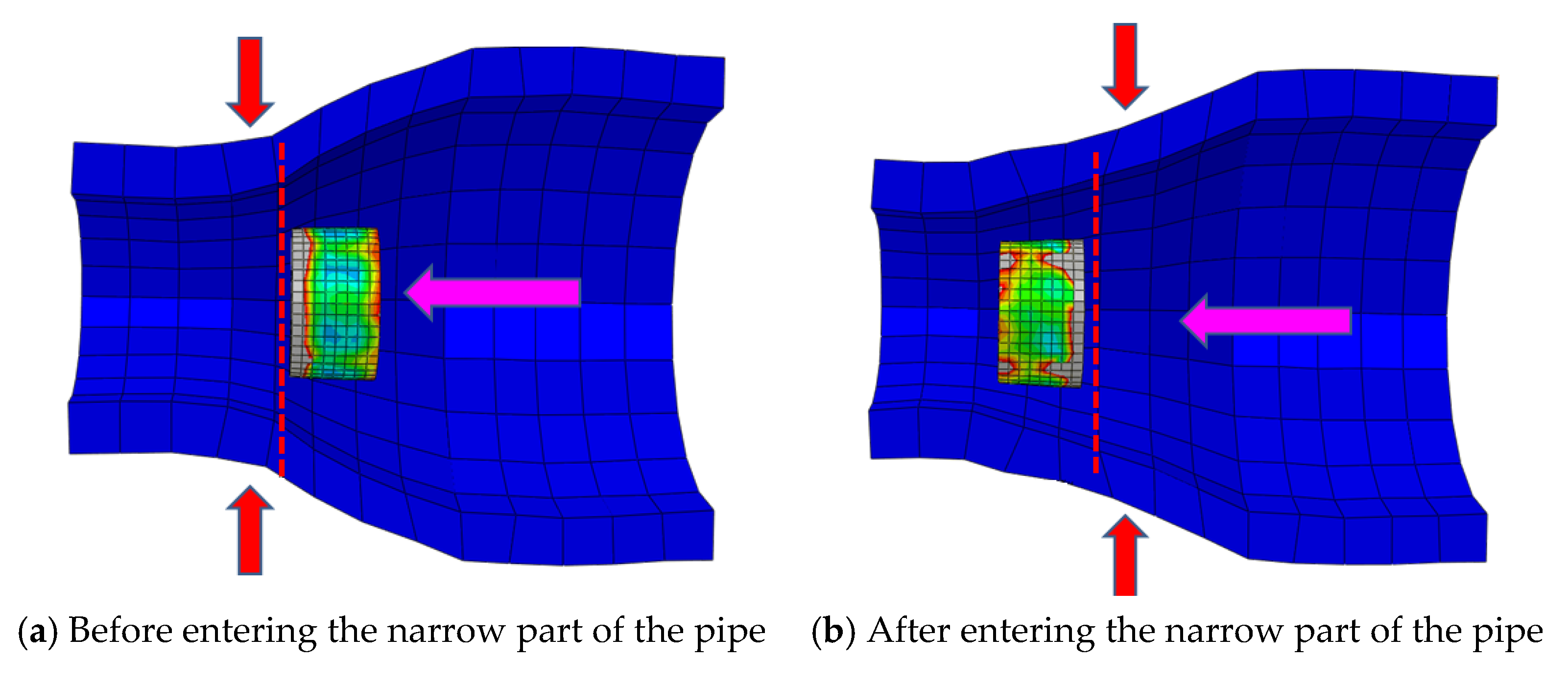
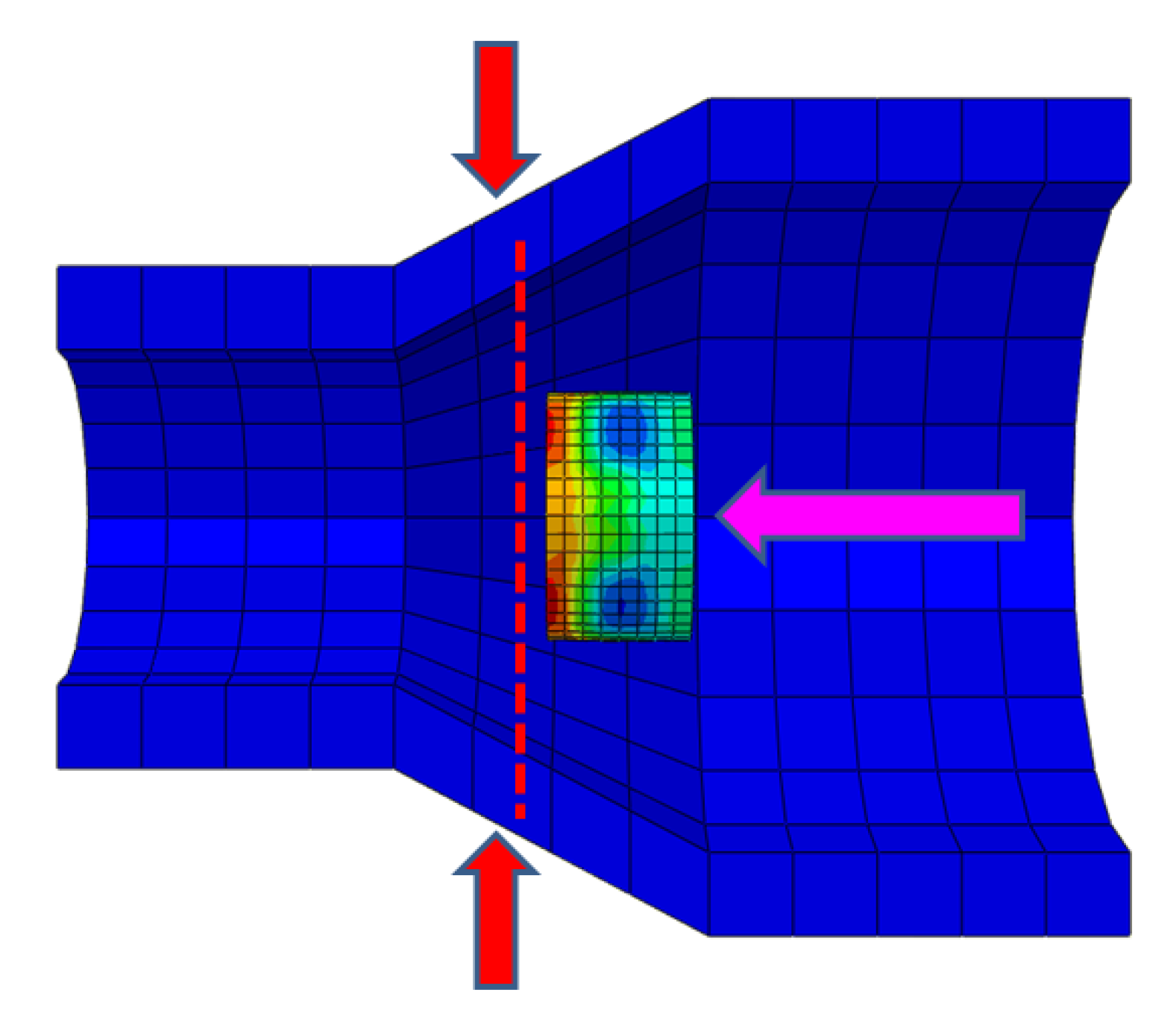
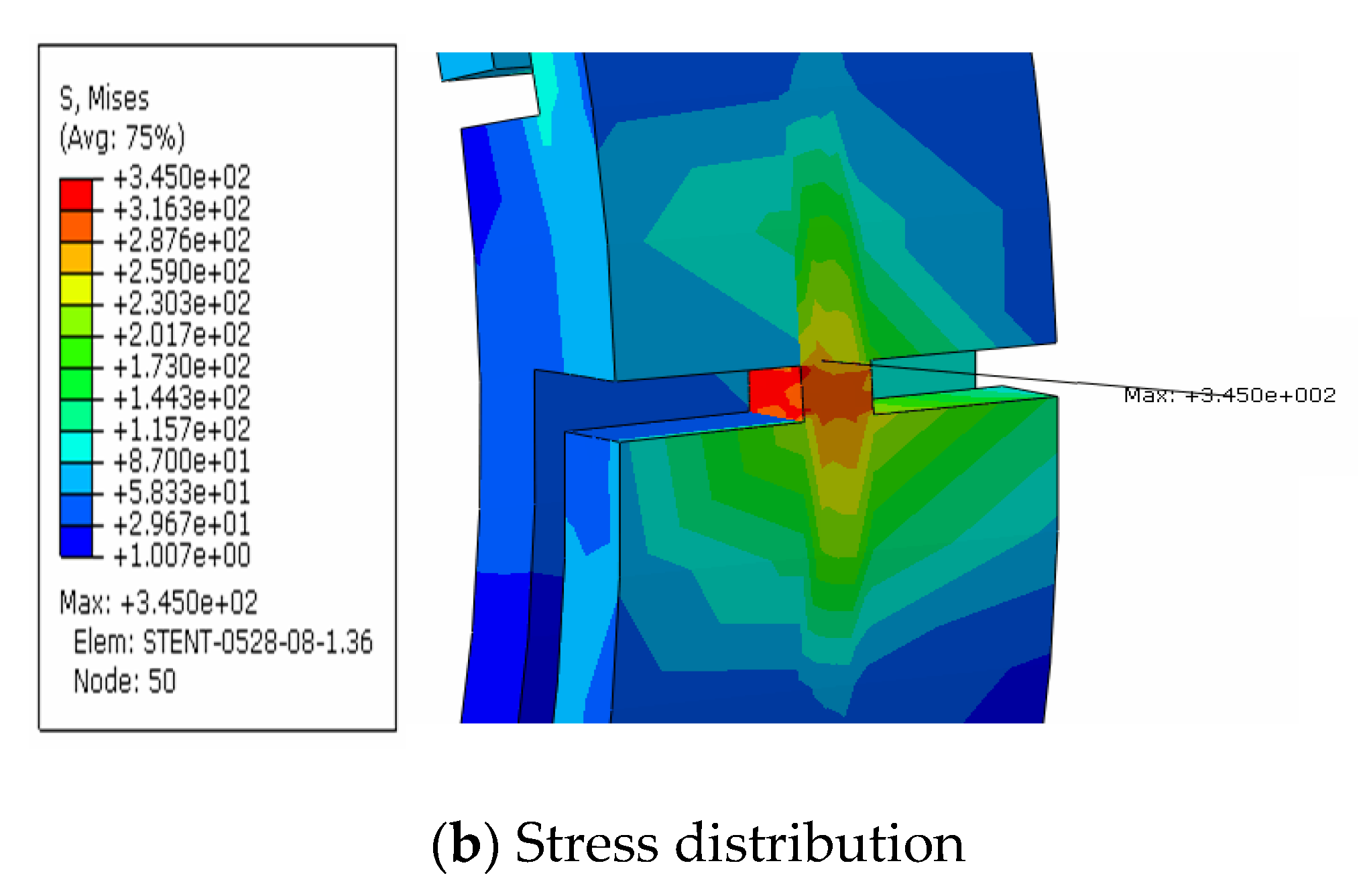
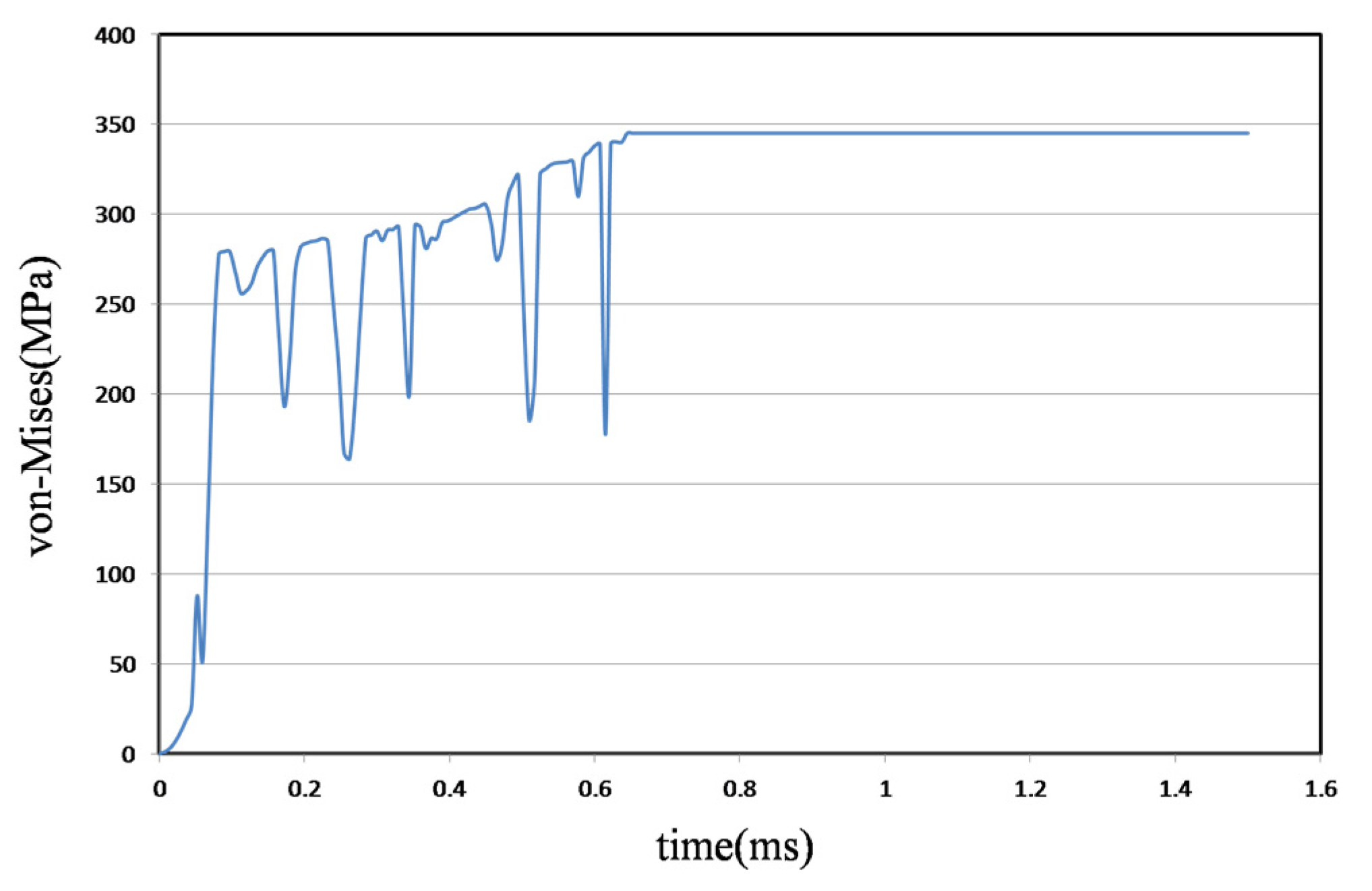
4.1. Metal Stent Stress Analysis
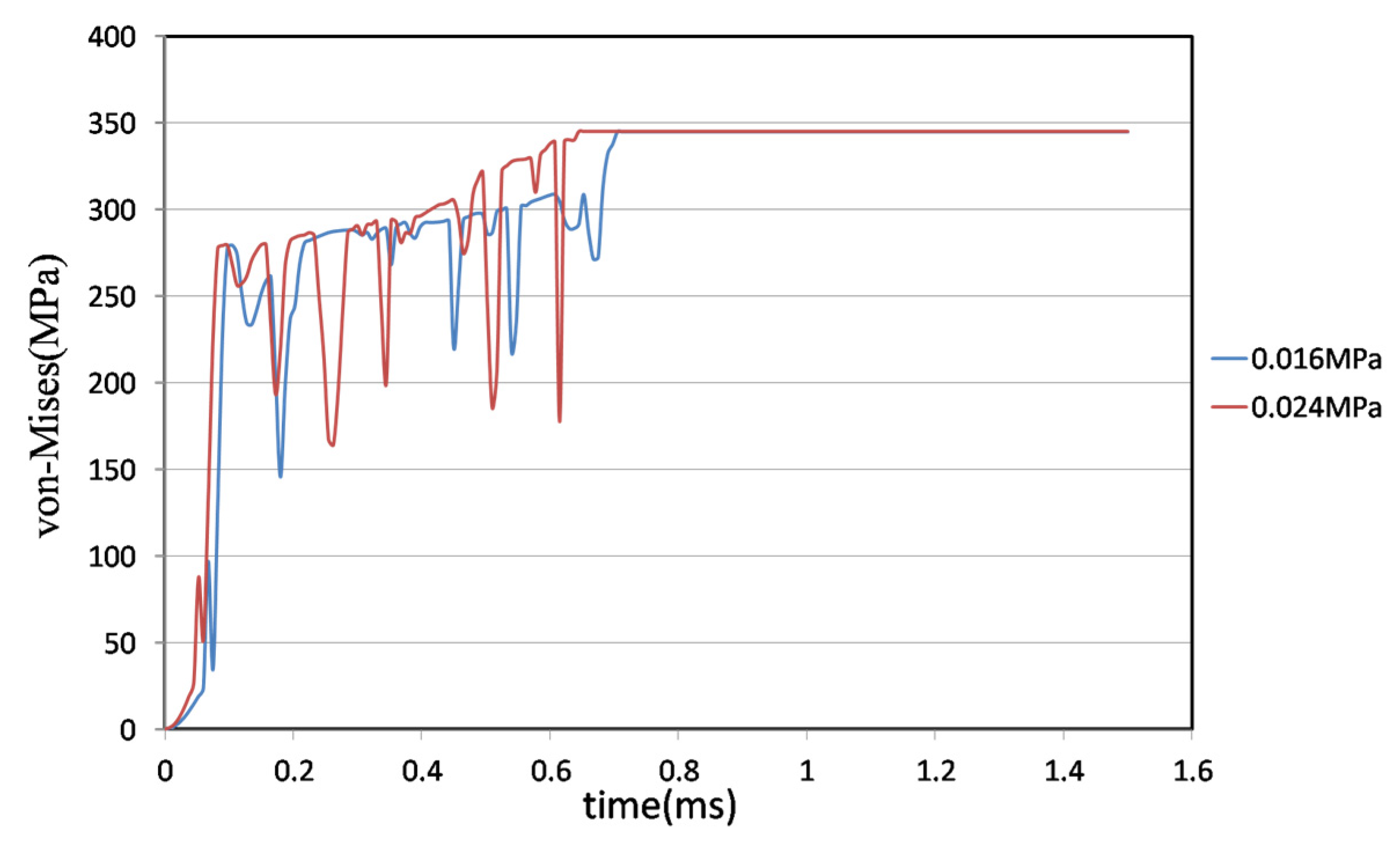
4.2. Comparison of Different Liquid Pressures
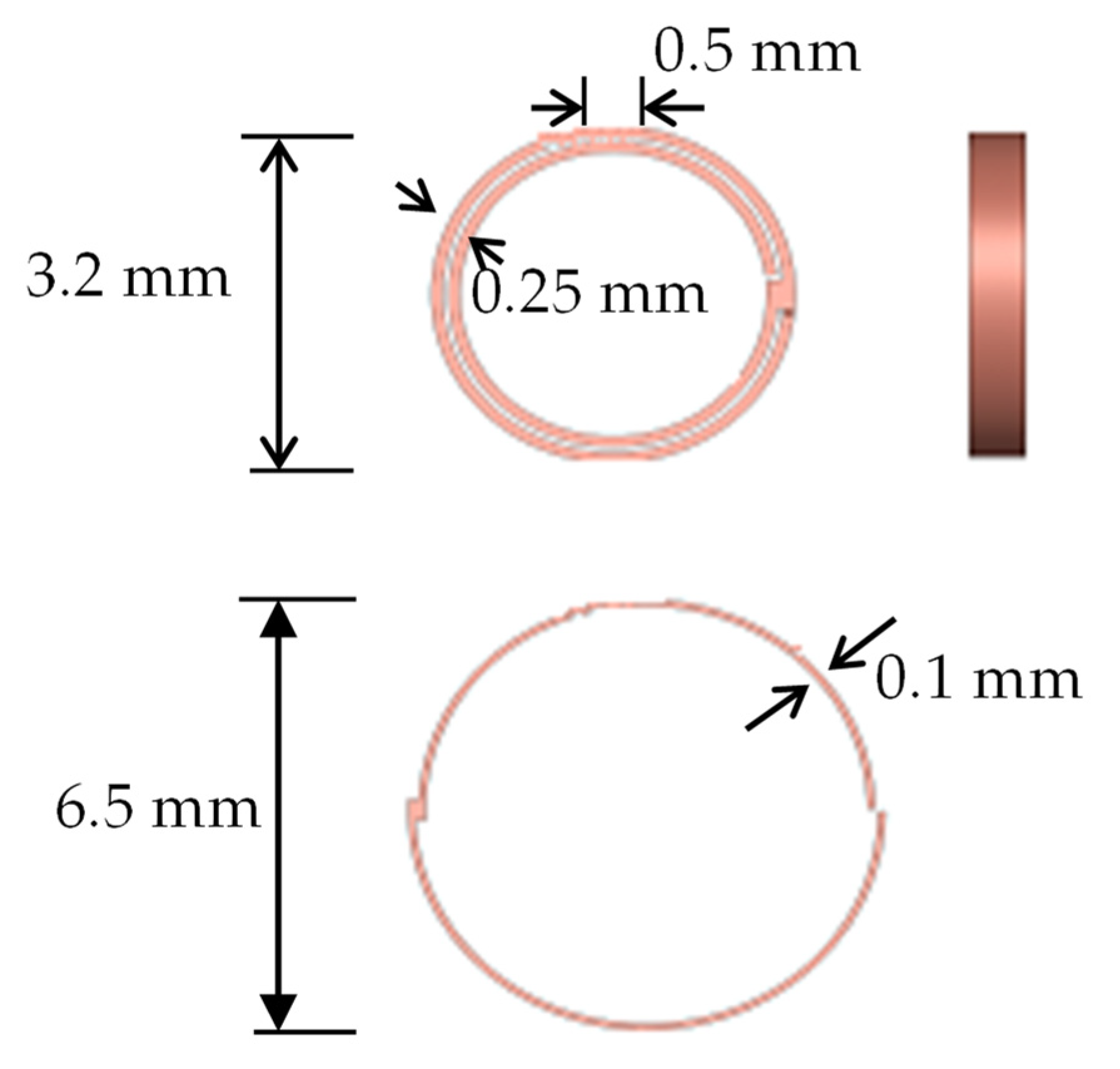
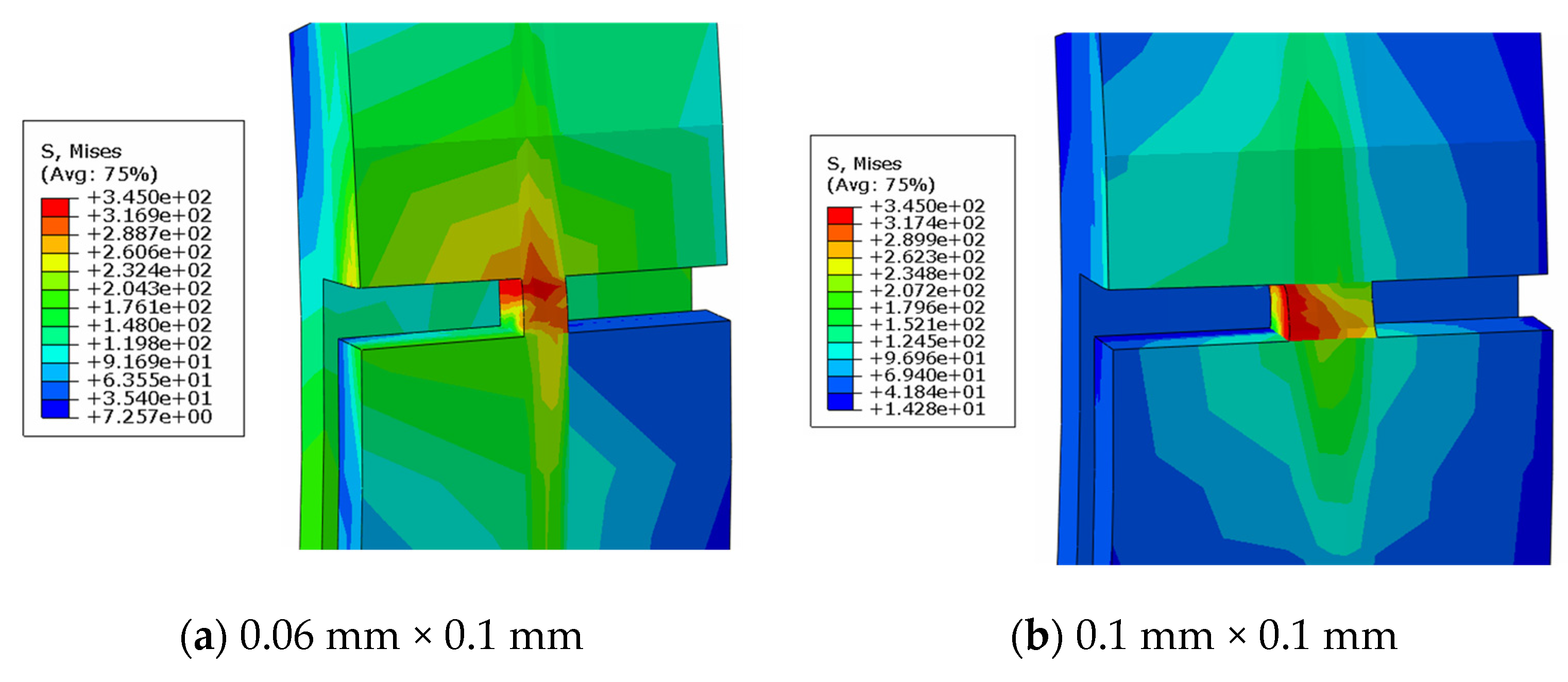
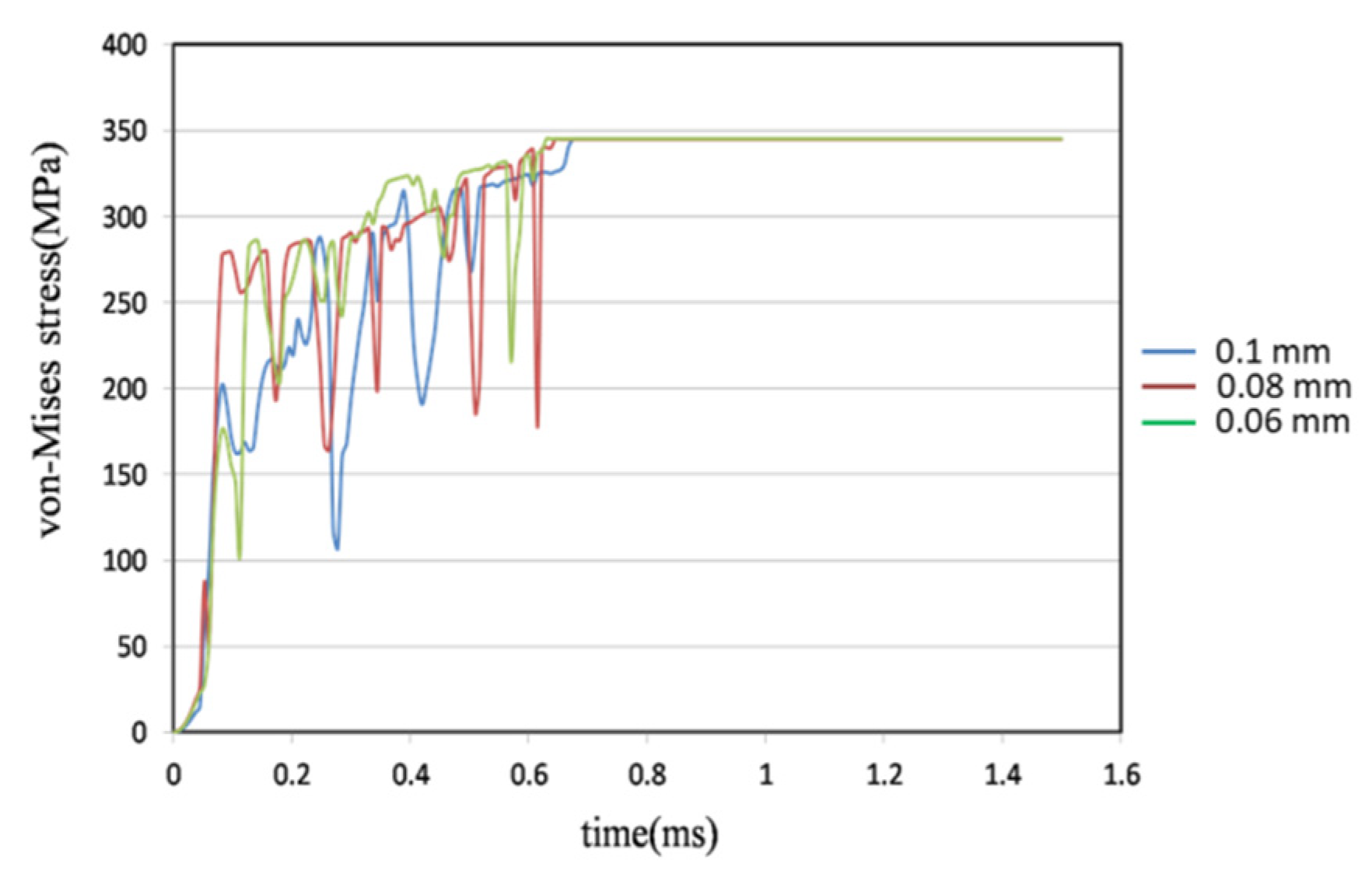
4.3. Comparison of Different Metal Joint Widths
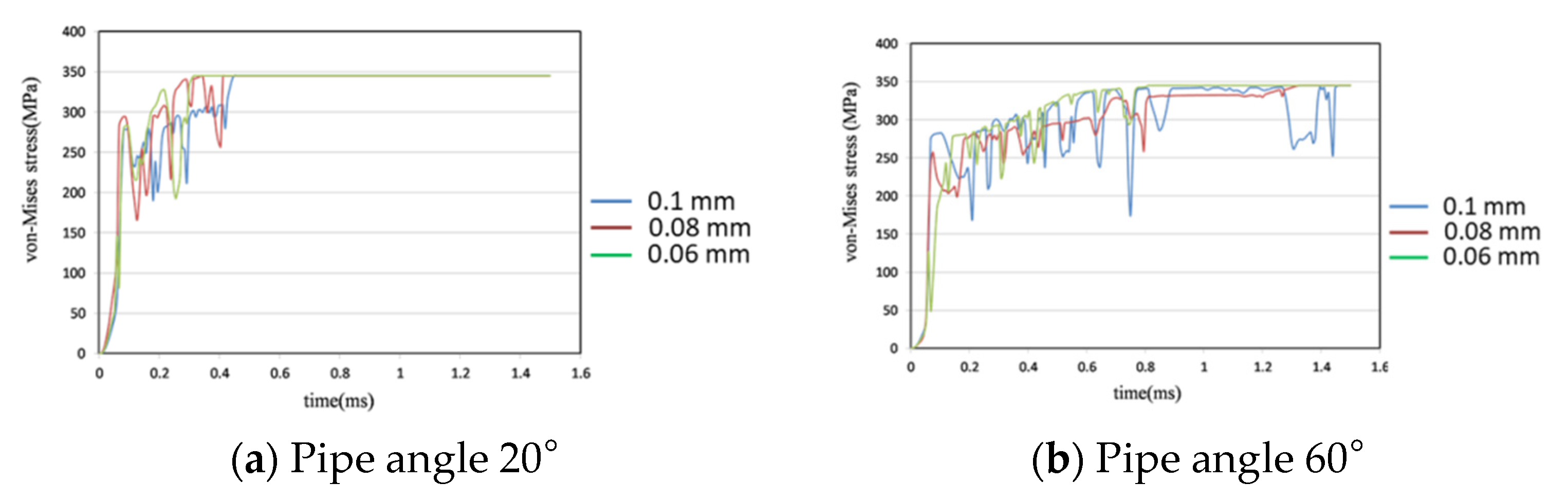
4.4. Comparison of Different Pipe Angles
5. Conclusions and Suggestions
- Two different pressures were investigated—0.016 MPa and 0.024 MPa—using the same pipe model and a metal stent with a 0.08 mm × 0.1 mm joint. Under the lower pressure, the joint broke 4% later than at the higher pressure.
- Three different joint widths—0.06 mm, 0.08 mm, and 0.1 mm—were simulated and compared at a pipe angle of 40°. The 0.06 mm joint broke 1% sooner than the 0.08 mm joint and the 0.1 mm model broke 2.53% later than the 0.08 mm model. The breakage time of the metal stent joint is dependent on width.
- Three different pipe angles—20°, 40°, and 60°—with three different joint widths—0.06 mm, 0.08 mm, and 0.1 mm—were compared. At 20°, the 0.06 mm joint broke 6% earlier than the 0.08 mm joint and the 0.1 mm joint broke 3.46% later than the 0.08 mm joint. At 60°, the 0.06 mm joint broke 34.53% faster than the 0.08 mm joint and the 0.1 mm broke 9% more slowly than the 0.08 mm joint. The metal stent joints break more slowly with a shallower pipe angle.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nuttall, J.B. Emergency Escape from Aircraft and Spacecraft. In Aerospace Medicine; Randel, H.W., Ed.; Williams and Wilkins Co.: Baltimore, MD, USA, 1971. [Google Scholar]
- Pollard, F.B.; Klotz, G.D. Method for Improving Helicopter Crew and Passenger Survivability. In Proceedings of the Ninth Annual SAFE Symposium, Las Vegas, NV, USA, 27 November–1 October 1971. [Google Scholar]
- Cragg, A.; Lund, G.; Rysavy, J.; Castaneda, F.; Castaneda, Z.W.; Amplatz, K. Non-Surgical Placement of Arterial Endoprosthesis: A New Technique Using Nitinol Wire. Radiology 1983, 147, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.C.; Wallace, S.; Charnsangavej, C.; Carrasco, C.H.; Gianturco, C. Percutaneous Endovascular Stents: An Experimental Evaluation. Radiology 1985, 156, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, H.; Puel, J.; Joffre, F. Self-Expanding Endovascular Prosthesis: An Experimental Study. Radiology 1987, 164, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.N.D.; Mac Donald, B.J.; Hashmi, M.S.J. Finite element simulation of slotted tube (stent) with the presence of plaque and artery by balloon expansion. J. Mater. Process. Technol. 2004, 155–156, 1772–1779. [Google Scholar] [CrossRef]
- Xia, Z.H.; Ju, F.; Sasaki, K. A general finite element analysis method for balloon expandable stents based on repeated unit cell (RUC) model. Finite Elem. Anal. Des. 2007, 43, 649–658. [Google Scholar] [CrossRef]
- Etave, F.; Finet, G.; Boivin, M.; Boyer, J.; Rioufol, G.; Thollet, G. Mechanical properties of coronary stents determined by using finite element analysis. J. Biomech. 2001, 34, 1065–1075. [Google Scholar] [CrossRef]
- Dumoulin, C.; Cochelin, B. Mechanical behaviour modelling of balloon expandable stents. J. Biomech. 2000, 33, 1461–1470. [Google Scholar] [CrossRef]
- Chen, Y.A. A Vascular Stent with a Compensating Mechanism. CN2787169Y, 8 September 2004. [Google Scholar]
- García, C.J.; González, H.F.; Juzgado, D.; Igea, F.; Pérez, M.M.; López, R.L.; Rodríguez, A.; González, C.P.; Yuguero, L.; Espinós, J.; et al. Use of self-expanding metal stents to treat malignant colorectal obstruction in general endoscopic practice (with videos). Gastrointest. Endosc. 2006, 64, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kang, D.H.; Kim, G.H.; Park, W.I.; Kim, H.W.; Park, J.H. Self-expanding metallic stents for gastric outlet obstruction resulting from stomach cancer: A preliminary study with a newly designed double-layered pyloric stent. Gastrointest. Endosc. 2007, 66, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Ananthasuresh, G.K. A kinematic theory for radially foldable planar linkages. Int. J. Solids Struct. 2007, 44, 6279–6298. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.; Lewis, H.; Hogan, B.; Burroughs, A.; Patch, D.; O’Beirne, J. A self-expanding metal stent for complicated variceal hemorrhage: Experience at a single center. Am. Soc. Gastrointest. Endosc. 2010, 71, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.Z. Plastic Stenting Analysis of Vascular Stents. Master’s Thesis, Department of Mechanical and Mechanical Engineering, National Sun Yat-Sen University, Kaohsiung, Taiwan, 2004. [Google Scholar]
- Ni, Z.H.; Gu, X.Z.; Wang, Y.X. Research on Rapid Prediction Method of Nonlinear Expansion Process of Medical Vascular Stent; School of Mechanical Engineering, Southeast University: Nanjing, China, 2008. [Google Scholar]
- Li, N.; Zhang, H.W.; Ouyang, H.J. Shape optimization of coronary artery stent based on a parametric model. Finite Elem. Anal. Des. 2009, 45, 468–475. [Google Scholar] [CrossRef]
- Malvè, M.; Pérez del Palomar, A.; Mena, A.; Trabelsi, O.; López-Villalobos, J.L.; Panadero, A.; Ginel, F.; Doblaré, M. Numerical modeling of a human stented trachea under different stent designs. Int. Commun. Heat Mass Transf. 2011, 38, 855–862. [Google Scholar] [CrossRef]
- Yu, H.; Liu, X.; Zhao, X.; Kusaba, Y. FEM analysis for V-H rolling process by updating geometric method. J. Mater. Process. Technol. 2006, 180, 323–327. [Google Scholar] [CrossRef]
- Kutryk, M.; Serruys, P.W. Coronary Stenting Current Perspectives; Martin Dunitz Ltd.: London, UK, 1999; ISBN 1-85317-693-1. [Google Scholar]
- Pelton, A.R.; Schroeder, V.; Mitchell, M.R.; Gong, X.Y.; Barney, M.; Robertson, S.W. Fatigue and durability of Nitinol stents. J. Mech. Behav. Biomed. Mater. 2008, 1, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mustafa, I.A.; Gerasimos, S.F.; Ekundayo, O.J.; Inmaculada, B.A.; Thomas, E.L.; Navin, C.N.; George, L.B.; Gregg, C.F.; Wilbert, S.A.; et al. Uncontrolled hypertension and increased risk for incident heart failure in older adults with hypertension: Findings from a propensity-matched prospective population study. J. Am. Soc. Hypertens. 2010, 4, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Les, P.P. An introduction to coiled springs (mainsprings) as a power source. Int. J. Fatigue 2011, 33, 1017–1024. [Google Scholar] [CrossRef]



| Aluminum Alloy 6061-T6 | |
|---|---|
| Elasticity coefficient | 68,900 (MPa) |
| Poisson’s ratio | 0.33 |
| Material density | 2700 (kg/m3) |
| Yield Stress (MPa) | Plastic Strain |
|---|---|
| 277 | 0 |
| 295 | 0.012 |
| 315 | 0.03 |
| 335 | 0.059 |
| 345 | 0.088 |
| PVC | |
|---|---|
| Elasticity coefficient | 3400 MPa |
| Poisson’s ratio | 0.33 |
| Material density | 1380 (kg/m3) |
| Liquid | |
|---|---|
| Mass density | 996 (kg/m3) |
| Viscosity coefficient | 1 × 10−6 (kg·m−1·s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.-Y.; Huang, H.-H.; Lu, C.-K. Design of a Self-Expanding Stent Mechanism Enacted by Fluid Pressure Difference. Appl. Sci. 2021, 11, 10114. https://doi.org/10.3390/app112110114
Chang M-Y, Huang H-H, Lu C-K. Design of a Self-Expanding Stent Mechanism Enacted by Fluid Pressure Difference. Applied Sciences. 2021; 11(21):10114. https://doi.org/10.3390/app112110114
Chicago/Turabian StyleChang, Ming-Yen, Hsing-Hui Huang, and Chia-Kai Lu. 2021. "Design of a Self-Expanding Stent Mechanism Enacted by Fluid Pressure Difference" Applied Sciences 11, no. 21: 10114. https://doi.org/10.3390/app112110114
APA StyleChang, M.-Y., Huang, H.-H., & Lu, C.-K. (2021). Design of a Self-Expanding Stent Mechanism Enacted by Fluid Pressure Difference. Applied Sciences, 11(21), 10114. https://doi.org/10.3390/app112110114






