Biological Diabrotica Management and Monitoring of Metarhizium Diversity in Austrian Maize Fields Following Mass Application of the Entomopathogen Metarhizium brunneum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sites and Cultivation
2.2. Treatment with M. brunneum
2.3. Assessment of Metarhizium spp. Abundance in the Field
2.4. Evaluation of the Control Efficacy of the Entomopathogen M. brunneum
2.5. Genetic Identification of Metarhizium spp. Isolates
2.6. Data Analysis
3. Results
3.1. Evaluation of Metarhizium spp. Abundance
3.2. Pest Abundance and Plant Injury
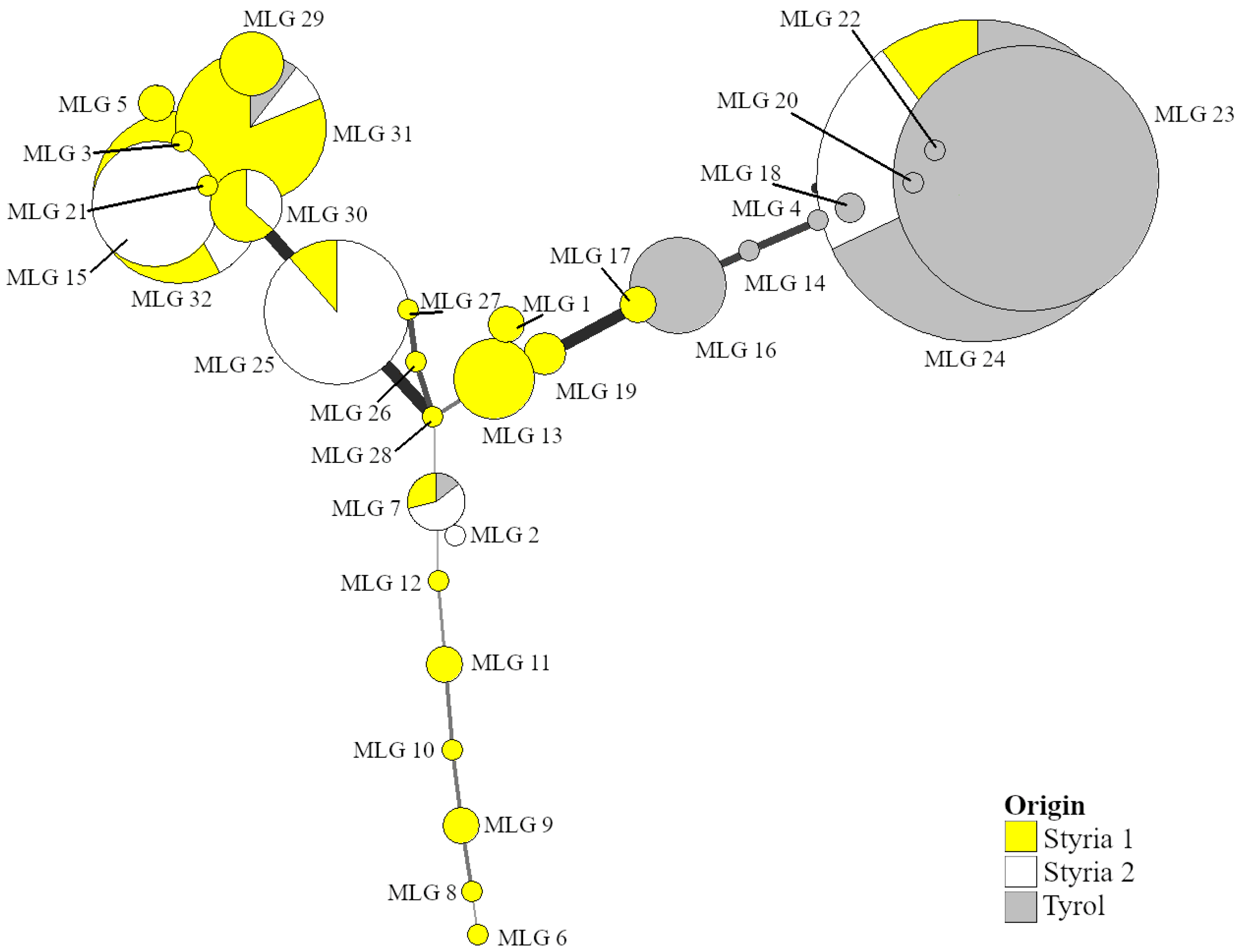
3.3. Metarhizium Genotyping
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rauch, H.; Steinwender, B.M.; Mayerhofer, J.; Sigsgaard, L.; Eilenberg, J.; Enkerli, J.; Zelger, R.; Strasser, H. Field efficacy of Heterorhabditis bacteriophora (Nematoda: Heterorhabditidae), Metarhizium brunneum (Hypocreales: Clavicipitaceae), and chemical insecticide combinations for Diabrotica virgifera virgifera larval management. Biol. Control 2017, 107, 1–10. [Google Scholar] [CrossRef]
- Petzold-Maxwell, J.L.; Jaronski, S.T.; Clifton, E.H.; Dunbar, M.W.; Jackson, M.A.; Gassmann, A.J. Interactions among Bt maize, entomopathogens, and rootworm species (Coleoptera: Chrysomelidae) in the field: Effects on survival, yield, and root injury. J. Econ. Entomol. 2013, 106, 622–632. [Google Scholar] [CrossRef] [Green Version]
- Meissle, M.; Pilz, C.; Romeis, J. Susceptibility of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) to the entomopathogenic fungus Metarhizium anisopliae when feeding on Bacillus thuringiensis Cry3Bb1-expressing maize. Appl. Environ. Microbiol. 2009, 75, 3937–3943. [Google Scholar] [CrossRef] [Green Version]
- Hajek, A.; Eilenberg, J. Augmentation: Inundative and inoculative biological control. In Natural Enemies: An Introduction to Biological Control, 2nd ed.; Cambridge University Press: Cambridge, UK, 2018; pp. 66–84. [Google Scholar]
- Köhl, J.; Booij, K.; Kolnaar, R.; Ravensberg, W.J. Ecological arguments to reconsider data requirements regarding the environmental fate of microbial biocontrol agents in the registration procedure in the European Union. BioControl 2019, 64, 469–487. [Google Scholar] [CrossRef] [Green Version]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Inglis, G.D.; Goettel, M.S.; Butt, T.M.; Strasser, H. Use of hyphomycetous fungi for managing insect pest. In Fungi as Bicontrol Agents: Progress, Problems and Potential; Butt, T.M., Jackson, C., Magan, N., Eds.; CABI: Wallingford, UK, 2001; pp. 23–69. [Google Scholar]
- Scheepmaker, J.W.A.; Butt, T.M. Natural and released inoculum levels of entomopathogenic fungal biocontrol agents in soil in relation to risk assessment and in accordance with EU regulations. Biocontrol Sci. Technol. 2010, 20, 503–552. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Eckard, S.; Hartmann, M.; Grabenweger, G.; Widmer, F.; Leuchtmann, A.; Enkerli, J. Assessing effects of the entomopathogenic fungus Metarhizium brunneum on soil microbial communities in Agriotes spp. biological pest control. FEMS Microbiol. Ecol. 2017, 93, 1–15. [Google Scholar] [CrossRef]
- Kepler, R.M.; Ugine, T.A.; Maul, J.E.; Cavigelli, M.A.; Rehner, S.A. Community composition and population genetics of insect pathogenic fungi in the genus Metarhizium from soils of a long-term agricultural research system. Environ. Microbiol. 2015, 17, 2791–2804. [Google Scholar] [CrossRef]
- Glare, T.R.; Scholte Op Reimer, Y.; Cummings, N.; Rivas-Franco, F.; Nelson, T.L.; Zimmermann, G. Diversity of the insect pathogenic fungi in the genus Metarhizium in New Zealand. New Zealand J. Bot. 2021, 59, 440–456. [Google Scholar] [CrossRef]
- Fernández-Bravo, M.; Gschwend, F.; Mayerhofer, J.; Hug, A.; Widmer, F.; Enkerli, J. Land-use type drives soil population structures of the entomopathogenic fungal genus Metarhizium. Microorg. 2021, 9, 1380. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Rauch, H.; Hartmann, M.; Widmer, F.; Gschwend, F.; Strasser, H.; Leuchtmann, A.; Enkerli, J. Response of soil microbial communities to the application of a formulated Metarhizium brunneum biocontrol strain. Biocontrol Sci. Technol. 2019, 29, 547–564. [Google Scholar] [CrossRef]
- Längle, T.; Pernfuss, B.; Seger, C.; Strasser, H. Field efficacy evaluation of Beauveria brongniartii against Melolontha melolontha in potato cultures. Sydowia 2005, 57, 54–93. [Google Scholar]
- Rauch, H.; Zelger, R.; Strasser, H. Highly efficient field emergence trap for quantitative adult western corn rootworm monitoring. J. Kans. Entomol. Soc. 2016, 89, 256–266. [Google Scholar] [CrossRef]
- Meier, U. Growth stages of mono and dicotyledonous plants. In BBCH monograph; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- Kepler, R.M.; Chen, Y.; Kilcrease, J.; Shao, J.; Rehner, S.A. Independent origins of diploidy in Metarhizium. Mycologia 2014, 108, 1091–1103. [Google Scholar]
- Mayerhofer, J.; Lutz, A.; Widmer, F.; Rehner, S.A.; Leuchtmann, A.; Enkerli, J. Multiplexed microsatellite markers for seven Metarhizium species. J. Invertebr. Pathol. 2015, 132, 132–134. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530. [Google Scholar] [CrossRef] [Green Version]
- Rehner, S.A. Genetic structure of Metarhizium species in western USA: Finite populations composed of divergent clonal lineages with limited evidence for recent recombination. J. Invertebr. Pathol. 2020, 177, 107491. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kumar, V.; Kakkar, G.; Seal, D.R.; McKenzie, C.L.; Osborne, L.S. Evaluation of insecticides for curative, preventive, and rotational use on Scirtothrips dorsalis Sout Asia 1 (Thysanoptera: Thripidae). Fla Entomol. 2018, 100, 634–646. [Google Scholar] [CrossRef] [Green Version]
- Pilz, C.; Keller, S.; Kuhlmann, U.; Töpfer, S. Comparative efficacy assessment of fungi, nematodes and insecticides to control western corn rootworm larvae in maize. BioControl 2009, 54, 671–684. [Google Scholar] [CrossRef]
- Töpfer, S.; Haye, T.; Erlandson, M.; Goettel, M.; Lundgren, J.; Kleespies, R.G.; Weber, D.; Jackson, J.; Peters, A.; Cabrera Walsh, G.; et al. A review of the natural enemies of beetles in the subtribe Diabroticina (Coleoptera: Chrysomelidaer): Implications for sustainable pest management. Biocontrol. Sci. Technol. 2009, 19, 1–65. [Google Scholar] [CrossRef]
- Benjamin, E.O.; Grabenweger, G.; Strasser, H.; Wesseler, J. The socioeconomic benefits of biological control of western corn rootworm Diabrotica virgifera virgifera and wireworms Agriotes spp. in maize and potatoes for selected European countries. J. Plant Dis. Prot. 2018, 125, 273–285. [Google Scholar] [CrossRef]
- Szalai, M.; Papp Komáromi, J.; Bazok, R.; Igrc Barcic, J.; Kiss, J.; Töpfer, S. Generational growth rate estimates of Diabrotica virgifera virgifera populations (Coleoptera: Chrysomelidae). J. Pest Sci. 2011, 84, 133–142. [Google Scholar] [CrossRef]
- Töpfer, S.; Zellner, M.; Kuhlmann, U. Food and oviposition preferences of Diabrotica v. virgifera in multiple-choice crop habitat situations. Entomologia 2013, 1, 60–68. [Google Scholar] [CrossRef]
- Sivčev, J.; Kljajić, P.; Kostić, M.; Sivčev, L.; Stanković, S. Management of western corn rootworm (Diabrotica virgifera virgifera). Pestic. Phytomed. 2012, 27, 189–201. [Google Scholar] [CrossRef]
- Stamm, D.E.; Mayo, Z.B.; Campbell, J.B.; Witkowki, J.F.; Andersen, L.W.; Kozub, R. Western corn rootworm (Coleoptera, Chrysomelidae) beetle counts as a means of making larval control recommendations in Nebraska. J. Econ. Entomol. 1985, 78, 794–798. [Google Scholar] [CrossRef]
- Jaber, L.R.; Enkerli, J. Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol. Control 2016, 103, 187–195. [Google Scholar] [CrossRef]
- Liao, X.; O’ Brien, T.R.; Fang, W.; St. Leger, R.J. The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl. Microbiol. Biotechnol. 2014, 98, 7089–7096. [Google Scholar] [CrossRef]
- Ahmad, I.; Jiménez-Gasco, M.; Luthe, D.S.; Shakeel, S.N.; Barbercheck, M.E. Endophytic Metarhizium robertsii promotes maize growth, supresses insect growth, and alters plant defense gene expression. Biol. Control 2020, 144, 104167. [Google Scholar] [CrossRef]
- Hu, S.; Bidochka, M.J. Root colonization by endophytic insect pathogenic fungi. J. Appl. Microbiol. 2021, 130, 570–581. [Google Scholar] [CrossRef]
- Bidochka, M.; Small, C. Phylogeopgraphy of Metarhizium, an insect pathogenic fungus. In Insect-Fungal Associations: Ecology and Evolution; Vega, F.E., Blackwell, M., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 28–50. [Google Scholar]
- Klingen, I.; Eilenberg, J.; Meadow, R. Effects of farming system, field margins and bait insect on the occurrence of insect pathogenic fungi in soils. Agric. Ecosyst. Environ. 2002, 91, 191–198. [Google Scholar] [CrossRef]
- Wyrebek, M.; Huber, C.; Sasan, R.K.; Bidochka, M.J. Three sympatrically occurring species of Metarhizium show plant rhizosphere specificity. Microbiology 2011, 157, 2904–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinwender, B.M.; Enkerli, J.; Widmer, F.; Eilenberg, J.; Thorup- Kristensen, K.; Meyling, N.V. Molecular diversity of the entomopathogenic fungal Metarhizium community within an agroecosystem. J. Invertebr. Pathol. 2014, 123, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Kepler, R.M. Species limits, phylogeography and reproductive mode in the Metarhizium anisopliae complex. J. Invertebr. Pathol. 2017, 148, 60–66. [Google Scholar] [CrossRef]
- Nishi, O.; Hasegawa, K.; Iiyama, K.; Yasunaga-Aoki, C.; Shimizu, S. Phylogenetic analysis of Metarhizium spp. isolated from soil in Japan. Appl. Entomol. Zool. 2011, 46, 301–309. [Google Scholar] [CrossRef]
- Rocha, L.F.; Inglis, P.W.; Humber, R.A.; Kipnis, A.; Luz, C. Occurrence of Metarhizium spp. in central Brazilian soils. J. Basic Microbiol. 2013, 53, 251–259. [Google Scholar] [CrossRef]
- Rezende, J.M.; Zanardo, A.B.R.; da Silva Lopes, M.; Delalibera, I.; Rehner, S.A. Phylogenetic diversity of Brazilian Metarhizium associated with sugarcane agriculture. BioControl 2015, 60, 495–505. [Google Scholar] [CrossRef]
- Keyser, C.A.; de Fine, H.H.; Steinwender, B.M.; Meyling, N.V. Diversity within the entomopathogenic fungal species Metarhizium flavoviride associated with agricultural crops in Denmark. BMC Microbiol. 2015, 15, 249. [Google Scholar] [CrossRef] [Green Version]
- Bidochka, M.; Kamp, A.M.; Lavender, T.M.; Dekoning, J.; de Croos, J.N.A. Habitat association in two genetic groups in the insect- pathogenic fungus Metarhizium anisopliae: Uncovering cryptic species? Appl. Environ. Microbiol. 2001, 67, 1335–1342. [Google Scholar] [CrossRef] [Green Version]
- Kryukov, V.; Yaroslavtseva, O.; Tyurin, M.; Akhanaev, Y.; Elisaphenko, E.; Wen, T.C.; Tomilova, O.; Tokarev, Y.; Glupov, V. Ecological preferences of Metarhizium spp. from Russia and neighboring territories and their activity against Colorado potato beetle larvae. J. Invertebr. Pathol. 2017, 149, 1–7. [Google Scholar] [CrossRef]
- Daoust, R.A.; Roberts, D.W. Studies on the prolonged storage of Metarhizium anisopliae conidia: Effect of temperature and relative humidity on conidial viability and virulence against mosquitoes. J. Invertebr. Pathol. 1983, 41, 143–150. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Pilz, C.; Enkerli, J.; Wegensteiner, R.; Keller, S. Establishment and persistence of the entomopathogenic fungus Metarhizium anisopliae in maize fields. J. Appl. Entomol. 2011, 135, 393–403. [Google Scholar] [CrossRef]
- Vidal, S.; Georg-August-University Göttingen, Göttingen, Lower Saxony, Germany. Personal communication. 2015. [Google Scholar]
- Strasser, H.; Rauch, H.; Zelger, R. Biological control of adult Diabrotica spray experiments with Metarhizium brunneum strain BIPESCO 5 under real farm conditions. In Microbial and Nematode Control of Invertebrate Pests, Proceedings of the 16th Meeting IOBC, Tiblisi, Georgia, 11–15 June 2017; Tarasco, E., Jehle, J.A., Burjanadze, M., Ruiu, L., Puza, V., Quesada-Moraga, E., Lopes-Ferber, M., Stephan, D., Eds.; IOBC: Darmstadt, Germany, 2017; Volume 129, pp. 84–87. [Google Scholar]




| Bad Radkersburg (Styria) | Oberndorf/ St. Johann I. T. (Tyrol) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Mean N° beetles total | Min beetles per week | Max beetles per week | Increase to previous year | cw of monitoring | cw of max catching | Mean N° beetles total | Minbeetles per week | Max beetles per week | Increase to previous year | cw of monitoring | cw of max catching |
| 2016 | x | <250 | >250 | - | 27/41 | - | 260 | 0 | 226 | - | 27/38 | 36 |
| 2017 | 4429 | 0 | 980 | - | 27/38 | 36 | 749 | 0 | 549 | 2.88 | 27/37 | 31 |
| 2018 | 7336 | 0 | 1488 | 1.66 | 27/38 | 36 | 1008 | 0 | 475 | 1.35 | 27/38 | 32 |
| 2019 | 4588 | 10 | 1041 | 0.63 | 27/38 | 36 | 753 | 0 | 250 | 0.75 | 28/40 | 37 |
| 2020 | 7023 | 72 | 1193 | 1.53 | 27/38 | 37 | y | - | - | - | - | - |
| BIPESCO 5 | M. Brunneum | M. Robertsii | M. Lepidiotae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Origin | Year | N | N | MLG | SG | N | MLG | SG | N | MLG | SG |
| Styria 1 | 2016 | 0 | 25 | 1 | 0 | 47 | 7 | 2 | 2 | 2 | 2 |
| Styria 1 | 2017 | 15 | 10 | 1 | 0 | 34 | 8 | 3 | 13 | 8 | 5 |
| Styria 1 | 2018 | 0 | 8 | 2 | 0 | 16 | 3 | 2 | 1 | 1 | 1 |
| Styria 2 | 2016 | 11 | 28 | 2 | 0 | 33 | 4 | 0 | 0 | 0 | 0 |
| Styria 2 | 2017 | 24 | 29 | 2 | 0 | 19 | 4 | 3 | 0 | 0 | 0 |
| Styria 2 | 2018 | 12 | 3 | 1 | 0 | 11 | 1 | 1 | 0 | 0 | 0 |
| Tyrol | 2016 | 20 | 130 | 5 | 3 | 5 | 1 | 0 | 0 | 0 | 0 |
| Tyrol | 2017 | 101 | 42 | 5 | 3 | 1 | 1 | 1 | 0 | 0 | 0 |
| Tyrol | 2018 | 30 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zottele, M.; Mayerhofer, J.; Embleton, H.; Wechselberger, K.; Enkerli, J.; Strasser, H. Biological Diabrotica Management and Monitoring of Metarhizium Diversity in Austrian Maize Fields Following Mass Application of the Entomopathogen Metarhizium brunneum. Appl. Sci. 2021, 11, 9445. https://doi.org/10.3390/app11209445
Zottele M, Mayerhofer J, Embleton H, Wechselberger K, Enkerli J, Strasser H. Biological Diabrotica Management and Monitoring of Metarhizium Diversity in Austrian Maize Fields Following Mass Application of the Entomopathogen Metarhizium brunneum. Applied Sciences. 2021; 11(20):9445. https://doi.org/10.3390/app11209445
Chicago/Turabian StyleZottele, Maria, Johanna Mayerhofer, Hannah Embleton, Katharina Wechselberger, Jürg Enkerli, and Hermann Strasser. 2021. "Biological Diabrotica Management and Monitoring of Metarhizium Diversity in Austrian Maize Fields Following Mass Application of the Entomopathogen Metarhizium brunneum" Applied Sciences 11, no. 20: 9445. https://doi.org/10.3390/app11209445
APA StyleZottele, M., Mayerhofer, J., Embleton, H., Wechselberger, K., Enkerli, J., & Strasser, H. (2021). Biological Diabrotica Management and Monitoring of Metarhizium Diversity in Austrian Maize Fields Following Mass Application of the Entomopathogen Metarhizium brunneum. Applied Sciences, 11(20), 9445. https://doi.org/10.3390/app11209445






