Featured Application
Tire, conveyor belt.
Abstract
The objective of the study was to investigate the effect of the partial replacement of carbon black (CB) by nanodiamonds (NDs) on the vulcanization, mechanical and dynamic properties of a natural rubber—butadiene rubber compound, a typical elastomer compound found in several applications (the tire and mining industry, for example). A studied hybrid filler system resulted in a 28% increase in tensile strength and 29% increase in 300% modulus at low ND loadings even though the total weight fraction of the filler system was kept constant at 25 parts per hundred rubber. The hybrid filler system improved dispersion of both fillers as was proven by scanning electron microscopy and the Payne effect study. In addition, the replacement of 2.5 and 5 phr CB by NDs resulted in 62% improvement in wear resistance. The DMA study showed that a certain ND-CB filler combination has a positive effect on tire properties such as wet grip and rolling resistance.
1. Introduction
A rubber compound consists of several components. One of the most important additives are fillers that are added for several purposes, such as to enhance the mechanical properties of rubber, reduce costs or improve a specific property, e.g., electrical conductivity. Carbon black (CB) has been the main reinforcing filler in rubbers over a century. A high reinforcing ability of CB is related to small particles and aggregate size and high structure (shape and level of branching of aggregates) of the filler resulting in a large surface area available to interact with polymer chains and create chemical and physical bonding with rubber [1]. However, CB has a negative influence on dynamic properties compared to silica, the second traditional reinforcing filler, which is currently used, for example, in tire treads. This, together with a high carbon footprint of the CB manufacturing process, increases the requirement for new filler solutions [2].
Nanosized fillers, such as carbon nanotubes (CNTs) and layered silicates, have been widely studied during the last decades [3,4,5,6,7,8,9,10,11]. They are promising fillers due to their small particle size and high surface area, and thus they have, in theory, a high reinforcing potential already at low filler concentrations. The practice has shown that the mixing of these nanofillers into rubber is challenging due to the high surface energy and their tendency to form large agglomerates. Several publications have claimed that solution mixing is required for a good dispersion of CNTs [3,12,13], but it is not yet an industrially viable method. Instead, the CB-nanofiller hybrid filler systems have been found to lead to improved filler dispersion and improved mechanical properties [14,15,16]. For example, by combining up to 5 parts per hundred rubber (phr) of CNTs with CB, a good dispersion of CNTs is achieved in a conventional rubber mixing process [14,16]. In addition, the hybrid filler system improves the CB dispersion and results in improved mechanical properties [17,18] leaving, however, the dynamic properties still compromised [14].
Furthermore, natural rubber latex containing a filler combination comprising of CNTs, graphite oxide and CB was found to have better fatigue, crack growth resistance and mechanical properties than the single CB filler system [19]. This was also related to the improved dispersion and synergistic effect. Similar conclusions were made in a study where a graphene nanoplatelet and CB hybrid filler system was studied in ethylene propylene diene rubber [20]. The partial replacement of CB by graphene nanoplatelets was mentioned to reduce the CB aggregation. Moreover, a surface modified expanded graphite was reported to improve the mechanical properties of the CB-filled NR composite [21] as well as an intercalated graphene—CB hybrid filler system in styrene-butadiene rubber (SBR) [22].
A less studied nanofiller type, nanodiamonds (NDs), are considered as a non-reinforcing filler but together with CB it could result in good dynamic properties, as they have been found to decrease the losses of rubbers [23], including low mechanical hysteresis [24]. Such an effect may be related to the reduced internal friction due to the dry lubricating behavior of NDs facilitating the orientation of macromolecules upon the applied stress [23,25]. Single detonation-produced NDs are 4–6 nm spherical particles consisting of sp3-carbon core, thin sp2-carbon transition layer and an active surface mostly containing carboxyls, hydroxyls, lactones, ketones and ethers [26,27]. NDs are known to exhibit synergistic effect with other carbon-based fillers in SBR [28] and epoxy, and the addition of NDs to some rubbers has been shown to improve e.g., elongation at break [29,30]. The CNT-ND hybrid filler system improved the dispersion of individual filler particles but had a negative influence on mechanical properties compared to CNT filled SBR [28].
In this study, CB is partially replaced with a small amount of NDs in a natural rubber (NR)—butadiene rubber (BR) compound. Up to 5 phr of NDs is used as the earlier studies conclude percolation threshold below 4 phr [29]. The main aim of the study is to increase polymer-filler interaction and dispersion of fillers and thus improve the mechanical and dynamic properties of rubber. Carboxylated NDs contain a high amount of surface carboxyl groups, as well as some lactones, phenols and anhydrides, groups that match to functional groups of CB [31]. The presence of these polar groups is expected to have limited interaction with non-polar elastomers but to contribute to the possible dry lubricating action of NDs. The effect of ND-CB combinations on the filler dispersion, vulcanization, mechanical and dynamic properties of NR-BR compound is investigated.
2. Materials and Methods
The studied NDs were carboxylated NDs (uDiamond® Vox P) from Carbodeon Oy, Vantaa, Finland. According to the manufacturer, the diameter of the NDs varied between 2 and 6 nm. The other materials used in the compound and formulation are presented in Table 1.

Table 1.
The formulation and mixing sequence of the studied rubber compounds.
Elastomers and other ingredients were mixed in a Brabender 350E mixer (Brabender GmbH, Duisburg, Germany) for six minutes. The mixing sequence is presented in Table 1. The starting temperature was 50 °C and the rotor speed was 60 rpm. The reference compound (ND0) contained only CB as filler in a concentration of 25 phr. In other compounds, 0.5, 1, 2.5 or 5 phr of CB was replaced by the same weight amount of NDs. The name of the compounds is related to the ND loading.
Curing characteristics were measured with an Advanced Polymer Analyzer, APA 2000 from Alpha Technologies (Hudson, OH, USA). The measurements were carried out at 150 °C for 25 min. The compounds were then vulcanized to their t’90 value, which means the time required to reach 90% of the maximum rheometric torque.
The apparent crosslink densities were studied by a swelling test. Approximately 0.4 g vulcanized rubber pieces were soaked in toluene for 72 h. The apparent crosslink density was determined from the swelling coefficient by 1/Q:
where w0 is the initial weight of the sample and wt is the weight at time t, and are densities of the solvent and unswollen vulcanizate, respectively [32]. Three measurements per compound were done and the averages and standard deviations were calculated.
Bound rubber (BDR) measurements were performed by dissolving 0.2 g of uncured rubber in toluene for 96 h. Toluene was exchanged every 24 h. After immersion, the samples were dried and weighed. The BDR content was calculated by the formula:
where is the rubber content in the sample, is the combined weight of the bag and the sample, is the weight of the dried bag and the sample, is the weight of the dry sample, and cpd is the total amount of rubber and filler in the compound in phr [33]. Three measurements per compound were done and the averages and standard deviations were calculated.
The Payne effect was studied with a dynamic mechanical analyzer (DMA, DMA/SDTA861e from Mettler Toledo, Columbus, OH, USA). The measurements were conducted for a circular sample with a diameter of 6 mm in strain sweep from 0.5 to 1200 µm with a frequency of 1 Hz at room temperature.
The state of dispersion of the CB and ND particles in the compounds was investigated by scanning electron microscopy (Zeiss ULTRAplus, Oberkochen, Germany). The fracture surfaces of the tensile test specimens were coated with a thin carbon layer to ensure the conductivity of the samples.
Tensile tests of the samples were carried out by testing 5 specimens/rubber compound with Instron 5967 universal tester (Instron, Darmstadt, Germany) according to ISO 37 sample type 3 [34]. Tear resistance was tested with the same equipment according to ISO 34 with trouser type specimens (3 specimen/rubber compound) [35]. The crosshead speeds for the tensile and tear tests were 200 mm/min and 100 mm/min, respectively.
Wear resistance was determined with CETR UMT-2 pin-on disk (Bruker Co, Billerica, MA, USA). Rubber samples with a diameter of 10 mm were rotated on a smooth AISI304 steel plate for 60 min with a force of 40 N. The rotation speed was 60 rpm. The change in mass was measured. The mass loss was converted to volume loss using the density of the compounds for more comparable results. The density of the compound was determined with a Wallace X21B electronic densimeter (H.W. Wallace & Co Ltd., Dorking, Surrey, England) by weighing the samples in air and in water.
Shore A hardness was determined with a Bareis hardness tester (Bareis Prüfgerätebau, Oberdischingen, Germany) according to ASTM D 2240 [36]. The Shore A value was recorded instantaneously after dropping durometer. Five measurements were performed for each sample.
Dynamic properties were studied with a Pyris Diamond DMA from PerkinElmer Instruments (Waltham, MA, USA), operating in tension mode. The measurements were done from −80 to +80 °C with a heating rate of 3 K/min, an amplitude of 40 μm, and a frequency of 1 Hz.
The standard deviation in the results were determined by:
where xi is the measured value, is the average and n is the number of measurements.
3. Results and Discussion
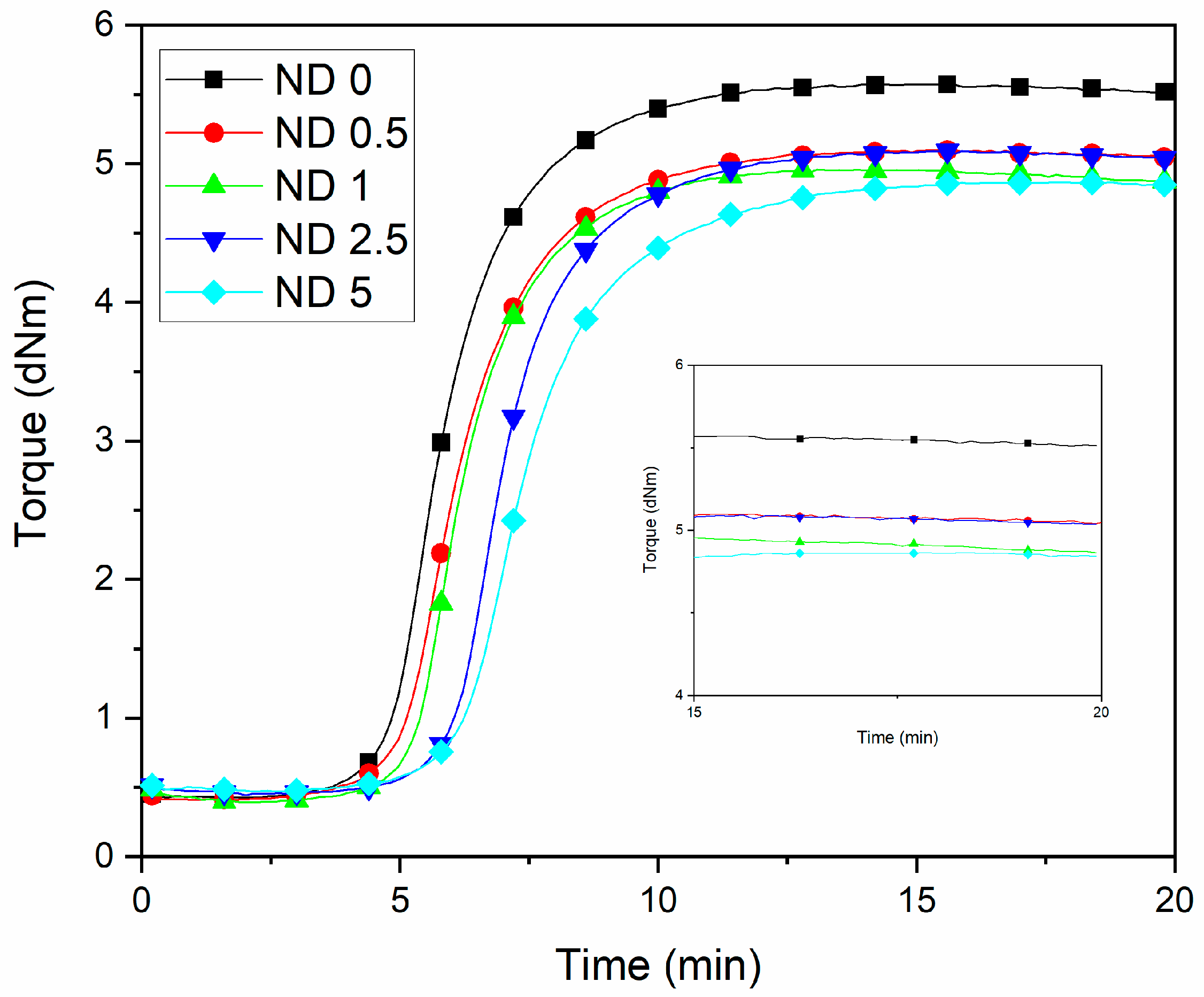
The replacement of small amounts of CB by NDs can be expected to shorten the vulcanization time due to the high thermal conductivity of NDs [37,38] and thus due to the more efficient thermal energy transfer and faster temperature increase in rubber. This kind of behavior has been observed for CNTs [39] and for the CNT-CB filler combinations [14] as well as in the earlier ND studies [40]. However, the effect of NDs was opposite in the current case. Figure 1 and Table S1 (Supporting Information) shows that both the scorch time and the vulcanization time increases when the concentration of NDs increases. The carboxyl functional groups are known to adsorb the accelerator [41]. Further, NDs decrease the maximum torque values (Table S1). Although NDs have similar functional groups on the surface than CB, the surface of NDs is more polar. This has a negative influence on the polymer-filler interaction and affects the maximum torque together with the adsorption of curatives. This indicates a lower reinforcing ability of NDs.
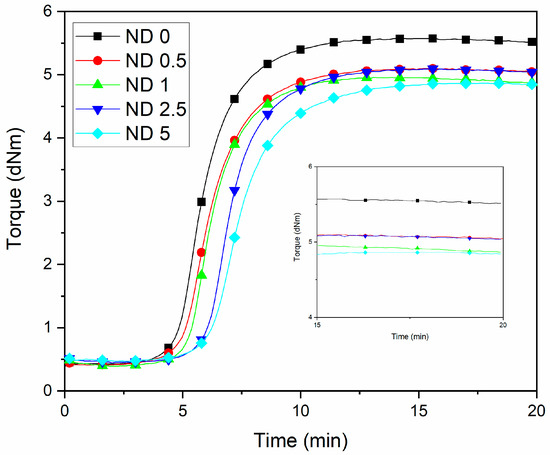
Figure 1.
Rheometric curves of NR/BR compounds at different ND/CB concentrations.
Although the rheometric torque indicated lower networking for the compounds containing NDs, the apparent crosslink density determined by the swelling study increases marginally when a part of CB was replaced by NDs, as seen in Table 2. Although swelling study is commonly accepted as a qualitative method for crosslink density, the filler volume and filler dispersion influence the swelling study results [42] as the swelling behavior of bound rubber and occluded rubber differ from the unfilled rubber. Replacing CB by the same weight amount of NDs decreases the total volume fraction of fillers as NDs have higher particle density than CB [43,44], but probably increases the total interfacial area through the improved CB dispersion, which leads to the increased apparent crosslink density determined by the swelling study.

Table 2.
Apparent crosslink density, bound rubber content and the Payne effect of the rubber compounds.
The polymer-filler interaction was studied by the bound rubber content. The bound rubber describes the polymer fraction adhered to filler by strong covalent bonds or by weak physical bonds. The bound rubber decreases the mobility of the polymer chains and makes the adhered polymer insoluble to solvents and thus reinforces the polymer. The higher the bound rubber content, the better the polymer-filler interaction. Table 2 shows that the bound rubber content decreases slightly at 0.5 and 1 phr ND loadings but increases back to the same level with the compound containing only CB at higher ND loadings. However, on the basis of the standard deviation, the direct conclusions are impossible to make. In this study, the physical and chemical bonds were not separated. Thus, the bound rubber results contain also the physically bonded or mechanically trapped rubber, i.e., occluded rubber. Due to the polar functional groups on the surface of NDs, covalent bonds with non-polar NR and BR are not likely to occur [24,45], and the rubber chains could slide along the surface of NDs showing some plasticizing effect. Thus, a decrease in bound rubber content is natural, especially if the ND particles are well dispersed and distributed. CB exists in aggregates and polymer chains can be trapped into the voids of these aggregates. In well dispersed NDs, these voids do not exist, and the amount of occluded rubber is reduced.
The state of dispersion was analyzed by the Payne Effect and SEM. The Payne Effect is determined by the difference between the complex shear modulus at low strain and at high strain. At low strain, filler-filler interaction is strong, and fillers stay in agglomerated form. When the strain is increased, the filler particles are pulled apart and the filler agglomerates are broken into smaller aggregates, and the modulus of rubber decreases. Hence, the Payne effect describes the filler-filler interaction. The higher the Payne Effect, the higher the filler-filler interaction and, in the case of nanofillers, the more likely the formation of a three-dimensional filler network instead of an aggregated filler structure [46]. Table 2 shows that the Payne effect increases after addition of NDs. The change can partly be explained by the increased total surface area of the fillers but NDs may also suppress the interfacial interaction of CB particles by locating between them. This improves the dispersion of CB and enables the formation of a filler network. At 5 phr ND loading, the Payne effect decreases again indicating worse filler dispersion.
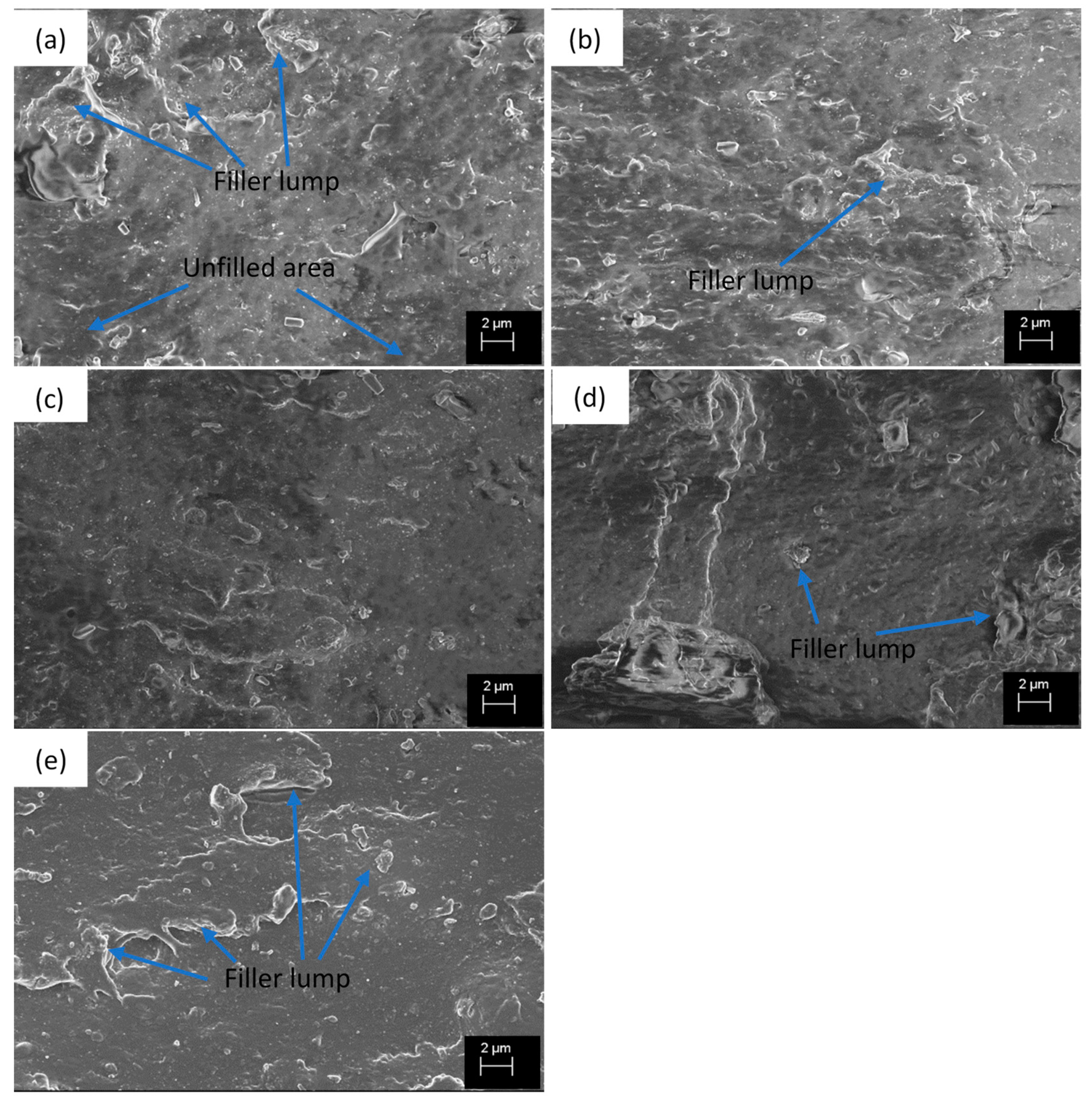
The SEM images (Figure 2) show some differences in filler dispersion and distribution in different compounds, although ND particles cannot be distinguished separately. The reference compound (Figure 2a) containing only CB as filler has well dispersed areas but also unfilled and agglomerated areas. In addition, the compound containing 0.5 phr (Figure 2b) NDs has an irregular surface caused by the large agglomerates, which is evidence of insufficient dispersion. Figure 2c,d shows that the filler dispersion is improved. The surface is rather smooth, large filler agglomerates do not exist (except for the two filler agglomerates in Figure 2d), and the fillers seem to be more evenly distributed than in the reference compound. The improved dispersion explains the earlier Payne effect results. At 5 phr ND concentration, the filler dispersion becomes poorer again and the large agglomerates are found (Figure 2e), which is the reason for the decreased filler-filler interaction observed from the Payne effect.
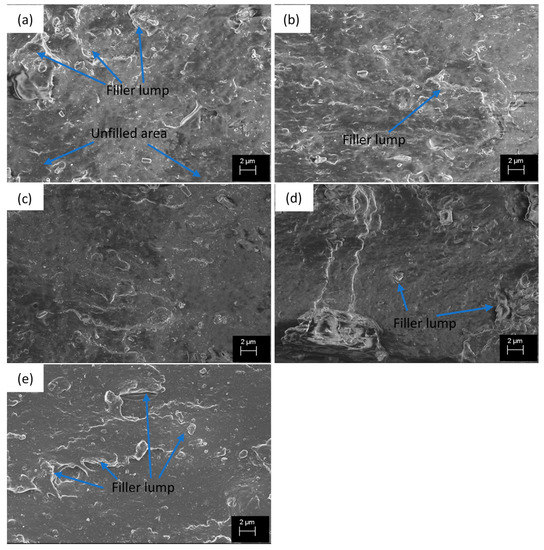
Figure 2.
SEM images from the fracture surface (tensile test) of the compounds (a) ND0, (b) ND0.5, (c) ND1, (d) ND2.5, (e) ND5.
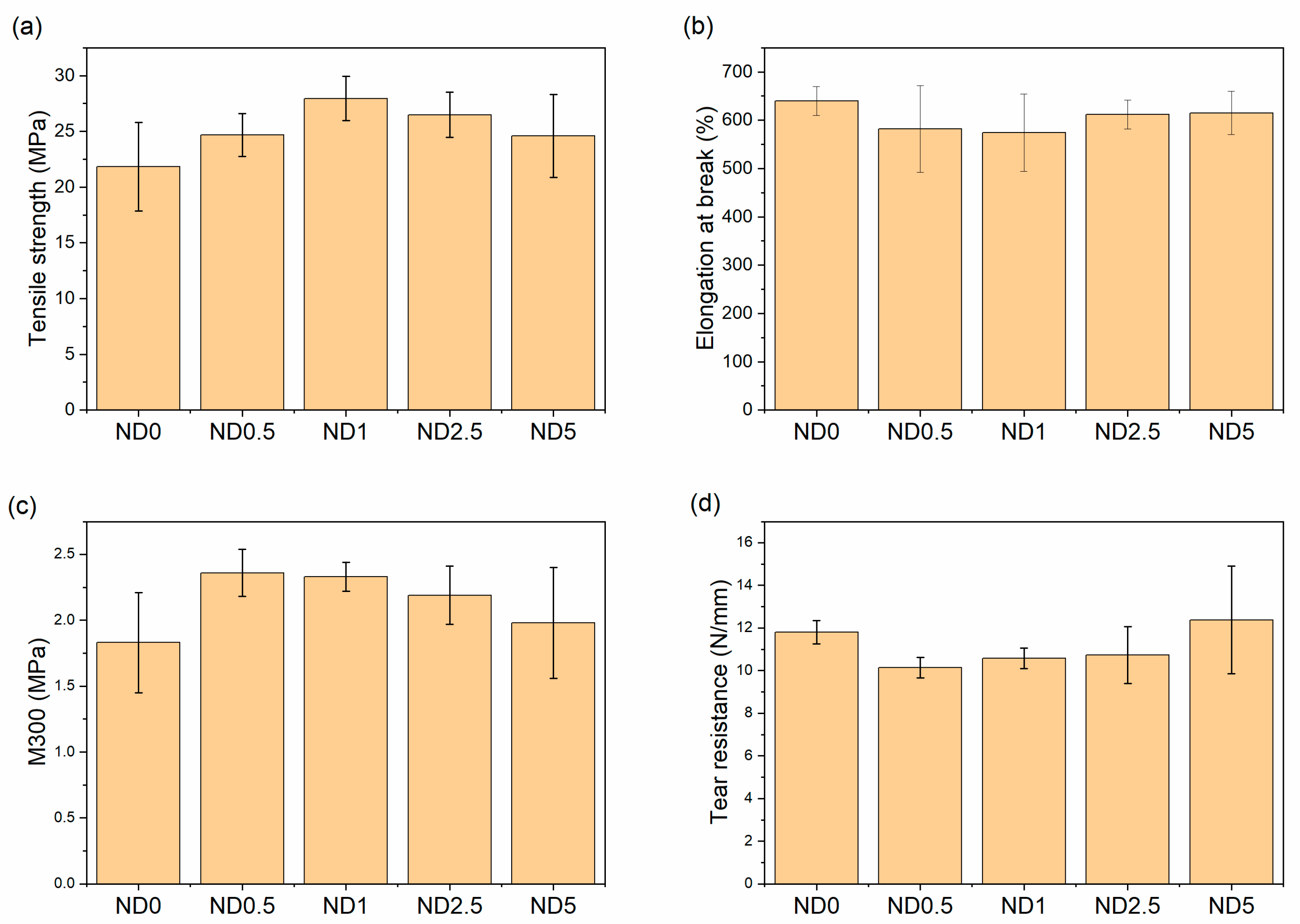
Although the clear evidence of improved polymer-filler interaction was not obtained from the bound rubber results, the tensile strength of the rubber compound is improved remarkably after addition of NDs as seen in Figure 3. This is new evidence of the improved dispersion. The benefits of the hybrid filler system are partly lost with higher ND concentrations (>2.5 phr) due to the worse filler dispersion and decreased polymer-filler interaction, but the tensile strength is still at a higher level than without NDs. Furthermore, the standard deviation is the highest for the compounds containing 0 or 5 phr NDs, which indicates inhomogeneous compounds due to the poorer filler dispersion. Hence, the tensile strength results support the SEM analysis. The tensile modulus at 300% elongation (M300) increases first but decreases when the amount of NDs is increased. Moreover, a minor decrease in elongation at break is observed after the addition of NDs for the compounds with the highest M300, but the changes are between the error bars. The decreased elongation at break is commonly attributed to the restricted mobility of the polymer chains.
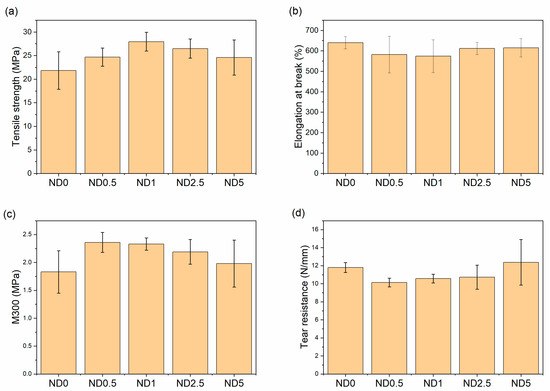
Figure 3.
The effect of NDs on the mechanical properties (a) tensile strength, (b) elongation at break, (c) 300% modulus, and (d) tear resistance.
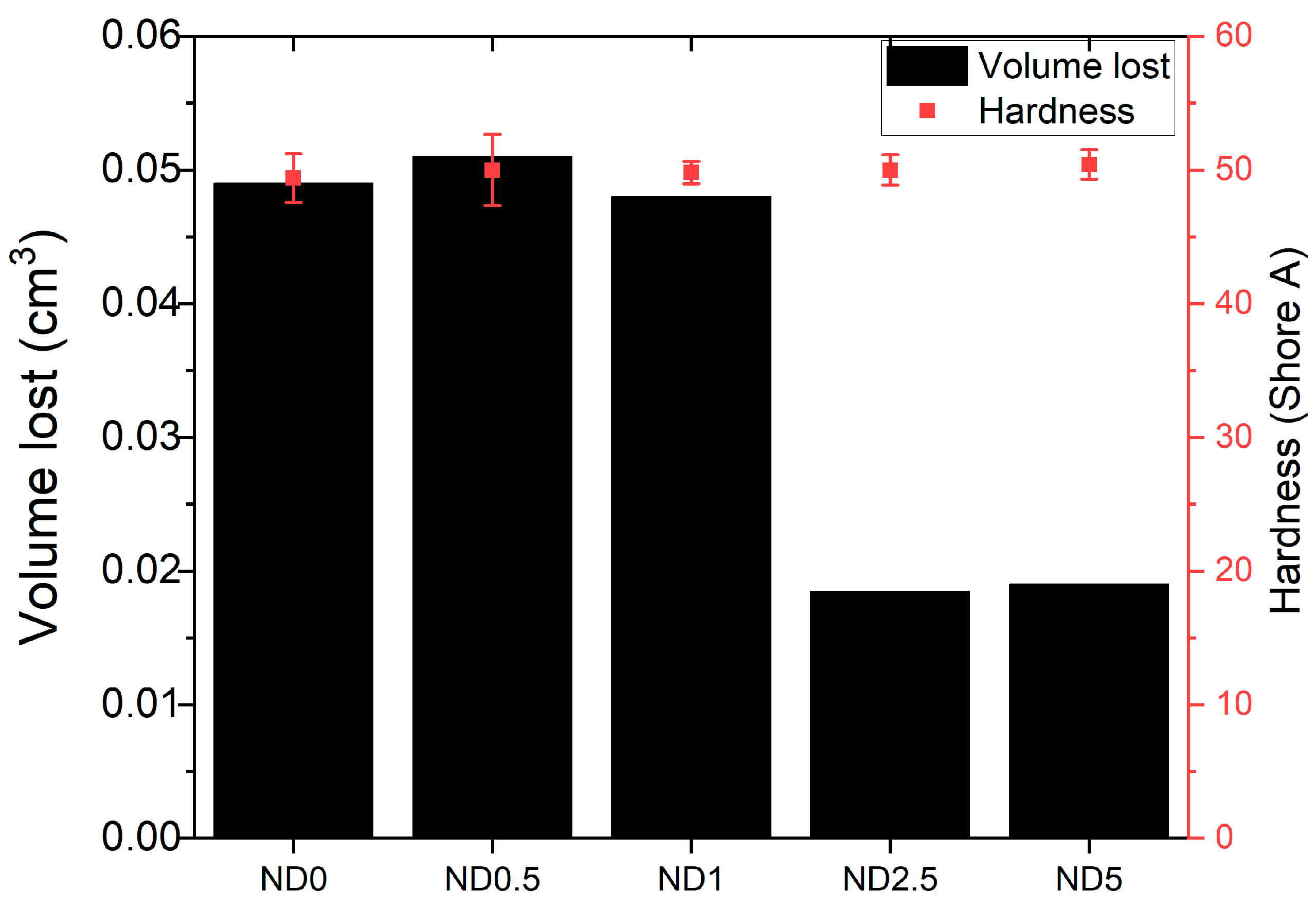
In tear resistance results the trend is opposite (Figure 3d). The tear resistance decreases when 0.5–2.5 phr CB is replaced by NDs but increases again at 5 phr NDs. This is related to the plasticizing effect of NDs and filler dispersion. The poor interface between NDs and rubber as well as the reduced mechanical trapping of polymer chains weakens the tear resistance at low ND loadings. The big standard deviation at higher ND loadings also indicates the poor dispersion of fillers. In addition, wear resistance is improved after addition of 2.5 and 5 phr NDs, although change in hardness is nonexistent (Figure 4). The harder surface of NDs helps rubber to slide on the smooth steel surface. The improvements in wear resistance as well as reduction in friction coefficient after addition of NDs has also been found for acrylonitrile butadiene rubber, fluororubber and epoxy [25,45,47]
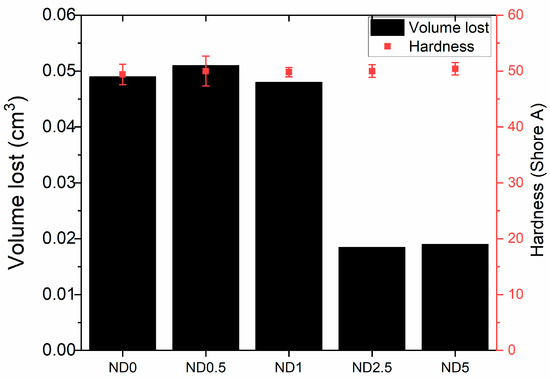
Figure 4.
Wear resistance of ND-CB filled rubber compound.
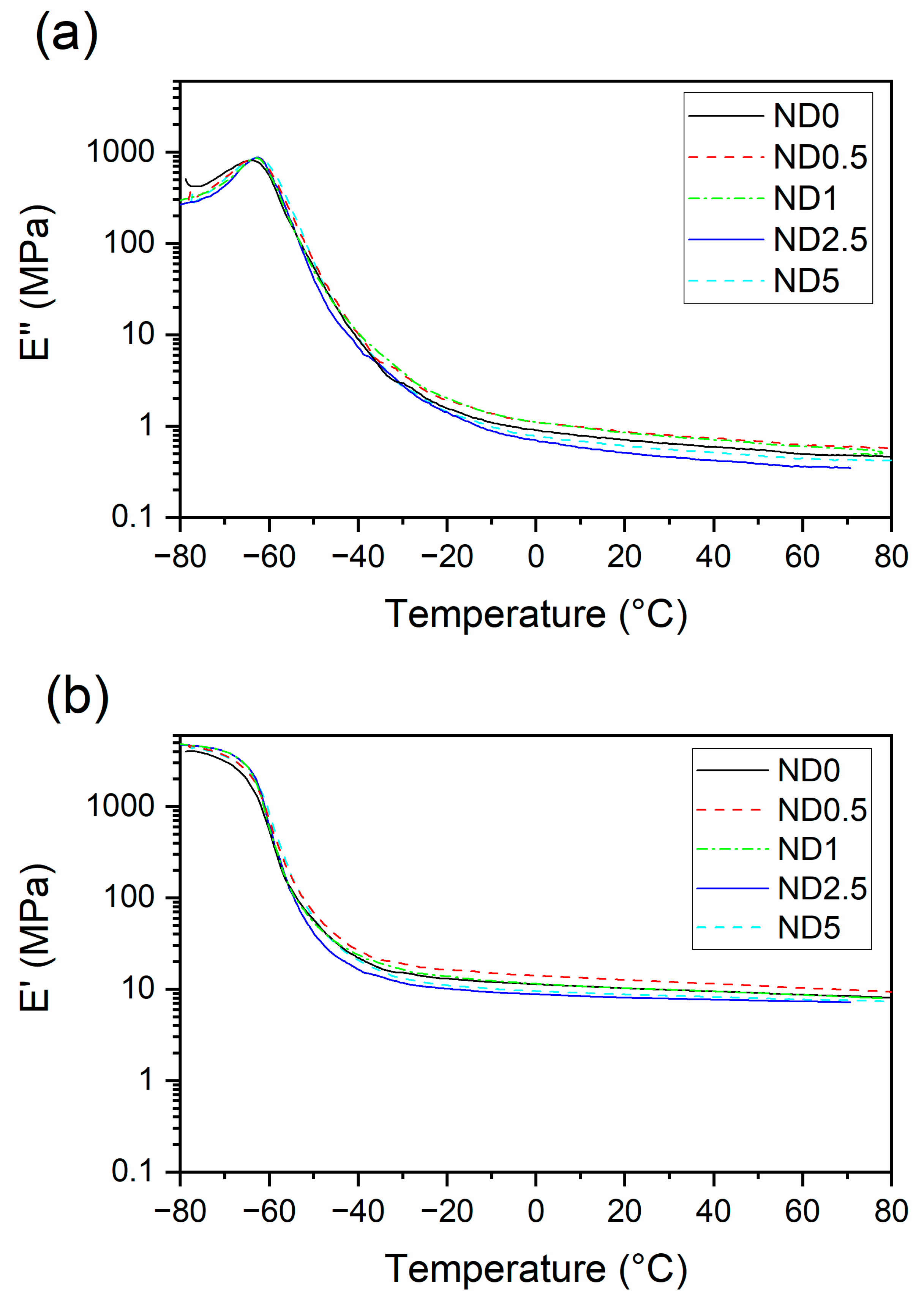
Dynamic properties play an important role in several rubber applications, such as tires or dampers. Figure 5 presents the storage and loss moduli of the compounds as a function of temperature. The storage modulus curve can be divided into three stages: the glassy region, glass transition and the rubbery region. In the rubbery plateau region, the storage modulus is the highest when 0.5 phr CB is replaced by NDs, possibly due to the improved filler dispersion. When the ND loading increases, the storage modulus decreases. At the same time, a reduction in loss modulus is observed for higher ND loadings due to the plasticizing effect [25] and decreased polymer-filler interaction.
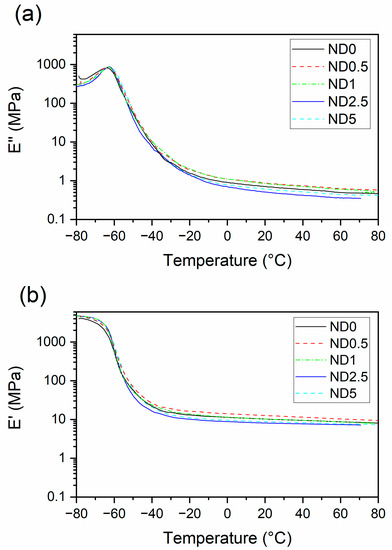
Figure 5.
The effect of partial replacement of CB by NDs on (a) storage modulus, (b) loss modulus.
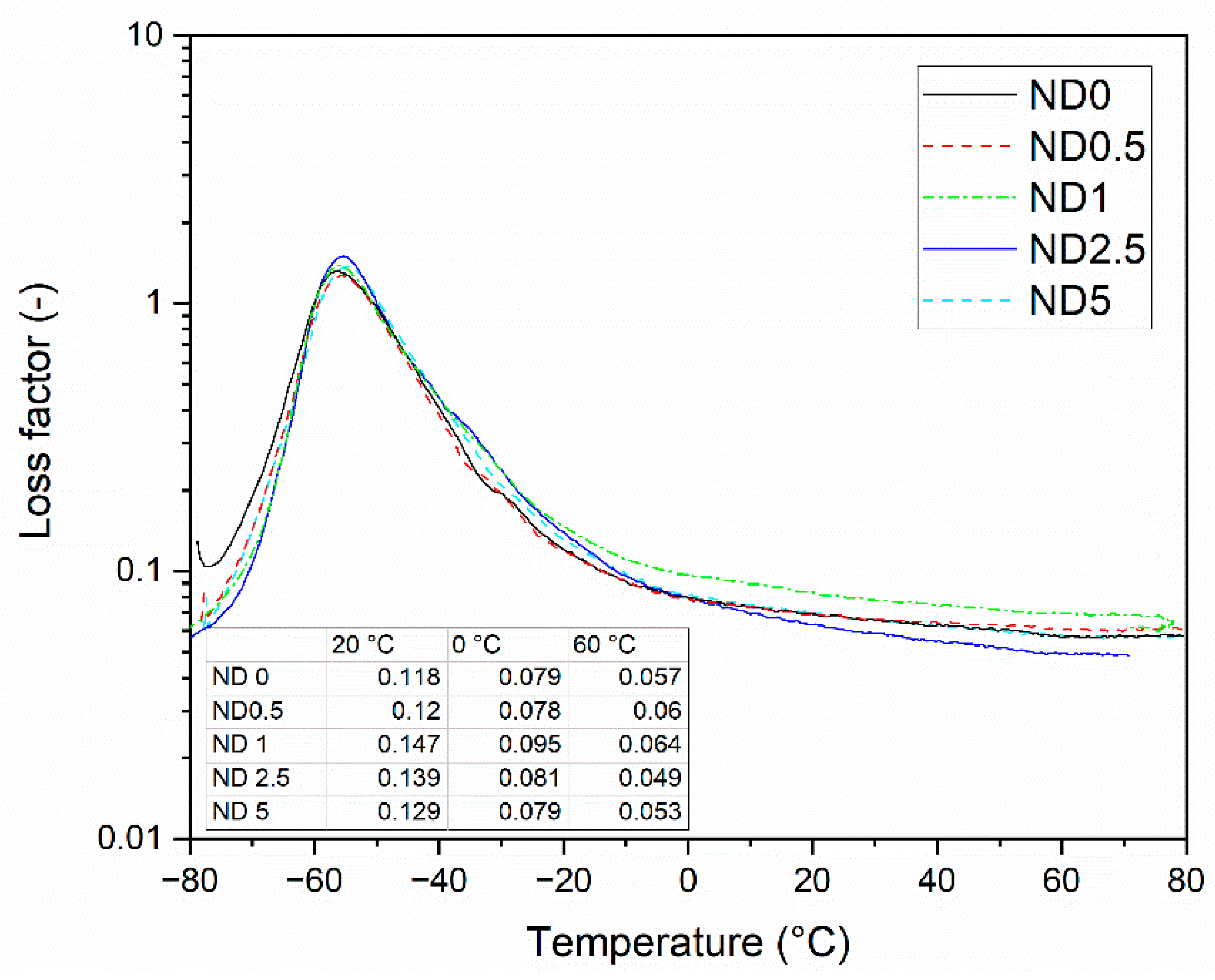
The loss factor of the compounds is presented in Figure 6. The gradual replacement of CB by NDs has only minor influence on the peak intensity. This behavior differs from the behavior of CNTs. The height of the loss factor peak decreases when CB is partially replaced by them due to the restricted mobility of the polymer chains [14]. In this case, only the compound containing 0.5 phr NDs show this effect, and still the effect is minimal. The addition of the higher amount of NDs results in the higher peak, thus NDs improve the mobility of the polymer chains. However, they do not affect the glass transition temperature. The loss factor curve is used to predict the tire properties, such as wet grip and rolling resistance. At −20 °C, the loss factor is improved when 1 or 2.5 phr CB is replaced by NDs, which indicates better grip on ice. At 0 °C, the temperature used to predict the wet grip, the compound containing 1 phr NDs shows the highest loss factor and thus the best wet grip in tire applications. The loss factor values at 60 °C are used as an indicator for the rolling resistance. At this temperature the loss factor is increasing when 0.5 or 1 phr CB is replaced by NDs, indicating the poorer polymer-filler interaction of NDs and the chain slipping of polymers. However, at a 2.5 phr ND concentration, the loss factor is remarkably lower than without NDs. Hence, the ND-CB filler combination has potential to reduce the rolling resistance in tires with proper concentrations.
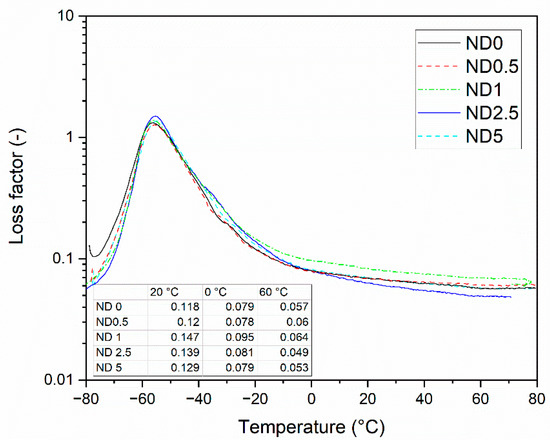
Figure 6.
The loss factor of the rubber compounds as a function of temperature.
4. Conclusions
The effect of a nanodiamond—carbon black hybrid filler system on the properties of a natural rubber—butadiene rubber compound was studied by gradual replacement of a small amount of carbon black by nanodiamonds. Significant enhancement in tensile strength was achieved by replacing 1 phr carbon black by nanodiamonds, which was due to the improved filler dispersion. The hybrid filler system facilitated the dispersion of both fillers, and the stronger filler network was achieved. In addition, the hybrid filler system improved the wear resistance of the compounds after addition of 2.5 and 5 phr NDs. Hence, the studied hybrid filler systems have a great potential for improved performance in several industrial sectors, such as mining and paper manufacturing, although further studies are still required. Moreover, the partial replacement of carbon black by nanodiamonds has the potential to increase the wet grip as well as to decrease the rolling resistance in tire tread compounds.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app112110085/s1, Table S1: The curing parameters of the compounds obtained from the rheometric curves.
Author Contributions
Conceptualization. M.P.; methodology. M.P. and E.S.; formal analysis. M.P.; investigation. M.P.; data curation. M.P.; writing—original draft preparation. M.P.; writing—review and editing. A.S. and E.S.; visualization. M.P.; supervision. M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Tampere Microscopy Center is acknowledged for allowing the use of SEM.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection. analyses. or interpretation of data; in the writing of the manuscript. or in the decision to publish the results.
References
- Fan, Y.; Fowler, G.D.; Zhao, M. The past. present and future of carbon black as a rubber reinforcing filler—A review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Verdejo, R.; López-Manchado, M.A.; Hernández Santana, M. Sustainable mobility: The route of tires through the circular economy model. Waste Manag. 2021, 126, 309–322. [Google Scholar] [CrossRef]
- Subramaniam, K.; Das, A.; Heinrich, G. Development of conducting polychloroprene rubber using imidazolium based ionic liquid modified multi-walled carbon nanotubes. Comp. Sci. Technol. 2011, 71, 1441–1449. [Google Scholar] [CrossRef] [Green Version]
- Le, H.H.; Parsaker, M.; Sriharish, M.N.; Henning, S.; Menzel, M.; Wießner, S.; Das, A.; Do, Q.K.; Heinrich, G.; Radusch, H.J. Effect of rubber polarity on selective wetting of carbon nanotubes in ternary blends. Express Polym. Lett. 2015, 9, 960–971. [Google Scholar] [CrossRef]
- Kummerlöwe, C.; Vennemann, N.; Yankova, E.; Wanitschek, M.; Grös, C.; Heider, T.; Haberkorn, F.; Siebert, A. Preparation and properties of carbon nanotube composites with nitrile- and styrene-botadiene rubebrs. Polym. Eng. Sci. 2013, 53, 849–856. [Google Scholar] [CrossRef]
- Ghosh, S.; Sengupta, R.A.; Heinrich, G. High Performance Nanocomposite based on Organoclay and Blends of Different Types of SBR with BR. Kautsch. Gummi Kunstst. 2011, 1–2, 48–54. [Google Scholar]
- Cadambi, R.M.; Ghassemieh, E. Optimized Process for the Inclusion of Carbon Nanotubes in Elastomers with Improved Thermal and Mechanical Properties. J. Appl. Polym. Sci. 2012, 124, 4993–5001. [Google Scholar] [CrossRef]
- Cataldo, F. Reinforcing effect of multiwall carbon nanotubes in carbon black filled NR compounds. Rubber Fibres Plast. Int. 2009, 4, 78–83. [Google Scholar]
- Basu, D.; Das, A.; Stöckelhuber, K.W.; Wagenknecht, U.; Heinrich, G. Advances in layered double hydroxide (LDH)-based elastomer composites. Prog. Polym. Sci. 2014, 39, 594–626. [Google Scholar] [CrossRef]
- Bokobza, L. Multiwall carbon nanotube elastomeric composites: A review. Polymer 2007, 48, 4907–4920. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.-X.; Ni, Q.-Q.; Natsuki, T. Effect of carbon nanotubes on the properties of natural rubber composites. Key Eng. Mater. 2011, 464, 660–662. [Google Scholar] [CrossRef]
- Bokobza, L. Enhanced electrical and mechanical properties of multiwall carbon nanotube rubber composites. Polym. Adv. Technol. 2012, 23, 1543–1549. [Google Scholar] [CrossRef]
- De Falco, A.; Goyanes, S.; Rubiolo, H.; Mondragon, I.; Marzocca, A. Carbon nanotubes as reinforcement of styrene-butadiene rubber. Appl. Surf. Sci. 2007, 254, 262–265. [Google Scholar] [CrossRef]
- Poikelispää, M.; Das, A.; Dierkes, W.; Vuorinen, J. The effect of partial replacement of carbon black by carbon nanotubes on the properties of natural rubber/butadiene rubber compound. J. Appl. Polym. Sci. 2013, 54, 402–410. [Google Scholar] [CrossRef]
- Galimberti, M.; Cipolletti, M.; Coombs, M.; Riccò, T.; Agnelli, S.; Pandini, S. The role of nanofillers in promoting hybrid filler networking and synergism with carbon black in a hydrocarbon rubber. Kautsch. Gummi Kunstst. 2013, 7–8, 31–36. [Google Scholar]
- Shahamatifard, F.; Rodrigue, D.; Park, K.W.; Frikha, S.; Mighri, F. Natural rubber nanocomposites: Effect of carbon black/multi-walled carbon nanotubes hybrid fillers on the mechanical properties and thermal conductivity. Polym.-Plast. Technol. Mater. 2021, 60, 1686–1696. [Google Scholar]
- Wang, G.X.; Yu, Q.Z.; Hu, Y.M.; Zhao, G.Y.; Chen, J.W.; Li, H.; Jiang, N.; Hu, D.W.; Xu, Y.Q.; Zhu, Y.T.; et al. Influence of the filler dimensionality on the electrical, mechanical and electromagnetic shielding properties of isoprene rubber-based flexible conductive composites. Compos. Commun. 2020, 21, 100417. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Mishra, Y.K. Nanocarbon Reinforced Rubber Nanocomposites: Detailed Insights about Mechanical, Dynamical Mechanical Properties, Payne, and Mullin Effects. Nanomater. 2018, 8, 945. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Fu, X.; Luo, M.; Xie, Z.; Huang, C.; Zhou, J.; Zhu, Y.; Huang, G.; Wu, J. Synergistic effect of CB and GO/CNT hybrid fillers on the mechanical properties and fatigue behavior of NR composites. RSC Adv. 2018, 8, 10573–10581. [Google Scholar] [CrossRef] [Green Version]
- Valentini, L.; Bittolo Bon, S.; Lopez-Manchado, M.A.; Verdejo, R.; Pappalardo, L.; Bolognini, A.; Alvino, A.; Borsini, S.; Berardo, A.; Pugno, N.M. Synergistic effect of graphene nanoplatelets and carbon black in multifunctional EPDM nanocomposites. Compos. Sci. Technol. 2016, 128, 123–130. [Google Scholar] [CrossRef]
- Malas, A.; Das, C.K.; Das, A.; Heinrich, G. Development of expanded graphite filled natural rubber vulcanizates in presence and absence of carbon black: Mechanical, thermal and morphological properties. Mater. Des. 2012, 39, 410–417. [Google Scholar] [CrossRef]
- Roy, A.; Kar, S.; Ghosal, K.; Naskar, K.; Bhowmick, A.K. Flourishing an Electrochemical Synthetic Route toward Carbon Black-Intercalated Graphene as a Neoteric Hybrid Nanofiller for Multifunctional Polymer Nanocomposites. Ind. Eng. Chem. Res. 2021, 60, 5758–5769. [Google Scholar] [CrossRef]
- Shakun, A.; Anyszka, R.; Sarlin, E.; Blume, A.; Vuorinen, J. Influence of surface modified nanodiamonds on dielectric and mechanical properties of silicone composites. Polymers 2019, 11, 1104. [Google Scholar] [CrossRef] [Green Version]
- Mokhireva, K.A.; Svistkov, A.L.; Solod’ko, V.N.; Komar, L.A.; Stöckelhuber, K.W. Experimental analysis of the effect of carbon nanoparticles with different geometry on the appearance of anisotropy of mechanical properties in elastomeric composites. Polym. Test. 2017, 59, 46–54. [Google Scholar] [CrossRef]
- Dolmatov, V. Polymer-diamond composites based on detonation nanodiamonds. Part 2. J. Superhard Mater. 2007, 29, 65–75. [Google Scholar] [CrossRef]
- Krueger, A. The structure and reactivity of nanoscale diamond. J. Mater. Chem. 2008, 18, 1485–1492. [Google Scholar] [CrossRef]
- Dolmatov, V.Y.; Fujimura, T. Physical and chemical problems of modification of detonation nanodiamond surface properties. In Synthesis, Properties and Applications of Ultrananocrystalline Diamond; Gruen, D., Shenderova, O., Vul’, A., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 192, pp. 217–230. [Google Scholar]
- Jafarpour, E.; Shojaei, A.; Ahmadijokani, F. High-performance styrene-butadiene rubber nanocomposite based on carbon nanotube/nanodiamond hybrid filler with synergistic thermal conduction characteristic and electrical insulating properties. Polymers 2020, 196, 122470. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Composition Materials Based on Elastomer and Polymer Matrices Filled with Nanodiamonds of Detonation Synthesis. Nanotechnol. Russ. 2009, 4, 556–575. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Gogotsi, Y. Nanodiamond–polymer composites. Diam. Relat. Mater. 2015, 58, 161–171. [Google Scholar] [CrossRef]
- Nagornaya, M.N.; Razdyakonova, G.I.; Khodakova, S.Y. The Effect of Functional Groups of Carbon Black on Rubber Properties. Procedia Eng. 2016, 15, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Zielińska, M.; Seyger, R.; Dierkes, W.K.; Bielinski, D.; Noordermeer, J.W.M. Swelling of EPDM Rubbers for Oil-Well Applications as Influenced by Medium Composition and Temperature I: Literature and theoretical background. Elastomery 2016, 20, 6–17. [Google Scholar]
- Leblanc, J.L.; Hardy, P. Evolution of bound rubber during the storage of uncured compounds. Kautsch. Gummi Kumstst. 1991, 44, 1119–1124. [Google Scholar]
- ISO 37:2017. Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- ISO 34-1:2010. Rubber, Vulcanized or Thermolastic—Determination of Tear Strength—Part 1: Trouser, Angle and Crescent Test Pieces; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- ASTM D 2240-05. Standard Test Method for Durometer Hardness; ASTM: West Conshehocken, PA, USA, 2010. [Google Scholar]
- Kidalov, S.V.; Shakhov, F.M.; Vul, A.Y.; Ozerin, A.N. Grain-boundary heat conductance in nanodiamond composites. Diam. Relat. Mater. 2010, 19, 976–980. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Hoikkanen, M.; Poikelispää, M.; Das, A.; Reuter, U.; Dierkes, W.; Vuorinen, J. Evaluation of mechanical and dynamic mechanical properties of multiwalled carbon nanotube-based ethylene-propylene copolymer composites mixed by masterbatch dilution. J. Comp. Mater. 2016, 50, 4093–4101. [Google Scholar] [CrossRef]
- Rabiei, S.; Shojaei, A. Vulcanization kinetics and reversion behavior of natural rubber/styrene-butadiene rubber blend filled with nanodiamond—the role of sulfur curing system. Eur. Polym. J. 2016, 81, 98–113. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Bhowmick, A.K. Influence of novel electronbeam modified surface treated dual phase filler on rheometric andmechanical properties of styrene butadiene rubber vulcanizates. Rubber Chem Technol. 2003, 76, 299–317. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef]
- Carbodeon uDiamond® Vox P Specific Characteristics. Available online: http://www.carbodeon.net/index.php/en/nanomaterials/udiamond-functionalised-powders/udiamond-vox-p (accessed on 28 October 2021).
- Growney, D.J.; Fowler, P.W.; Mykhaylyk, O.O.; Fielding, L.A.; Derry, M.J.; Aragrag, N.; Lamb, G.D.; Armes, S.P. Determination of effective particle density for sterically stabilized carbon black particles: Effect of diblock copolymer stabilizer composition. Langmuir 2015, 31, 8764–8773. [Google Scholar] [CrossRef]
- Shakun, A.; Vuorinen, J.; Hoikkanen, M.; Poikelispää, M.; Das, A. Hard nanodiamonds in soft rubbers: Past. present and future —A review. Compos. Part A Appl Sci. Manuf. 2014, 64, 49–69. [Google Scholar] [CrossRef]
- Das, A.; Stöckelhuber, K.W.; Rooj, S.; Wang, D.-Y.; Heinrich, G. Synergistic effects of expanded nanoclay and carbon black on natural rubber compounds. Kautsch. Gummi Kunstst. 2010, 63, 296–302. [Google Scholar]
- Neitzel, I.; Mochalin, V.; Bares, J.; Carpick, R.W.; Erdemir, A.; Gogotsi, Y. Tribological Properties of Nanodiamond—Epoxy Composites. Tribol. Lett. 2012, 47, 195–202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).