A Laser Irradiation Method for Controlling Pieris rapae Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Laser Irradiation Experiment Equipment
2.3. Test of the Effect of Laser Irradiation on Pieris rapae Larvae
2.3.1. Test Process
- Before the test, the working parameters (laser power, irradiation area, laser opening time) were adjusted to the target value by adjusting the laser irradiation device;
- When the experiment was performed, P. rapae larva was placed in a petri dish. After the P. rapae stopped moving, the dish was moved such that the irradiation position of the P. rapae was directly above the intersection of the cross marks;
- The laser was started and stopped after the set time to complete the laser strike. The larvae were not fixed during the test. Then, these larvae were placed on the observation platform after laser irradiation, and normal larvae (i.e., those without laser irradiation) were added as the control group (CK);
- The antifeedant percentage and mortality rate of the larvae after 24 h, 48 h and 72 h were calculated.
2.3.2. Experiment 1: Single Working Parameter Tests
2.3.3. Experiment 2: Combinations of Different Working Parameters Tests
2.3.4. Dependent Variables
2.4. Statistical Analysis
3. Results
3.1. Experiment 1: Effects of Single Working Parameters on the Antifeedant Percentage and Mortality Rate of Pieris rapae Larvae
3.1.1. Laser Power
3.1.2. Irradiation Area
3.1.3. Laser Opening Time
3.1.4. Irradiation Position
3.1.5. Dependent Variables
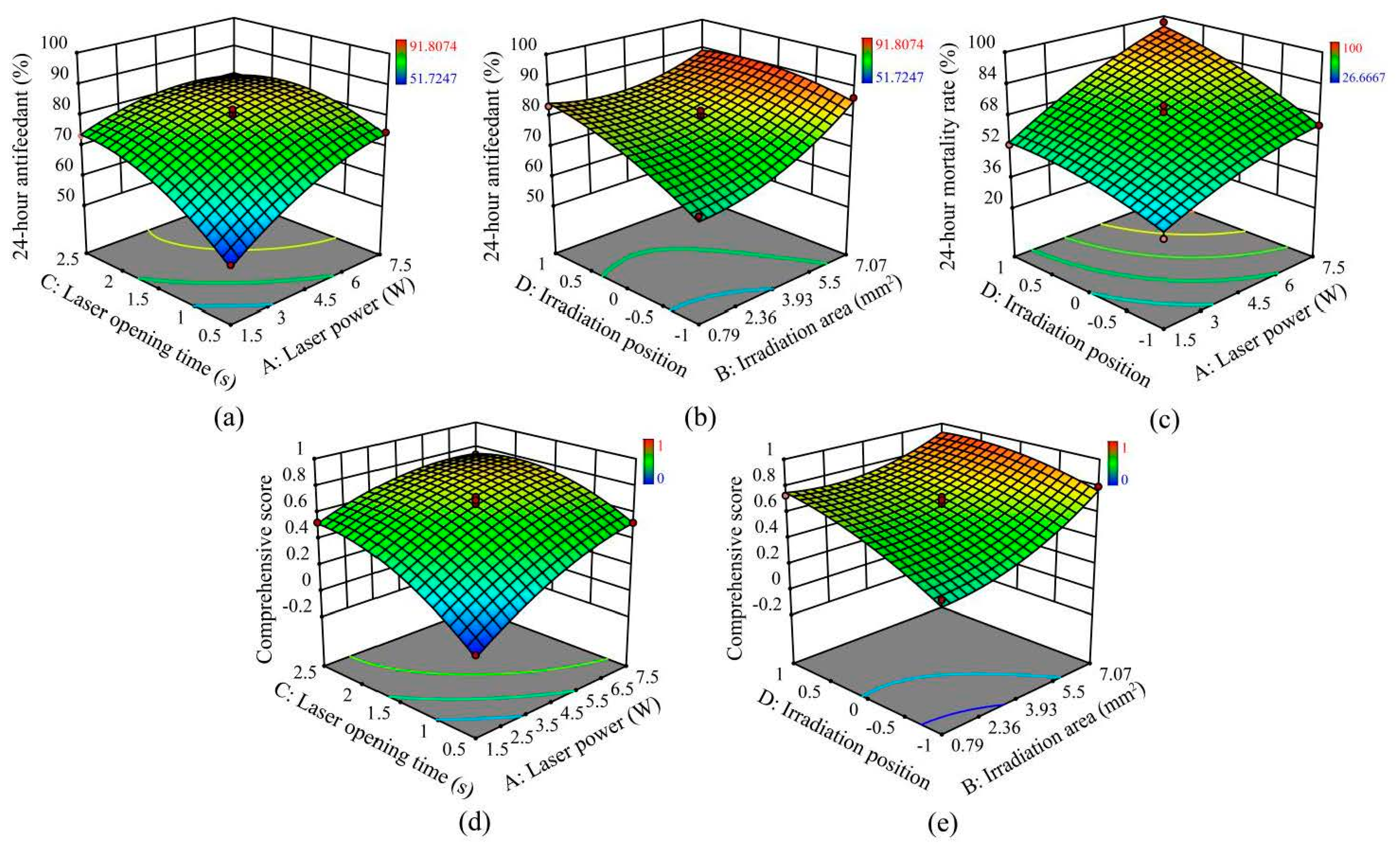
3.2. Experiment 2: Effects of Different Working Parameter Combinations on the Antifeedant Percentage and Mortality Rate of Pieris rapae Larvae
Establishment of Regression Model
- 1.
- 24 h antifeedant percentage (%) Y1:
- 2.
- 24 h mortality rate (%) Y2:
- 3.
- Comprehensive score K:
3.3. Experiment 3: Optimization of Working Parameters and Validation
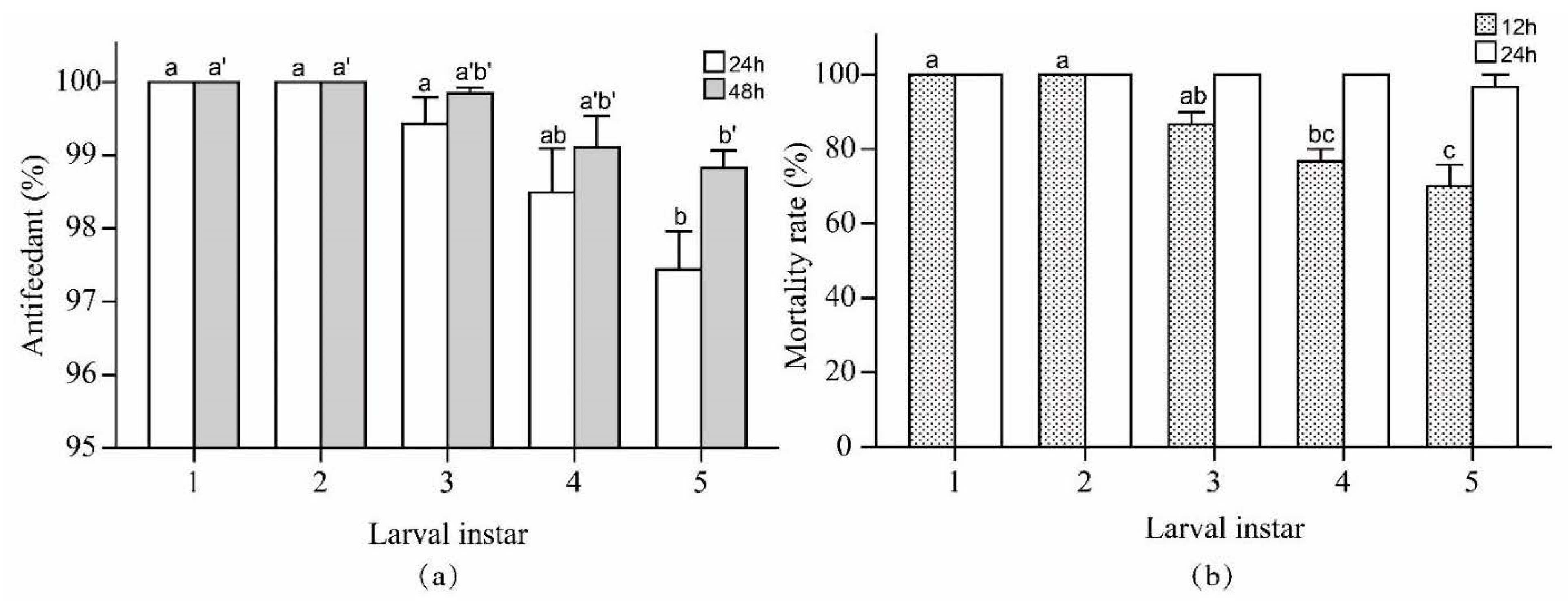
3.4. Experiment 4: Verification Test with 1st- to 5th-Instar Pieris rapae Larvae
4. Discussion
5. Conclusions
- The optimal combination for the maximum comprehensive score was as follows: laser power, 7.5 W; irradiation area, 6.189 mm2; laser opening time, 1.177 s; and irradiation position, middle abdomen.
- The optimal combination identified based on the observations was used to verify the experiment. The results showed that the decrease in the antifeedant percentage of P. rapae larvae after 24 h was 98.49%, whereas the 24 h mortality rate was 100%.
- The combination of the experimental parameters was suitable for 1st- to 5th-instar P. rapae larvae, and the mortality rate of the 5th-instar larvae at 36 h also was 100%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, S.F.; Lombaert, E.; Espeset, A.; Vila, R.; Talavera, G.; Dincă, V.; Doellman, M.M.; Renshaw, M.A.; Eng, M.W.; Hornett, E.A.; et al. Global invasion history of the agricultural pest butterfly Pieris rapae revealed with genomics and citizen science. Proc. Natl. Acad. Sci. USA 2019, 116, 20015–20024. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ren, W.; Kang, Z.; Jiang, J.H.; Zhao, X.J.; Du, L.F. A trypsin inhibitor from Cassia obtusifolia seeds: Isolation, characterization and activity against Pieris rapae. Biotechnol. Lett. 2007, 29, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Mnif, I.; Ghribi, D. Potential of bacterial derived biopesticides in pest management. Crop Prot. 2015, 77, 52–64. [Google Scholar] [CrossRef]
- Liu, J.L.; Wei, X.Q.; Guo, H. Breeding of an L-Valine Producing Strain by Laser Mutation. Chin. J. Laser 2016, 43, 178–184. [Google Scholar] [CrossRef]
- Kaierle, S.; Marx, C.; Rath, T.; Hustedt, M. Find and Irradiate—Lasers Used for Weed Control: Chemical free elimination of unwanted plants. Laser Tech. J. 2013, 10, 44–47. [Google Scholar] [CrossRef]
- Podleśny, J.; Stochmal, A.; Podleśna, A.; Misiak, L.E. Effect of laser light treatment on some biochemical and physiological processes in seeds and seedlings of white lupine and faba bean. Plant Growth Regul. 2012, 67, 227–233. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Li, J.T.; Zhang, M.M.; Bi, Z.Z.; Li, Z.L. He–Ne laser pretreatment protects wheat seedlings against cadmium-induced oxidative stress. Ecotoxicol. Environ. Saf. 2013, 88, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Abou-Dahab, A.D.M.; Mohammed, T.A.; Heikal, A.A.; Taha, L.S.; Gabr, A.M.G.; Metwally, S.A.; Ali, A.I.R. In vitro laser radiation induces mutation and growth in Eustoma grandiflorum plant. Bull. Nat. Res. Cent. 2019, 43, 3. [Google Scholar] [CrossRef]
- Spalding, E.P.; Folta, K.M. Illuminating topics in plant photobiology. Plant Cell Environ. 2005, 28, 39–53. [Google Scholar] [CrossRef]
- Perveen, R.; Jamil, Y.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He–Ne Laser-Induced Improvement in Biochemical, Physiological, Growth and Yield Characteristics in Sunflower (Helianthus annuus L.). Photochem. Photobiol. 2011, 87, 1453–1463. [Google Scholar] [CrossRef]
- Wöltjen, C.; Haferkamp, H.; Rath, T.; Herzog, D. Plant growth depression by selective irradiation of the meristem with CO2 and diode lasers. Biosyst. Eng. 2008, 101, 316–324. [Google Scholar] [CrossRef]
- Wöltjen, C.; Rath, T.; Herzog, D. Investigations about the Technical Basics of Laser Beam Use for Plant Manipulation. Acta Hortic. 2008, 801, 587–594. [Google Scholar] [CrossRef]
- Nasim, H.; Jamil, Y. Diode lasers: From laboratory to industry. Opt. Laser Technol. 2014, 56, 211–222. [Google Scholar] [CrossRef]
- Astatkie, T.; Rifai, M.N.; Havard, P.; Adsett, J.; Lacko-Bartosova, M.; Otepka, P. Effectiveness of hot water, infrared and open flame thermal units for controlling weeds. Biol. Agric. Hortic. 2007, 25, 1–12. [Google Scholar] [CrossRef]
- Heisel, T.; Schou, J.; Christensen, S.; Andreasen, C. Cutting weeds with a CO2 laser. Weed Res. 2001, 41, 19–29. [Google Scholar] [CrossRef]
- Heisel, T.; Schou, J.; Andreasen, C.; Christensen, S. Using laser to measure stem thickness and cut weed stems. Weed Res. 2002, 42, 242–248. [Google Scholar] [CrossRef]
- Coleman, G.R.Y.; Stead, A.; Rigter, M.P.; Xu, Z.; Johnson, D.; Brooker, G.M.; Sukkarieh, S.; Walsh, M.J. Using energy requirements to compare the suitability of alternative methods for broadcast and site-specific weed control. Weed Res. 2019, 33, 633–650. [Google Scholar] [CrossRef]
- Cornwell, P.B. The Entomology of Radiation Disinfestation of Grain, 1st ed.; Pergamon Press: London, UK, 1969; pp. 47–52. [Google Scholar]
- Ramos Elorduy de Conconi, J.; Elorduy, C.; Oxley, T.; Barry, S. Laser light as a new potential method for pest control in preserved foods. In Biodeterioration 5, Proceedings of the 5th International Biodeterioration Symposium, Aberdeen, UK, 7–11 September 1981; Oxley, T.A., Barry, S., Eds.; Wiley: Chichester, NH, USA, 1983; pp. 592–608. [Google Scholar]
- He, Y.; Zhang, K.; Xiao, B.; Hou, T.P. Study on the Antifeedant Activity and Mechanism for Bioactive Compound from Stellera chamaejasme against Larvae of Pieris rapae. Chin. J. Biol. Control 2006, 22, 33–37. [Google Scholar] [CrossRef]
- Neimz, M.H. Laser-Tissue Interactions: Fundamentals and Applications, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Obayashi, K.; Sato, K.; Ito, N.; Wang, X.L.; Takagi, S. Physical pest control of drosophila using laser, 1: Effects of laser emissions on pest. J. Jpn. Soc. Agric. Mach. 2006, 67, 93–100. [Google Scholar] [CrossRef]
- Faruki, S.I.; Das, D.R.; Khan, A.R.; Khatun, M. Effects of ultraviolet (254 nm) irradiation on egg hatching and adult emergence of the flour beetles, Tribolium castaneum, T. confusum and the almond moth, Cadra cautella. J. Insect Sci. 2007, 7, 36. [Google Scholar] [CrossRef]
- Keller, M.D.; Leahy, D.J.; Norton, B.J.; Mullen, E.R.; Marvit, M.; Makagon, A. Laser induced mortality of Anopheles stephensi mosquitoes. Sci. Rep. 2016, 6, 20936. [Google Scholar] [CrossRef]
- Sorungbe, A.A.; Badmus, H.A.; Sulaimon, A.M. Effect of ultraviolet irradiation on egg hatching of tropical warehouse moth (Ephestia cautella), development of its adult and mortality. Int. J. Res. Pharm. Biosci. 2016, 3, 23–27. [Google Scholar]
- Mathiassen, S.K.; Bak, T.; Christensen, S.; Kudsk, P. The effect of laser treatment as a weed control method. Biosyst. Eng. 2006, 95, 497–505. [Google Scholar] [CrossRef]
- Ai, S.R.; Yao, M.Y.; Huang, L.; Wu, R.M. Analyzeand Compare the Thermal Effect on Locusts and Host Plants Tissue by Semiconductor Laser Irradiation. Appl. Laser 2010, 30, 236–239. [Google Scholar] [CrossRef]
- Ma, X.L.; Zhu, Z.; Li, Y.; Yang, G.D.; Pei, Y.X. Expressing a modified cowpea trypsin inhibitor gene to increase insect tolerance against Pieris rapae in Chinese cabbage. Hortic. Environ. Biotechnol. 2017, 58, 195–202. [Google Scholar] [CrossRef]
- Hao, C.; Fan, X. Breeding Pieris rapae L. as Experinent Insect and Observation of Feeding Activity of the Larvae. J. Shanxi Agric. Univ. 1998, 18, 30–32. [Google Scholar]
- Chen, Y.N.; Ma, J. Study on the larva age markers of three important vegetable pests. J. Changjiang Veg. 1994, 2, 17–18. [Google Scholar]
- Powell, J.A. Lepidoptera. In Encyclopedia of Insects, 1st ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: New York, NY, USA, 2009; pp. 559–586. [Google Scholar]
- Pan, L.; Ren, L.; Chen, F.; Feng, Y.Q.; Luo, Y.Q. Antifeedant activity of Ginkgo biloba secondary metabolites against Hyphantria cunea larvae: Mechanisms and applications. PLoS ONE 2016, 11, e0155682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Liu, Z.Q.; Lei, F.J.; Fu, J.F.; Zhang, X.X.; Ma, W.L.; Zhang, L.X. Antifeedant and oviposition-deterring activity of total ginsenosides against Pieris rapae. Saudi J. Biol. Sci. 2017, 24, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Fan, Y.; Jing, B.N.; Yu, L.Q.; Wang, W.; Wang, D.D.; Zhao, T.Z. The synergistic effect of natural Celastrus angulatus and natural Vitex negundo on Pieris rapae and Ectropis oblique hypulina. Jiangsu Agri. Sci. 2018, 46, 63–66. [Google Scholar] [CrossRef]
- Zeng, W.A.; Tan, J.C.; Tan, L.; Chen, J.Z. Biological activities of crude extracts from Polygonum hydropiper L. against Pieris rapae L. J. Hunan Agric. Univ. (Nat. Sci.) 2007, 33, 76–78. [Google Scholar] [CrossRef]
- Li, Y.Y.; Hu, C.R. Experiment Design and Data Processing, 3rd ed.; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
- Xie, F.P.; Liu, M.Z.; Yang, M.M.; Liu, D.W.; Wang, X.S.; Ren, S.G. Design of ordered fertilizer device for bagged slow-release fertilizer. Trans. Chin. Soc. Agric. Eng. 2019, 35, 40–49. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Hadzieva, J.; Mladenovska, K.; Simonoska, C.M.; Glavaš, D.M.; Dimchevska, S.; Geškovski, N.; Grozdanov, A.; Popovski, E.; Petruševski, G.; Chachorovska, M.; et al. Lactobacillus casei encapsulated in soy protein isolate and alginate microparticles prepared by spray drying. Food Technol. Biotechnol. 2017, 55, 173–186. [Google Scholar] [CrossRef]
- Ellis, K.; Silvestrini, R.; Varela, B.; Alharbi, N.; Hailstone, R. Modeling setting time and compressive strength in sodium carbonate activated blast furnace slag mortars using statistical mixture design. Cem. Concr. Compos. 2016, 74, 1–6. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Das, N. Process optimization for microencapsulation of probiotic yeasts. Front. Biol. 2018, 13, 197–207. [Google Scholar] [CrossRef]
- Madhumita, M.; Guha, P.; Nag, A. Optimization of the exhaustive hydrodistillation method in the recovery of essential oil from fresh and cured betel leaves (Piper betle L.) using the Box–Behnken design. J. Food Process. Preserv. 2019, 43, e14196. [Google Scholar] [CrossRef]
- Gao, R.; Gao, C.; Tian, X.; Yu, X.Y.; Di, X.D.; Xiao, H.; Zhang, X. Insecticidal activity of deoxypodophyllotoxin, isolated from Juniperus sabina L., and related lignans against larvae of Pieris rapae L. Pest Manag. Sci. 2004, 60, 1131–1136. [Google Scholar] [CrossRef]
- Zeng, T.; Li, L.F.; Wei, D.W.; Chen, H.S.; Liu, Y. Antifeeding activity of some plant extracts against the larvae of Pieris rapae. J. Guangxi Agric. Biol. Sci. 2006, 1, 38–42. [Google Scholar]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. The effects of Artemisia annua L. and Achillea millefolium L. crude leaf extracts on the toxicity, development, feeding efficiency and chemical activities of small cabbage Pieris rapae L. (Lepidoptera: Pieridae). Pestic. Biochem. Physiol. 2011, 99, 244–249. [Google Scholar] [CrossRef]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. Effect of milk thistle, Silybium marianum, extract on toxicity, development, nutrition, and enzyme activities of the small white butterfly, Pieris rapae. J. Insect Sci. 2013, 13, 146. [Google Scholar] [CrossRef][Green Version]
- Wang, P.; Lu, Y.; Dong, J.; Jing, L.; Yuan, Z.Q.; Yang, J.G.; Qiao, Y. Control Effect of 13 Pesticides on Pieris rapae in the Cauliflower Field. Agrochemicals 2017, 56, 300–302. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.M.; Wei, X.; Wei, Z.L. Control Effect of Frequency Vibrating Insecticidal Lamp on Sugarcane Pest in Laibin Sugarcane Area. J. Anhui Agric. Sci. 2018, 46, 126–127, 151. [Google Scholar] [CrossRef]
- Ye, S.G.; Xu, F.C.; Wu, Y.H.; Chen, Z.L. Control effect of frequency vibrating insecticidal lamp on pests in vegetable field. Plant Prot. 2000, 26, 45–46. [Google Scholar]
- Zhang, G.X.; Zheng, G.; Li, X.J.; Bu, J. Application of frequency vibrating insecticidal lamp from the perspective of biodiversity protection. Entomol. Knowl. 2004, 41, 532–535. [Google Scholar]
- Ren, K.; Tu, K.; Li, H.W. Control Effects of Semiconductor Laser on Drosophila Melanogaster. Chin. J. Laser 2006, 33, 1148–1152. [Google Scholar]
- Sumesh, N.; Chang, C.; Hsu, F.; Su, C.; Chen, S. Rapid laser pest control system with 3D small object detection. In Proceedings of the International Society for Optical Engineering (SPIE 11299)—AI and Optical Data Sciences, San Francisco, CA, USA, 25 March 2020. [Google Scholar] [CrossRef]
- Xiang, Y.; Lin, J.W.; Li, Y.J.; Xiong, Y.; Hu, Z.F.; Chen, Y.Q. A Laser Pest Control Robot Based on Machine Vision. CN. Patent 209,473,426 U, 11 October 2019. [Google Scholar]
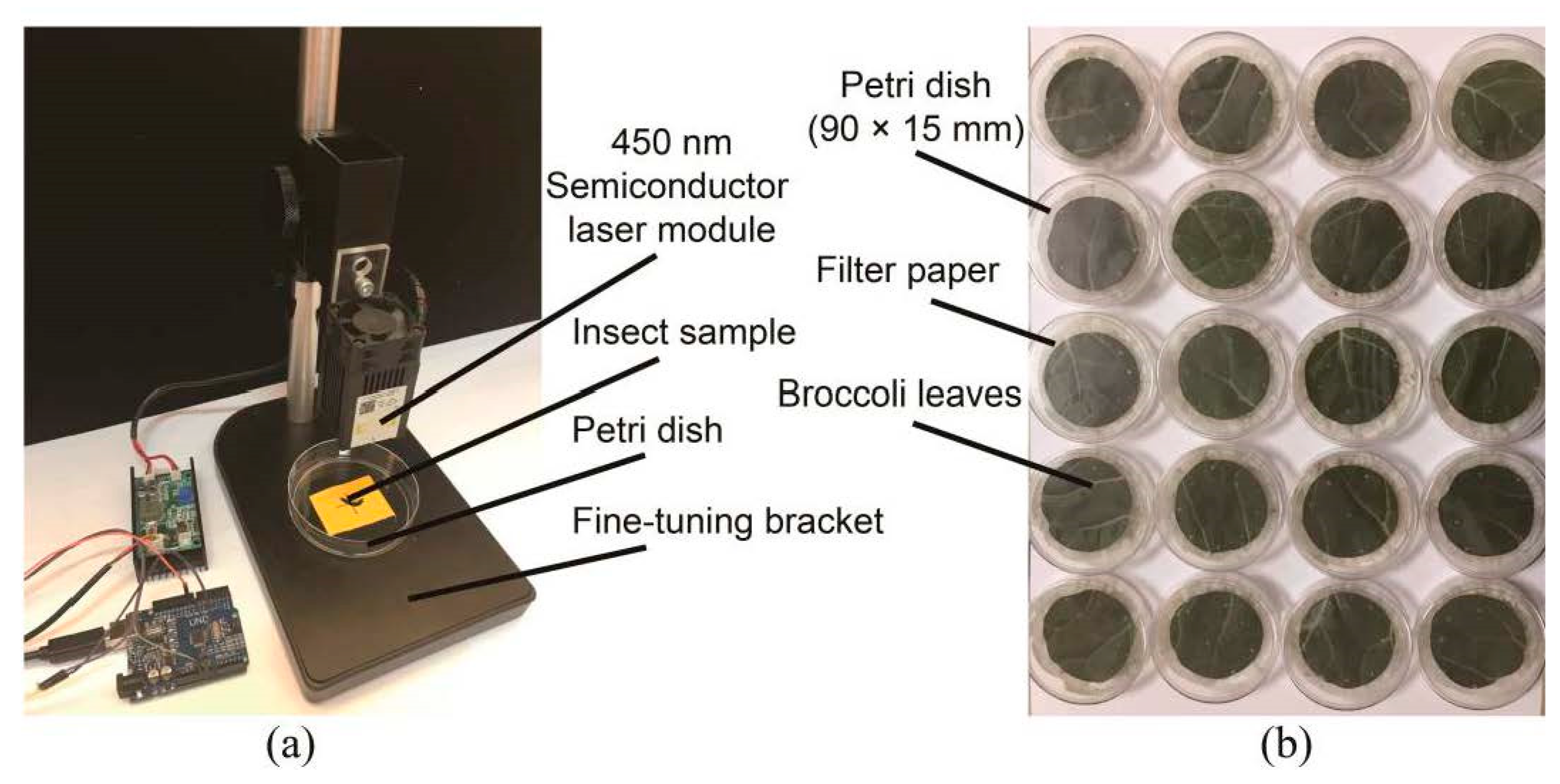

| Experiment Number 1 | Laser Power/W | Irradiation Area 2/mm2 | Laser Opening Time/s | Irradiation Position 3 |
|---|---|---|---|---|
| 1 | 1 | 0.01 | 2 | Middle |
| 2 | 2.5 | 0.01 | 2 | Middle |
| 3 | 5 | 0.01 | 2 | Middle |
| 4 | 7.5 | 0.01 | 2 | Middle |
| 5 | 10 | 0.01 | 2 | Middle |
| 6 | 5 | 0.01 | 2 | Middle |
| 7 | 5 | 0.79 | 2 | Middle |
| 8 | 5 | 3.14 | 2 | Middle |
| 9 | 5 | 7.07 | 2 | Middle |
| 10 | 5 | 0.01 | 0.5 | Middle |
| 11 | 5 | 0.01 | 1 | Middle |
| 12 | 5 | 0.01 | 2 | Middle |
| 13 | 5 | 0.01 | 3 | Middle |
| 14 | 5 | 0.01 | 2 | Head |
| 15 | 5 | 0.01 | 2 | Mesothorax |
| 16 | 5 | 0.01 | 2 | Middle |
| 17 | 5 | 0.01 | 2 | End |
| Control group (CK) | – | – | – | – |
| Factors | Factor Levels 1 | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Laser power/W | 1.5 | 4.5 | 7.5 |
| Irradiation area/mm2 | 0.79 | 3.93 | 7.07 |
| Laser opening time/s | 0.5 | 1.5 | 2.5 |
| Irradiation position 2 | Head | Mesothorax | Middle of abdomen |
| Experiment Number 1 | Working Parameters 2 | Mean (%) 24 h Antifeedant Percentage | Mean (%) 24 h Mortality Rate | Comprehensive Score 3 | |||
|---|---|---|---|---|---|---|---|
| A | B | C | D | ||||
| 1 | 1 | 0 | 0 | 1 | 87.59 ± 4.25 | 96.67 ± 3.33 | 0.9127 |
| 2 | 0 | 0 | 0 | 0 | 80.54 ± 3.55 | 66.67 ± 3.33 | 0.6668 |
| 3 | 1 | −1 | 0 | 0 | 77.79 ± 8.22 | 73.33 ± 3.33 | 0.6461 |
| 4 | 0 | 1 | 1 | 0 | 89.15 ± 1.32 | 90.00 ± 0.00 | 0.9127 |
| 5 | 1 | 0 | 0 | −1 | 75.58 ± 3.96 | 63.33 ± 3.33 | 0.5665 |
| 6 | 0 | 0 | −1 | 1 | 69.21 ± 5.83 | 53.33 ± 3.33 | 0.4144 |
| 7 | 0 | 0 | −1 | −1 | 53.34 ± 2.78 | 26.67 ± 3.33 | 0.0282 |
| 8 | −1 | 0 | 1 | 0 | 73.36 ± 4.33 | 63.33 ± 3.33 | 0.5279 |
| 9 | 0 | 1 | 0 | 1 | 87.77 ± 3.31 | 90.00 ± 0.00 | 0.8886 |
| 10 | 0 | 0 | 0 | 0 | 73.90 ± 1.77 | 73.33 ± 3.33 | 0.5781 |
| 11 | 0 | 0 | 0 | 0 | 82.03 ± 6.58 | 73.33 ± 3.33 | 0.7202 |
| 12 | −1 | −1 | 0 | 0 | 64.17 ± 7.23 | 46.67 ± 3.33 | 0.2991 |
| 13 | −1 | 0 | 0 | −1 | 58.21 ± 8.36 | 36.67 ± 3.33 | 0.1542 |
| 14 | 0 | 0 | 0 | 0 | 82.18 ± 4.54 | 66.67 ± 3.33 | 0.6955 |
| 15 | 0 | −1 | 1 | 0 | 80.4 ± 5.36 | 66.67 ± 3.33 | 0.6644 |
| 16 | 0 | −1 | 0 | 1 | 83.37 ± 4.02 | 70.00 ± 0.00 | 0.7300 |
| 17 | 0 | 0 | 0 | 0 | 77.05 ± 8.23 | 70.00 ± 0.00 | 0.6195 |
| 18 | −1 | 1 | 0 | 0 | 78.81 ± 3.00 | 63.33 ± 3.33 | 0.6230 |
| 19 | −1 | 0 | 0 | 1 | 70.23 ± 6.58 | 53.33 ± 3.33 | 0.4323 |
| 20 | 0 | 0 | 1 | −1 | 73.07 ± 5.94 | 63.33 ± 3.33 | 0.5228 |
| 21 | 0 | 0 | 1 | 1 | 85.19 ± 4.18 | 90.00 ± 0.00 | 0.8436 |
| 22 | −1 | 0 | −1 | 0 | 51.72 ± 6.36 | 26.67 ± 3.33 | 0.0000 |
| 23 | 1 | 0 | −1 | 0 | 74.84 ± 3.51 | 56.67 ± 3.33 | 0.5264 |
| 24 | 0 | −1 | 0 | −1 | 66.59 ± 3.35 | 53.33 ± 3.33 | 0.3687 |
| 25 | 1 | 0 | 1 | 0 | 79.03 ± 1.98 | 90.00 ± 0.00 | 0.7359 |
| 26 | 1 | 1 | 0 | 0 | 91.81 ± 2.20 | 100.00 ± 0.00 | 1.0000 |
| 27 | 0 | 1 | −1 | 0 | 81.24 ± 0.60 | 66.67 ± 3.33 | 0.6791 |
| 28 | 0 | 1 | 0 | −1 | 86.43 ± 2.36 | 73.33 ± 3.33 | 0.7971 |
| 29 | 0 | −1 | −1 | 0 | 64.81 ± 3.91 | 40.00 ± 5.77 | 0.2830 |
| Value | Optimal Working Parameters 1 | Mean (%) 24 h Antifeedant Percentage | Mean (%) 24 h Mortality Rate | Comprehensive Score 3 | Desirability 4 | |||
|---|---|---|---|---|---|---|---|---|
| A | B2 | C | D | |||||
| Predicted 5 | 5.222 | 7.070 | 1.585 | 1.000 | 91.01 | 100.00 | 0.979 | 0.980 |
| Actual 6 | 5.222 | 7.070 | 1.585 | 1.000 | 98.33 ± 0.41a | 96.67 ± 3.33a | ||
| Bias 7 (%) | 7.44 | −3.45 | ||||||
| Predicted 5 | 7.500 | 6.189 | 1.177 | 1.000 | 88.50 | 100.50 | 0.922 | 0.952 |
| Actual 6 | 7.500 | 6.158 | 1.177 | 1.000 | 98.49 ± 0.60a | 100 ± 0.00a | ||
| Bias 7 (%) | 10.14 | −0.5 | ||||||
| Predicted 5 | 7.500 | 4.961 | 1.539 | 1.000 | 86.76 | 100.00 | 0.891 | 0.918 |
| Actual 6 | 7.500 | 4.908 | 1.539 | 1.000 | 97.91 ± 0.72a | 100 ± 0.00a | ||
| Bias 7 (%) | 11.39 | 0.00 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiang, Y.; Yang, Z.; Han, X.; Lin, J.; Hu, Z. A Laser Irradiation Method for Controlling Pieris rapae Larvae. Appl. Sci. 2021, 11, 9533. https://doi.org/10.3390/app11209533
Li Y, Xiang Y, Yang Z, Han X, Lin J, Hu Z. A Laser Irradiation Method for Controlling Pieris rapae Larvae. Applied Sciences. 2021; 11(20):9533. https://doi.org/10.3390/app11209533
Chicago/Turabian StyleLi, Yajun, Yang Xiang, Zhongxia Yang, Xiongzhe Han, Jiewen Lin, and Zhengfang Hu. 2021. "A Laser Irradiation Method for Controlling Pieris rapae Larvae" Applied Sciences 11, no. 20: 9533. https://doi.org/10.3390/app11209533
APA StyleLi, Y., Xiang, Y., Yang, Z., Han, X., Lin, J., & Hu, Z. (2021). A Laser Irradiation Method for Controlling Pieris rapae Larvae. Applied Sciences, 11(20), 9533. https://doi.org/10.3390/app11209533







