Abstract
At present, chemical pesticides remain the main approach for controlling Pieris rapae (L.) (Lepidoptera: Pieridae). This research proposes a novel laser irradiation method for managing P. rapae larvae as an alternative to chemical control. The effectiveness of controlling larvae and the influencing factors of lasers were studied to estimate optimal parameter combinations. Tests using the antifeedant effect and mortality of the larvae as dependent variables showed that the laser power, irradiation area, laser opening time and irradiation position were positively correlated with the P. rapae controlling effect. The optimal parameters for each factor were the following: laser power, 7.5 W; irradiation area, 6.189 mm2; laser opening time, 1.177 s; and irradiation position, middle of the abdomen. Based on these observations, a validation experiment was performed using the optimal combination of parameters, and the results showed that the antifeedant percentage of P. rapae larvae within 24 h posttreatment was 98.49%, whereas the mortality rate was 100%. The optimal parameter combination identified in the study was suitable for P. rapae larvae from the first- to fifth-instar stages, and a more effective controlling effect was observed with the younger larvae. These results can provide a theoretical basis for future pest control using laser pest-killing robots.
1. Introduction
Adult Pieris rapae (L.) (Lepidoptera: Pieridae) is one of the most numerous and tenacious butterflies in the world [1], and the larvae are a notorious pest of Cruciferae. In some serious cases, the larvae consume all the leaves and can even cause soft rot [2]. At present, chemical control remains the main control method for these larvae. The long-term use of chemical pesticides has caused harm to humans and the environment and forms a vicious cycle [3]. Therefore, the identification of an eco-friendly control method is urgently needed to support the demand for a greater quantity cruciferous plant of higher quality.
Lasers are light sources with high energy density and good monochromaticity and directionality [4]. Since their invention, lasers have been used for many applications; in fact, lasers are a high-tech tool used to meet the challenges of sustainable agriculture [5]. For instance, lasers can be used as biostimulator devices, and studies have shown that laser irradiation can stimulate various plant organs and tissues [6,7,8]. The irradiation of seeds, seedlings or plants by a low-intensity laser can improve their yield and quality due to stimulation of their biochemical and physiological processes [9,10]. In addition, lasers are biological inhibition devices, and some studies have shown that the thermal effects of lasers can damage or cause localized mortality in biological tissues [11,12,13]. Compared with traditional treatments [14], the use of laser irradiation for weed control has shown good results in terms of effectiveness, efficiency and environmental protection [15,16,17].
Similarly, lasers can also be used in agricultural pest control. The effect of lasers for pest extermination or as a pest deterrent has been investigated since the 1960s [18,19,20]. These studies prove that the laser-based pest control uses the laser’s thermal effect to damage or kill larvae. When the laser irradiation intensity exceeds the absorption level of the epidermis, heat damages the local exoskeleton and the internal tissue under it [21]. Obayashi et al. [22] conducted the laser irradiation experiment of drosophila and demonstrated the irradiation of mosquitos or fly pests can cause death or infertility. Faruki et al. [23] studied the effect of 254 nm laser irradiation on egg hatching and adult emergence of the stored-grain pest eggs. The significantly reduced hatching and adult emergence caused by laser irradiation in the experimental pests proved that irradiation is an efficacious method for pest control. Keller et al. [24] used the short laser pulses to kill flying pests and studied the effect of laser pulses with different working parameters on the mortality of mosquitoes. Sorungbe et al. [25] proved that laser irradiation can kill arthropod pests, including eggs, nymphs and adults, and had a significant impact on eggs hatching, adult emergence and mortality of the tropical warehouse moth Ephestia cautella (Walker).
Studies have shown that the biological effects of laser-controlled biological tissue are related to the wavelength, laser power, irradiation area, laser opening time and irradiation position [26,27]. In this study, the controlling effect of 450 nm laser on P. rapae larvae and the experimental factors of lasers (laser power, irradiation area, laser opening time and irradiation position) were studied. Furthermore, the optimal combination of working parameters was estimated. Ultimately, through verification test, the universality of its ability to prevent the P. rapae at different instars attack was evaluated.
2. Materials and Methods
2.1. Insects
In this study, eggs of P. rapae were collected from Yunyuan Scientific Research Base, Hunan Agricultural University, Changsha, Hunan Province, China, and reared in a laboratory (Hunan Province Key Laboratory of Intelligent Agricultural Machinery and Equipment, Changsha, China) to obtain different instars of P. rapae. During collection, leaves with eggs were placed in a growth chamber with a temperature of 25 °C and 85% relative humidity (RH) [28]. After hatching, the larvae were lured to fresh broccoli leaves and placed on a petri dish under the same temperature and RH conditions.
The total amount of food eaten by the 1st- to 3rd-instar P. rapae larvae accounted for only 2.53% of the total food consumption [29]. Therefore, fourth-instar larvae were used (~9 days after hatching).
2.2. Laser Irradiation Experiment Equipment
The laser irradiation device is shown in Figure 1a. The device consisted of a semiconductor laser module (Model No. 450 nm/10 W; New Legend Technology Co., Ltd., Wuhan, China), a fine-tuning bracket (Model No. T10; SunTime Technology Co., Ltd., Shenzhen, China), a 90 × 15 mm Petri dish (Model No. 82302-1090; Citorest Scientific Co., Ltd., Haimen, China) and a portable computer for laser control. The light spot of the semiconductor laser module was round, the focal length could be adjusted and the minimum focal diameter was over 0.1 mm. During the experiment, the semiconductor laser was fixed on the fine-tuning bracket and oriented vertically, facing downward. A yellow label with a cross mark was attached to the base of the fine-tuning bracket. The intersection of the cross mark was on the same vertical line as the output laser.
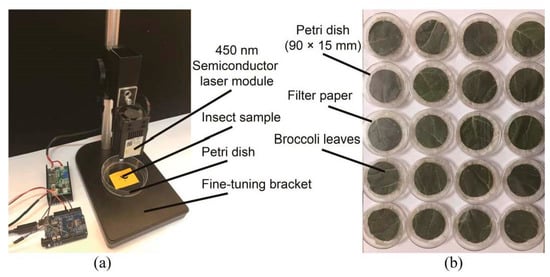
Figure 1.
Schematic design of laser irradiation test equipment. (a) Experimental device for laser irradiation. The 450 nm semiconductor laser module (10 W) regulated the irradiation power and opening time of the Pieris rapae larvae irradiation experiment. The fine-tuning bracket and petri dish were adjusted to control irradiation area and irradiation position, respectively. (b) Petri dishes for observing irradiated P. rapae. Observe the irradiated P. rapae larvae placed in the petri dish with moist filter paper and broccoli leaves at the bottom after the experiment. Each petri dish contains 10 vents (1 mm in diameter) for air circulation.
The observation platform was set to observe the changes of P. rapae after irradiation (Figure 1b). Chemical-free broccoli leaves collected from a field of broccoli plants were cut into a circular disc with a diameter of 56 mm. The filter paper and broccoli leaves were replaced every 24 h. One larva was placed in each petri dish. During culture, the dishes were maintained under conditions of 25 °C, 85% humidity and a 16 h light/8 h dark regime [28].
2.3. Test of the Effect of Laser Irradiation on Pieris rapae Larvae
2.3.1. Test Process
In this study, single working parameters (experiment 1) were first tested, and based on the experimental results, further tests were performed using combinations of different working parameters (experiment 2). Furthermore, the working parameters were optimized and verified (experiment 3), and the 1st- to 5th-instar P. rapae larvae were evaluated with the optimal working parameter combination (experiment 4). Each treatment included 20 P. rapae larvae and was replicated three times; a total of 120 1st-instar, 120 2nd-instar, 120 3rd-instar, 3240 4th-instar, and 180 5th-instar larvae were used. The larvae were tested using the following steps:
- Before the test, the working parameters (laser power, irradiation area, laser opening time) were adjusted to the target value by adjusting the laser irradiation device;
- When the experiment was performed, P. rapae larva was placed in a petri dish. After the P. rapae stopped moving, the dish was moved such that the irradiation position of the P. rapae was directly above the intersection of the cross marks;
- The laser was started and stopped after the set time to complete the laser strike. The larvae were not fixed during the test. Then, these larvae were placed on the observation platform after laser irradiation, and normal larvae (i.e., those without laser irradiation) were added as the control group (CK);
- The antifeedant percentage and mortality rate of the larvae after 24 h, 48 h and 72 h were calculated.
2.3.2. Experiment 1: Single Working Parameter Tests
The specific design of the single working parameter tests is shown in Table 1. There is no interaction between laser power, irradiation area, laser opening time and irradiation position. The average head widths of the 3rd-, 4th- and 5th-instar P. rapae larvae are 0.9000 mm, 1.5640 mm, and 2.7380 mm [30], respectively. Therefore, the diameter range of the irradiation area was set to 0.1–3 mm, and the irradiation areas were thus 0.01 mm2, 0.79 mm2, 3.14 mm2 and 7.07 mm2, respectively. Laser irradiation was performed without fixing the larvae, and four different laser opening times (0.5 s, 1 s, 2 s and 3 s) were tested in the tests.

Table 1.
List of working parameters used in 18 groups of single factor laser irradiation experiment.
The body of P. rapae larvae can be divided into the head (sensory and feeding center), thorax (movement center) and abdomen (metabolism and reproduction center) [31]. Therefore, four representative irradiation positions were selected: head, mesothorax, middle and end of the abdomen. The tests were repeated three times, and a total of 1080 4th-instar larvae ((17 + 1(CK)) groups × 20 larvae × 3 replicates = 1080 larvae) were thus used.
2.3.3. Experiment 2: Combinations of Different Working Parameters Tests
Based on the results of the single working parameter tests, the ranges of the four parameters were selected and used to perform tests of various combinations of different working parameters. The four parameters tested in this experiment exerted nonlinear effects on the antifeedant percentage and mortality rate of P. rapae larvae. Therefore, a Box–Behnken design (BBD) was used to perform surface analysis tests with four factors and three levels, as shown in Table 2. The experimental design was created with Design-Expert 10.0.4 (Stat-Ease Inc., Minneapolis, MN, USA), and 29 sets of tests were generated. Therefore, a total of 1800 4th-instar larvae ((29 + 1(CK)) groups × 20 larvae × 3 replicates = 1800 larvae) were thus used.

Table 2.
Combined experiment table based on the experimental results of a single working parameter designed by the Response Surface Methodology (RSM).
After the tests, optimization analysis on response surface was carried out by BBD experimental design method. It identified the optimal parameter combinations by the multiple quadratic regression equation fitting each independent variables and dependent variables.
2.3.4. Dependent Variables
In the test, the antifeedant percentage was used to evaluate the inhibition or hindrance effect of laser irradiation on the feeding behavior of P. rapae larvae. Every 24 h, the feeding areas of the larvae were measured using squared grid paper (1 × 1 mm). The average feeding area of the larvae after 24 h, 48 h and 72 h were calculated. The leaves and filter paper were replaced after every measurement. The following formula was used to calculate the antifeedant percentage (nonselective) [32,33]:
The mortality rate was selected as another indicator of the controlling effect of laser irradiation on larvae. Time measurements were obtained to estimate the antifeedant percentage, the number of dead larvae in each group was counted, and the mortality within 3 days was calculated. The larvae were gently touched with a brush. If no reaction was detected, the larvae were considered dead [34].
To estimate the best working parameter combination, the antifeedant percentage and mortality rate of the larvae were converted into their membership degrees (Wi and Ui, respectively), and a comprehensive score was calculated. The priority was to improve the antifeedant percentage of the larvae. Therefore, the weight of the antifeedant effect should be greater than that of the mortality. According to previous studies [35,36], the weights of the antifeedant percentage and mortality rate were 0.7 and 0.3, respectively, and the comprehensive score (Ki) was used as the evaluation indicator and calculated using the following formula [37]:
where a greater Ki value obtained from the formula indicated a better combination of working parameters.
2.4. Statistical Analysis
The data were statistical analyzed using SPSS Statistics 25.0 software (SPSS, Inc., Chicago, IL, USA). The data for insect antifeedant percentage (%), mortality rate (%) and comprehensive score were statistically investigated using Tukey’s honestly significant difference test and one-way analysis of variance (ANOVA). All percentage values were transformed by arcsine square root to improve the normality and homoscedasticity of data analysis [38], but the untransformed percentage values are shown in the figures and tables. The predicted and adjusted R2 within 0.2 of each other and adequate precision values greater than 4 suggested that the models can be used for navigation in the designed space [39].
3. Results
3.1. Experiment 1: Effects of Single Working Parameters on the Antifeedant Percentage and Mortality Rate of Pieris rapae Larvae
The results from the laser irradiation tests with single working parameters are shown in Figure 2. The mortality of the control group was not adjusted because no larvae died within 72 h. The larvae were collected for tests from 5 October 2019 to 2 April 2020.
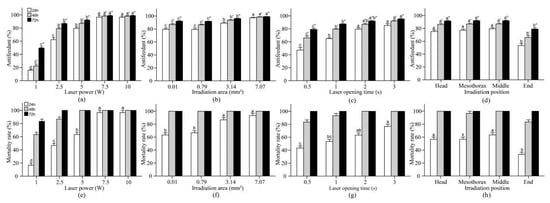
Figure 2.
Antifeedant percentages and mortality rates (Mean ± SE) of 4th-instar P. rapae larvae after 24 h, 48 h and 72 h under varying working parameter tests. We repeated the experiment 3 times of putting 20 P. rapae larvae into the petri dish in turn for laser irradiation and observation. Only one working parameter was tested in each experiment, and the other working parameters were fixed (Table 1). The tested working parameters were laser power (a,e), irradiation area (b,f), laser opening time (c,g) and irradiation position (d,h). Bars with the same letters indicate no significant difference among the tested groups within each observation time (ANOVA followed by Tukey’s honestly significant difference test, p > 0.05), while test between different observation times cannot be compared with each other.
3.1.1. Laser Power
As shown in Figure 2a,e, the antifeedant percentages and mortality rates of P. rapae larvae increased sharply as the laser power increased. All treatments showed significant differences in repellent activity at 24 h (F = 214.66, df = 4, 10, p < 0.001), 48 h (F = 297.28, df = 4, 10, p < 0.001) and 72 h (F = 289.40, df = 4, 10, p < 0.001) (Figure 2a). The mortality rate of 100% was detected 48 h posttreatment with a laser power of 5 W, and the same results were obtained with 7.5 W and 10 W (Figure 2e). However, when the laser power was greater than 7.5 W, no significant differences in the antifeedant percentages after 24 h (F = 0.004, df = 1, 4, p = 0.952), 48 h (F = 0.003, df = 1, 4, p = 0.960) and 72 h (F = 0.001, df = 1, 4, p = 0.971) (7.5 W vs. 10 W) or mortality rates (7.5 W vs. 10 W: after 24 h, Same, all 96.67%; 48 h: Same, all 100%) were detected. The antifeedant percentage (24 h, 15.84%; 48 h, 22.21%; 72 h, 49.56%) and mortality rate (24 h, 16.66%; 48 h, 63.33%; 72 h, 83.33%) obtained with a laser power of 1 W were extremely significantly different from those obtained with the other laser powers (Figure 2a,e). Therefore, the laser power range was adjusted to 1.5–7.5 W, and values within this range were used in the various combinations of different working parameters tested in subsequent tests.
3.1.2. Irradiation Area
This effectiveness of killing larvae using laser irradiation was significant at each 24 h interval (antifeedant percentage: 24 h, F = 22.04, df = 3, 8, p < 0.001; 48 h, F = 33.66, df = 3, 8, p < 0.001; 72 h, F = 35.04, df = 3, 8, p < 0.001) (Figure 2b). If the irradiation area was greater than 0.01 mm2, a mortality rate of 100% was observed 48 h posttreatment (Figure 2f). Moreover, at 24 h posttreatment, the antifeedant percentage and mortality rate obtained with an irradiation area of 0.01 mm2 were 79.14% and 66.67%, respectively. No significant difference was found between irradiation areas of 0.79 mm2 and 0.01 mm2 at all tested posttreatment times (0.79 mm2 vs. 0.01 mm2: antifeedant percentages, 24 h, F = 0.006, df = 1, 4, p = 0.943; 48 h, F = 0.066, df = 1, 4, p = 0.811; 72 h, F = 0.074, df = 1, 4, p = 0.799; mortality rate: 24 h, F = 0.500, df = 1, 4, p = 0.519; 48 h, Same, all 100%). Therefore, the diameter range of the irradiation area was set to 1–3 mm, which indicates that the irradiation area was between 0.79 and 7.07 mm2.
3.1.3. Laser Opening Time
The laser irradiation tests showed that the antifeedant percentages and mortality rates associated with laser opening time increased in an irradiation time-dependent manner (Figure 2c,g). The highest killing effect was observed at 72 h posttreatment with all laser opening times, and antifeedant percentages of 79.12%, 87.42%, 91.99% and 95.07% were detected at 0.5 s, 1 s, 2 s and 3 s, respectively (Figure 2c). A mortality rate of 100% was found at 72 h posttreatment with all laser opening times (Figure 2g). However, 76.67 ± 5.77% of the larvae left the irradiation area within 3 s, which indicated that the laser opening time was too long (data not shown). Moreover, no markedly significant difference was found between the laser opening times of 2 s and 3 s at all posttreatment times (2 s vs. 3 s: antifeedant percentage, 24 h, F = 2.819, df = 1, 4, p = 0.168; 48 h, F = 5.186, df = 1, 4, p = 0.085; 72 h, F = 5.375, df = 1, 4, p = 0.081; mortality rate: 24 h, F = 8.000, df = 1, 4, p = 0.047; 48 h, Same, all 100%). Therefore, the laser opening time range was adjusted to 0.5–2.5 s.
3.1.4. Irradiation Position
As shown in Figure 2d,h, the antifeedant percentages and mortality rates of the irradiation position were significant at each 24 h interval (Antifeedant percentage: 24 h, F = 26.91, df = 3, 8, p < 0.001; 48 h, F = 24.56, df = 3, 8, p < 0.001; 72 h, F = 26.66, df = 3, 8, p < 0.001; mortality rate: 24 h, F = 15.58, df = 3, 8, p = 0.001; 48 h, F = 11.33, df = 3, 8, p = 0.003; 72 h, Same, all 100%). Moreover, the larvae exhibited different reaction times when different body parts were irradiated. The head was the most sensitive, followed by the mesothorax and the middle and end of the abdomen (data not shown). However, the lowest killing effect was found with an irradiation position at the end of the abdomen (antifeedant percentages of 53.24%, 65.92% and 79.00% at 24 h, 48 h and 72 h; mortality rate of 33.33% at 24 h), and this effect was extremely significantly different from that obtained with the other irradiation positions (Figure 2d,h). To ensure the inclusion of three levels of each test factor for the response surface, the irradiation position was set as the head, mesothorax and middle of the abdomen.
3.1.5. Dependent Variables
The results from the single working parameter tests with 17 groups (Figure 2) showed that 11 of the groups exhibited a mortality rate of 100% at 48 h posttreatment, whereas a mortality rate of 100% was found at 72 h posttreatment with all 17 groups. Therefore, only results measured after 24 h were used.
3.2. Experiment 2: Effects of Different Working Parameter Combinations on the Antifeedant Percentage and Mortality Rate of Pieris rapae Larvae
The test results are shown in Table 3. The larvae were collected in the Hunan Province Key Laboratory of Intelligent Agricultural Machinery and Equipment, Changsha, China, from 10 April to 20 June 2020.

Table 3.
Antifeedant percentages and mortality rates (Mean ± SE) of 4th-instar Pieris rapae larvae after 24 h of irradiation in 29 groups of different working parameter combination tests.
Establishment of Regression Model
To simplify the regression equation, the nonsignificant terms based on the original equation were removed, the model was manually optimized, and the second-order polynomial equation is provided below [40,41]. The three-dimensional (3D) contour plot of an equation is the best method for expressing the linear, interaction and quadratic effects of working parameters on the responses, and the third and fourth variables were maintained at the zero level to obtain the 3D response surfaces [42]. The plots of the significant interaction terms for Y1, Y2 and K are illustrated in Figure 3.
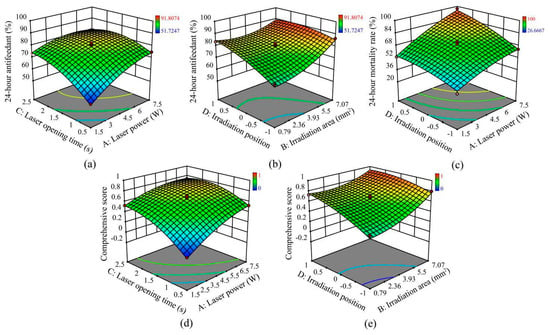
Figure 3.
Response surface plots showing the effects of significant interaction terms on the 24 h antifeedant percentage (a,b), 24 h mortality rate (b) and comprehensive score (d,e). Insets (a,d) show the prediction results of irradiation experiment at laser power of 1.5–7.5 W, irradiation area of 3.93 mm2, laser opening time of 0.5–2.5 s and irradiation position on mesothorax (0). Insets (b,e) show the prediction results of irradiation experiment at laser power of 4.5 W, irradiation area of 0.79–7.07 mm2, laser opening time of 1.5 s and irradiation position on head (−1), mesothorax (0) and middle of abdomen (1). Inset (c) shows the prediction results of irradiation experiment at laser power of 1.5–7.5 W, irradiation area of 3.93 mm2, laser opening time of 1.5 s and irradiation position on head (−1), mesothorax (0) and middle of abdomen (1). Use the red points to indicate those response values that are higher than the predicted value, while orange points mean the opposite.
- 1.
- 24 h antifeedant percentage (%) Y1:
In this case, A, B, C, D, AC, BD, A2, B2, C2 and D2 were found to be significant model terms (p < 0.05). The effect of laser power (A) on the antifeedant percentage was the most significant (F = 96.41, df = 1, 13, p < 0.0001). The interactions AC (laser power and laser opening time; F = 11.53, df = 1, 13, p = 0.0034; Figure 3a) and BD (irradiation area and irradiation position; F = 9.03, df = 1, 13, p = 0.0080; Figure 3b) had a more significant positive impact on the 24 h antifeedant percentage than the other interactions.
The R2 value was found to be 0.9616, which indicated a realistic fit of the model to the experimental data [40]. The predicted R2 (0.9061) and the adjusted R2 (0.9367) values were within 0.2 of each other, whereas adequate precision values were greater than 4, which indicated a satisfactory model fit [39].
- 2.
- 24 h mortality rate (%) Y2:
In this case, A, B, C, D, AD, A2, B2, C2 and D2 were found to be significant model terms (p < 0.001). The laser opening time (C) had the most significant effect on the mortality rate (F = 245.15, df = 1, 14, p < 0.0001). The interaction AD (laser power and irradiation position; F = 5.47, df = 1, 14, p = 0.0311; Figure 3c) had a more significant positive impact on the 24 h mortality rate than the other interactions.
The R2 value was found to equal 0.9777, which indicated a realistic fit of the model to the experimental data [40]. The predicted R2 (0.9467) and the adjusted R2 (0.9653) values were within 0.2 of each other, whereas adequate precision values were greater than 4, which indicated a satisfactory model fit [39].
- 3.
- Comprehensive score K:
A negative sign in front of the factors indicates an inverse relationship between the response of interest and the studied factors, and a positive sign indicates the opposite [39]. Therefore, the results showed that the K values of the working parameters with the linear effects of the variables A, B, C and D and the quadratic variable B2 exhibited a positive effect (Equation (5)). However, the quadratic variables A2, C2 and D2 and the interaction variables AC, BC and BD exerted a negative effect. Moreover, due to the quadratic effects of the variables, both the 24 h antifeedant percentages and the mortality rates of P. rapae larvae also decreased (Equations (3) and (4)).
In this case, A, B, C, D, AC, BD, A2, B2, C2 and D2 were found to be significant model terms (p < 0.05). The effect of laser power (A) on the comprehensive score was the most significant (F = 161.94, df = 1, 13, p < 0.0001). The interactions AC (laser power and laser opening time; F = 9.28, df = 1, 13, p = 0.0073; Figure 3d) and BD (irradiation area and irradiation position; F = 6.66, df = 1, 13, p = 0.0195; Figure 3e) exerted more significant positive impacts on the comprehensive score than the other interactions. The R2 value was found to equal 0.9740, which indicated a realistic fit of the model to the experimental data. This finding also indicated that 97.4% of the variation in response could be elucidated effectively and showed that 2.6% of the variations occurred when the tests were performed [40]. The predicted R2 (0.9241) and the adjusted R2 (0.9573) values were within 0.2 of each other, whereas adequate precision values were greater than 4, which indicated a satisfactory model fit [39].
3.3. Experiment 3: Optimization of Working Parameters and Validation
The derived correlations (Experiment 2) enabled optimization of the working parameters by means of maximization of all responses [39]. The combination of the response analysis and the analysis of the maximal comprehensive score determined the optimal batches with a predictive response, which are presented in Table 4. The larvae were collected in the Hunan Province Key Laboratory of Intelligent Agricultural Machinery and Equipment, Changsha, China, from 27 June to 10 July 2020.

Table 4.
Composition of optimized samples with estimated responses and the cross-validation of the model.
The cross-validation of the model, the estimated responses and the percentage of the relative error of the predicted and experimental values identified the optimal laser power (7.5 W), the optimal irradiation area (6.189 mm2), the optimal laser opening time (1.177 s) and the optimal irradiation position (middle of the abdomen). With the optimal combination of working parameters, the corresponding predicted response values for the 24 h antifeedant percentage and mortality rate of 4th-instar P. rapae larvae were 98.49% and 100%, respectively.
3.4. Experiment 4: Verification Test with 1st- to 5th-Instar Pieris rapae Larvae
In the field environment, 1st- to 5th-instar P. rapae larvae existed simultaneously. Therefore, to verify the universality of the working parameters in controlling the P. rapae larvae under field situations, the optimal combination of parameters was used to irradiate 1st- to 5th-instars. The larvae were collected in the Hunan Province Key Laboratory of Intelligent Agricultural Machinery and Equipment, Changsha, Hunan Province, China, from 13 July to 18 July 2020.
As shown in Figure 4, the 1st- to 3rd-instar larvae showed no significant differences in the antifeedant percentages (24 h, F = 8.00, df = 4, 10, p = 0.004; 48 h, F = 6.01, df = 4, 10, p = 0.010) or mortality rates (12 h, F = 16.50, df = 4, 10, p = 0.0002; 24 h, same, all 100%). The antifeedant percentages and mortality rates of the 5th-instar larvae were lower than those of the 4th-instar larvae, but no markedly significant difference was found between the 4th- and 5th-instar larvae (the antifeedant percentage: 24 h, F = 1.78, df = 1, 4, p = 0.254; 48 h, F = 0.32, df = 1, 4, p = 0.603; mortality rate, 12 h, F = 1.00, df = 1, 4, p = 0.374; 24 h, 100 ± 0.00%, 96.67 ± 3.33%). Moreover, the mortality rate of the 4th- and 5th-instar larvae at 36 h was 100%. Overall, the results showed that the laser test parameters were suitable for 1st- to 5th-instar larvae and that younger larvae exhibited higher antifeedant percentages and mortality rates.
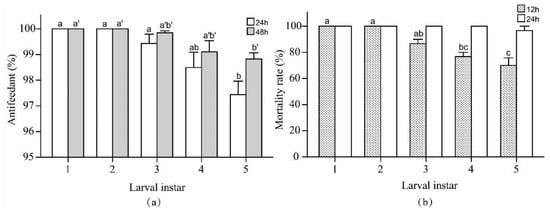
Figure 4.
Antifeedant percentages (a) and mortality rates (b) (Mean ± SE) of 1st- to 5th-instar P. rapae larvae in optimal working parameters tests. Bars with the same letters indicate no significant difference among the tested groups within each observation time (ANOVA followed by Tukey’s honestly significant difference test, p > 0.05), while test between different observation times cannot be compared with each other.
4. Discussion
This research proposes a laser irradiation method for managing P. rapae larvae as an alternative to chemical control. The effectiveness of controlling larvae and the influencing factors of 450 nm lasers were studied. Furthermore, the optimal combination of parameters was estimated. The results indicate the noncontact method of laser irradiation exhibits high efficiency at controlling P. rapae larvae.
It has been shown that many insecticides, such as 1.8% avermectin, 5% chlorobenzamide, 6% spinetoram and some plant extracts [43,44,45,46,47], have been commonly used to control P. rapae larvae in recent years. Most of these insecticides exhibited a pest reduction rate of less than 80% within 3 days after treatment, and the insecticidal effectiveness decreased over time [47]. Moreover, among the 74 plant extracts tested, the most effective plant extract caused an antifeedant percentage of 96.10% after 48 h [43]. After 24 h, the laser-based method used in this research showed the same insecticidal effectiveness on larvae as traditional chemical control for 3 days and the increase in the antifeedant percentage after 48 h reached 99.27%. Thus, the laser-based method for controlling larvae exhibits the merits of avoiding the development of resistance and effectively reducing the harm of pesticide residues in the environment to human beings.
At present, the frequency vibration insecticidal lamp is widely used in physical control methods [48]. The lamp uses the phototaxis of pests to lure pests and then kill them through the power grid. According to the investigation of Ye et al. [49], the lamp can trap and kill 30 species of pests in 5 orders and 17 families and can trap and kill pests in main orders and families in vegetable fields. However, the lamp has the same killing ability for beneficial insects and nontarget insects in the control area, threatening the natural ecological balance [50]. On the other hand, the above methods have a certain trapping and killing effect on adults such as moths but have no effect on larvae directly endangering vegetable crops.
The application of other laser technologies to control pests is mainly aimed at stored grain pests and adult pests such as flies, which cannot move by themselves and need long-term irradiation [25,51]. However, the control method proposed in this study directly affects larvae, and the laser opening time is only 1.117 s. This approach can achieve the target killing of crop pests through a noncontact method. Moreover, the results of the study on P. rapae can be used as a basis for laser control of other Lepidopteran pests. Unified approaches for killing a variety of pests remain to be studied further. As revealed from the mentioned research and the experimental results of the paper, the laser irradiation method exhibits high efficiency at controlling P. rapae larvae. In addition, the results here can provide support for eventually application in the field to replace or compliment insecticides and other pest management techniques. This paper also proposes a physical control method for controlling larvae by laser irradiation. In this method, machine vision is used to accurately identify and locate P. rapae larvae in a field or processing plant, and a laser actuator is then used to perform a precise strike to achieve efficient and pollution-free pest mortality. Presently, Sumesh et al. [52] and our team [53] both designed a laser pest control device based on machine vision. With this device, the researchers examined the strike accuracy of laser and control effect of laser irradiation in the laboratory or field. The achieved result can provide theoretical reference for the design and analysis of laser pest control device.
5. Conclusions
In the study, a physical control method of controlling P. rapae larvae by laser irradiation is proposed. By testing the controlling effect of 450 nm laser on P. rapae larvae and studying the influencing factors of lasers (laser power, irradiation area, laser opening time and irradiation position), the optimal combination of working parameters was obtained to improve the controlling performance of P. rapae larvae.
The main research conclusions were drawn:
- The optimal combination for the maximum comprehensive score was as follows: laser power, 7.5 W; irradiation area, 6.189 mm2; laser opening time, 1.177 s; and irradiation position, middle abdomen.
- The optimal combination identified based on the observations was used to verify the experiment. The results showed that the decrease in the antifeedant percentage of P. rapae larvae after 24 h was 98.49%, whereas the 24 h mortality rate was 100%.
- The combination of the experimental parameters was suitable for 1st- to 5th-instar P. rapae larvae, and the mortality rate of the 5th-instar larvae at 36 h also was 100%.
The results indicate the capacity of laser irradiation to serve as a high-efficiency and noncontact physical control method against P. rapae. Although the high antifeedant percentage and mortality rate are good predictors of field success in laser, the control effect of laser irradiation against P. rapae should be evaluated in the field. The approaches for this technology in processing plants or field remain to be studied further.
Author Contributions
Conceptualization, Y.L. and Y.X.; methodology, Y.L., J.L. and Y.X.; software, Y.L. and Z.H.; validation, Y.X.; investigation, Y.L. and Y.X.; resources, Y.X.; data curation, Y.L., J.L. and Y.X.; writing—origianal draft preparation, Y.L., Z.H. and Y.X.; writing—review and editing, Y.X., Z.Y. and X.H.; visualization, X.H.; supervision, Y.X.; funding acquisition, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Surface of the Natural Science Foundation of Hunan Province of China, grant number “2021JJ30363”, and the Scientific Research Fund of the Hunan Provincial Education Department of China, grant number “19A224”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented in this article in the form of figures and tables.
Acknowledgments
We gratefully acknowledge Mingliag Wu, Ying Xiong and anonymous referees for thoughtful review of this research as well as the assistance of Feiyan Li with statistical analyses. We also thank the Yunyuan Scientific Research Base (Hunan Agricultural University, Changsha) for providing us with insects and broccoli leaves.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ryan, S.F.; Lombaert, E.; Espeset, A.; Vila, R.; Talavera, G.; Dincă, V.; Doellman, M.M.; Renshaw, M.A.; Eng, M.W.; Hornett, E.A.; et al. Global invasion history of the agricultural pest butterfly Pieris rapae revealed with genomics and citizen science. Proc. Natl. Acad. Sci. USA 2019, 116, 20015–20024. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Ren, W.; Kang, Z.; Jiang, J.H.; Zhao, X.J.; Du, L.F. A trypsin inhibitor from Cassia obtusifolia seeds: Isolation, characterization and activity against Pieris rapae. Biotechnol. Lett. 2007, 29, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Mnif, I.; Ghribi, D. Potential of bacterial derived biopesticides in pest management. Crop Prot. 2015, 77, 52–64. [Google Scholar] [CrossRef]
- Liu, J.L.; Wei, X.Q.; Guo, H. Breeding of an L-Valine Producing Strain by Laser Mutation. Chin. J. Laser 2016, 43, 178–184. [Google Scholar] [CrossRef]
- Kaierle, S.; Marx, C.; Rath, T.; Hustedt, M. Find and Irradiate—Lasers Used for Weed Control: Chemical free elimination of unwanted plants. Laser Tech. J. 2013, 10, 44–47. [Google Scholar] [CrossRef]
- Podleśny, J.; Stochmal, A.; Podleśna, A.; Misiak, L.E. Effect of laser light treatment on some biochemical and physiological processes in seeds and seedlings of white lupine and faba bean. Plant Growth Regul. 2012, 67, 227–233. [Google Scholar] [CrossRef]
- Qiu, Z.B.; Li, J.T.; Zhang, M.M.; Bi, Z.Z.; Li, Z.L. He–Ne laser pretreatment protects wheat seedlings against cadmium-induced oxidative stress. Ecotoxicol. Environ. Saf. 2013, 88, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Abou-Dahab, A.D.M.; Mohammed, T.A.; Heikal, A.A.; Taha, L.S.; Gabr, A.M.G.; Metwally, S.A.; Ali, A.I.R. In vitro laser radiation induces mutation and growth in Eustoma grandiflorum plant. Bull. Nat. Res. Cent. 2019, 43, 3. [Google Scholar] [CrossRef]
- Spalding, E.P.; Folta, K.M. Illuminating topics in plant photobiology. Plant Cell Environ. 2005, 28, 39–53. [Google Scholar] [CrossRef]
- Perveen, R.; Jamil, Y.; Ashraf, M.; Ali, Q.; Iqbal, M.; Ahmad, M.R. He–Ne Laser-Induced Improvement in Biochemical, Physiological, Growth and Yield Characteristics in Sunflower (Helianthus annuus L.). Photochem. Photobiol. 2011, 87, 1453–1463. [Google Scholar] [CrossRef]
- Wöltjen, C.; Haferkamp, H.; Rath, T.; Herzog, D. Plant growth depression by selective irradiation of the meristem with CO2 and diode lasers. Biosyst. Eng. 2008, 101, 316–324. [Google Scholar] [CrossRef]
- Wöltjen, C.; Rath, T.; Herzog, D. Investigations about the Technical Basics of Laser Beam Use for Plant Manipulation. Acta Hortic. 2008, 801, 587–594. [Google Scholar] [CrossRef]
- Nasim, H.; Jamil, Y. Diode lasers: From laboratory to industry. Opt. Laser Technol. 2014, 56, 211–222. [Google Scholar] [CrossRef]
- Astatkie, T.; Rifai, M.N.; Havard, P.; Adsett, J.; Lacko-Bartosova, M.; Otepka, P. Effectiveness of hot water, infrared and open flame thermal units for controlling weeds. Biol. Agric. Hortic. 2007, 25, 1–12. [Google Scholar] [CrossRef]
- Heisel, T.; Schou, J.; Christensen, S.; Andreasen, C. Cutting weeds with a CO2 laser. Weed Res. 2001, 41, 19–29. [Google Scholar] [CrossRef]
- Heisel, T.; Schou, J.; Andreasen, C.; Christensen, S. Using laser to measure stem thickness and cut weed stems. Weed Res. 2002, 42, 242–248. [Google Scholar] [CrossRef]
- Coleman, G.R.Y.; Stead, A.; Rigter, M.P.; Xu, Z.; Johnson, D.; Brooker, G.M.; Sukkarieh, S.; Walsh, M.J. Using energy requirements to compare the suitability of alternative methods for broadcast and site-specific weed control. Weed Res. 2019, 33, 633–650. [Google Scholar] [CrossRef]
- Cornwell, P.B. The Entomology of Radiation Disinfestation of Grain, 1st ed.; Pergamon Press: London, UK, 1969; pp. 47–52. [Google Scholar]
- Ramos Elorduy de Conconi, J.; Elorduy, C.; Oxley, T.; Barry, S. Laser light as a new potential method for pest control in preserved foods. In Biodeterioration 5, Proceedings of the 5th International Biodeterioration Symposium, Aberdeen, UK, 7–11 September 1981; Oxley, T.A., Barry, S., Eds.; Wiley: Chichester, NH, USA, 1983; pp. 592–608. [Google Scholar]
- He, Y.; Zhang, K.; Xiao, B.; Hou, T.P. Study on the Antifeedant Activity and Mechanism for Bioactive Compound from Stellera chamaejasme against Larvae of Pieris rapae. Chin. J. Biol. Control 2006, 22, 33–37. [Google Scholar] [CrossRef]
- Neimz, M.H. Laser-Tissue Interactions: Fundamentals and Applications, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Obayashi, K.; Sato, K.; Ito, N.; Wang, X.L.; Takagi, S. Physical pest control of drosophila using laser, 1: Effects of laser emissions on pest. J. Jpn. Soc. Agric. Mach. 2006, 67, 93–100. [Google Scholar] [CrossRef]
- Faruki, S.I.; Das, D.R.; Khan, A.R.; Khatun, M. Effects of ultraviolet (254 nm) irradiation on egg hatching and adult emergence of the flour beetles, Tribolium castaneum, T. confusum and the almond moth, Cadra cautella. J. Insect Sci. 2007, 7, 36. [Google Scholar] [CrossRef]
- Keller, M.D.; Leahy, D.J.; Norton, B.J.; Mullen, E.R.; Marvit, M.; Makagon, A. Laser induced mortality of Anopheles stephensi mosquitoes. Sci. Rep. 2016, 6, 20936. [Google Scholar] [CrossRef]
- Sorungbe, A.A.; Badmus, H.A.; Sulaimon, A.M. Effect of ultraviolet irradiation on egg hatching of tropical warehouse moth (Ephestia cautella), development of its adult and mortality. Int. J. Res. Pharm. Biosci. 2016, 3, 23–27. [Google Scholar]
- Mathiassen, S.K.; Bak, T.; Christensen, S.; Kudsk, P. The effect of laser treatment as a weed control method. Biosyst. Eng. 2006, 95, 497–505. [Google Scholar] [CrossRef]
- Ai, S.R.; Yao, M.Y.; Huang, L.; Wu, R.M. Analyzeand Compare the Thermal Effect on Locusts and Host Plants Tissue by Semiconductor Laser Irradiation. Appl. Laser 2010, 30, 236–239. [Google Scholar] [CrossRef]
- Ma, X.L.; Zhu, Z.; Li, Y.; Yang, G.D.; Pei, Y.X. Expressing a modified cowpea trypsin inhibitor gene to increase insect tolerance against Pieris rapae in Chinese cabbage. Hortic. Environ. Biotechnol. 2017, 58, 195–202. [Google Scholar] [CrossRef]
- Hao, C.; Fan, X. Breeding Pieris rapae L. as Experinent Insect and Observation of Feeding Activity of the Larvae. J. Shanxi Agric. Univ. 1998, 18, 30–32. [Google Scholar]
- Chen, Y.N.; Ma, J. Study on the larva age markers of three important vegetable pests. J. Changjiang Veg. 1994, 2, 17–18. [Google Scholar]
- Powell, J.A. Lepidoptera. In Encyclopedia of Insects, 1st ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: New York, NY, USA, 2009; pp. 559–586. [Google Scholar]
- Pan, L.; Ren, L.; Chen, F.; Feng, Y.Q.; Luo, Y.Q. Antifeedant activity of Ginkgo biloba secondary metabolites against Hyphantria cunea larvae: Mechanisms and applications. PLoS ONE 2016, 11, e0155682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Liu, Z.Q.; Lei, F.J.; Fu, J.F.; Zhang, X.X.; Ma, W.L.; Zhang, L.X. Antifeedant and oviposition-deterring activity of total ginsenosides against Pieris rapae. Saudi J. Biol. Sci. 2017, 24, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Fan, Y.; Jing, B.N.; Yu, L.Q.; Wang, W.; Wang, D.D.; Zhao, T.Z. The synergistic effect of natural Celastrus angulatus and natural Vitex negundo on Pieris rapae and Ectropis oblique hypulina. Jiangsu Agri. Sci. 2018, 46, 63–66. [Google Scholar] [CrossRef]
- Zeng, W.A.; Tan, J.C.; Tan, L.; Chen, J.Z. Biological activities of crude extracts from Polygonum hydropiper L. against Pieris rapae L. J. Hunan Agric. Univ. (Nat. Sci.) 2007, 33, 76–78. [Google Scholar] [CrossRef]
- Li, Y.Y.; Hu, C.R. Experiment Design and Data Processing, 3rd ed.; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
- Xie, F.P.; Liu, M.Z.; Yang, M.M.; Liu, D.W.; Wang, X.S.; Ren, S.G. Design of ordered fertilizer device for bagged slow-release fertilizer. Trans. Chin. Soc. Agric. Eng. 2019, 35, 40–49. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Hadzieva, J.; Mladenovska, K.; Simonoska, C.M.; Glavaš, D.M.; Dimchevska, S.; Geškovski, N.; Grozdanov, A.; Popovski, E.; Petruševski, G.; Chachorovska, M.; et al. Lactobacillus casei encapsulated in soy protein isolate and alginate microparticles prepared by spray drying. Food Technol. Biotechnol. 2017, 55, 173–186. [Google Scholar] [CrossRef]
- Ellis, K.; Silvestrini, R.; Varela, B.; Alharbi, N.; Hailstone, R. Modeling setting time and compressive strength in sodium carbonate activated blast furnace slag mortars using statistical mixture design. Cem. Concr. Compos. 2016, 74, 1–6. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Das, N. Process optimization for microencapsulation of probiotic yeasts. Front. Biol. 2018, 13, 197–207. [Google Scholar] [CrossRef]
- Madhumita, M.; Guha, P.; Nag, A. Optimization of the exhaustive hydrodistillation method in the recovery of essential oil from fresh and cured betel leaves (Piper betle L.) using the Box–Behnken design. J. Food Process. Preserv. 2019, 43, e14196. [Google Scholar] [CrossRef]
- Gao, R.; Gao, C.; Tian, X.; Yu, X.Y.; Di, X.D.; Xiao, H.; Zhang, X. Insecticidal activity of deoxypodophyllotoxin, isolated from Juniperus sabina L., and related lignans against larvae of Pieris rapae L. Pest Manag. Sci. 2004, 60, 1131–1136. [Google Scholar] [CrossRef]
- Zeng, T.; Li, L.F.; Wei, D.W.; Chen, H.S.; Liu, Y. Antifeeding activity of some plant extracts against the larvae of Pieris rapae. J. Guangxi Agric. Biol. Sci. 2006, 1, 38–42. [Google Scholar]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. The effects of Artemisia annua L. and Achillea millefolium L. crude leaf extracts on the toxicity, development, feeding efficiency and chemical activities of small cabbage Pieris rapae L. (Lepidoptera: Pieridae). Pestic. Biochem. Physiol. 2011, 99, 244–249. [Google Scholar] [CrossRef]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. Effect of milk thistle, Silybium marianum, extract on toxicity, development, nutrition, and enzyme activities of the small white butterfly, Pieris rapae. J. Insect Sci. 2013, 13, 146. [Google Scholar] [CrossRef][Green Version]
- Wang, P.; Lu, Y.; Dong, J.; Jing, L.; Yuan, Z.Q.; Yang, J.G.; Qiao, Y. Control Effect of 13 Pesticides on Pieris rapae in the Cauliflower Field. Agrochemicals 2017, 56, 300–302. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.M.; Wei, X.; Wei, Z.L. Control Effect of Frequency Vibrating Insecticidal Lamp on Sugarcane Pest in Laibin Sugarcane Area. J. Anhui Agric. Sci. 2018, 46, 126–127, 151. [Google Scholar] [CrossRef]
- Ye, S.G.; Xu, F.C.; Wu, Y.H.; Chen, Z.L. Control effect of frequency vibrating insecticidal lamp on pests in vegetable field. Plant Prot. 2000, 26, 45–46. [Google Scholar]
- Zhang, G.X.; Zheng, G.; Li, X.J.; Bu, J. Application of frequency vibrating insecticidal lamp from the perspective of biodiversity protection. Entomol. Knowl. 2004, 41, 532–535. [Google Scholar]
- Ren, K.; Tu, K.; Li, H.W. Control Effects of Semiconductor Laser on Drosophila Melanogaster. Chin. J. Laser 2006, 33, 1148–1152. [Google Scholar]
- Sumesh, N.; Chang, C.; Hsu, F.; Su, C.; Chen, S. Rapid laser pest control system with 3D small object detection. In Proceedings of the International Society for Optical Engineering (SPIE 11299)—AI and Optical Data Sciences, San Francisco, CA, USA, 25 March 2020. [Google Scholar] [CrossRef]
- Xiang, Y.; Lin, J.W.; Li, Y.J.; Xiong, Y.; Hu, Z.F.; Chen, Y.Q. A Laser Pest Control Robot Based on Machine Vision. CN. Patent 209,473,426 U, 11 October 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).