Nanoscale Topographies for Corneal Endothelial Regeneration
Abstract
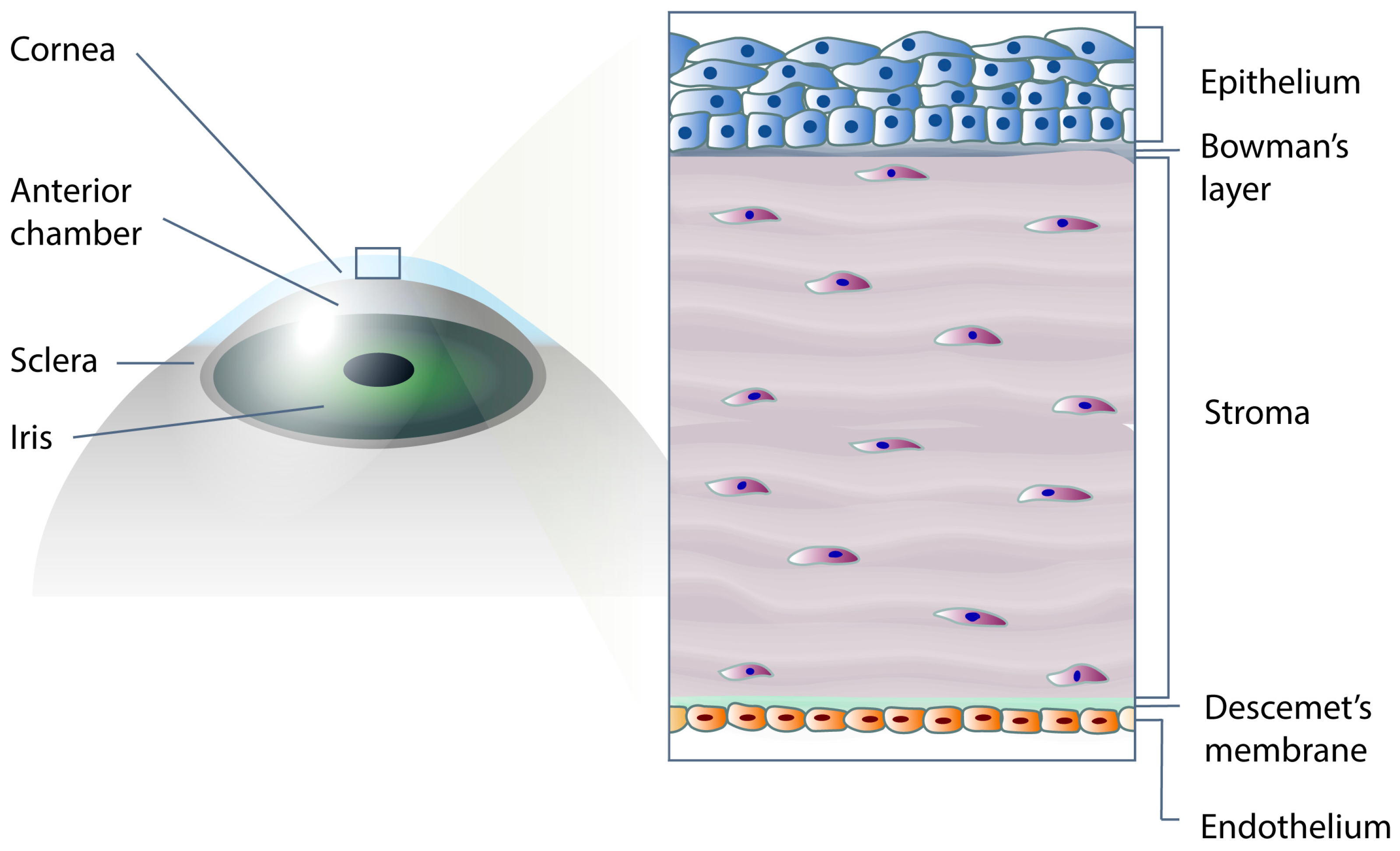
1. Introduction
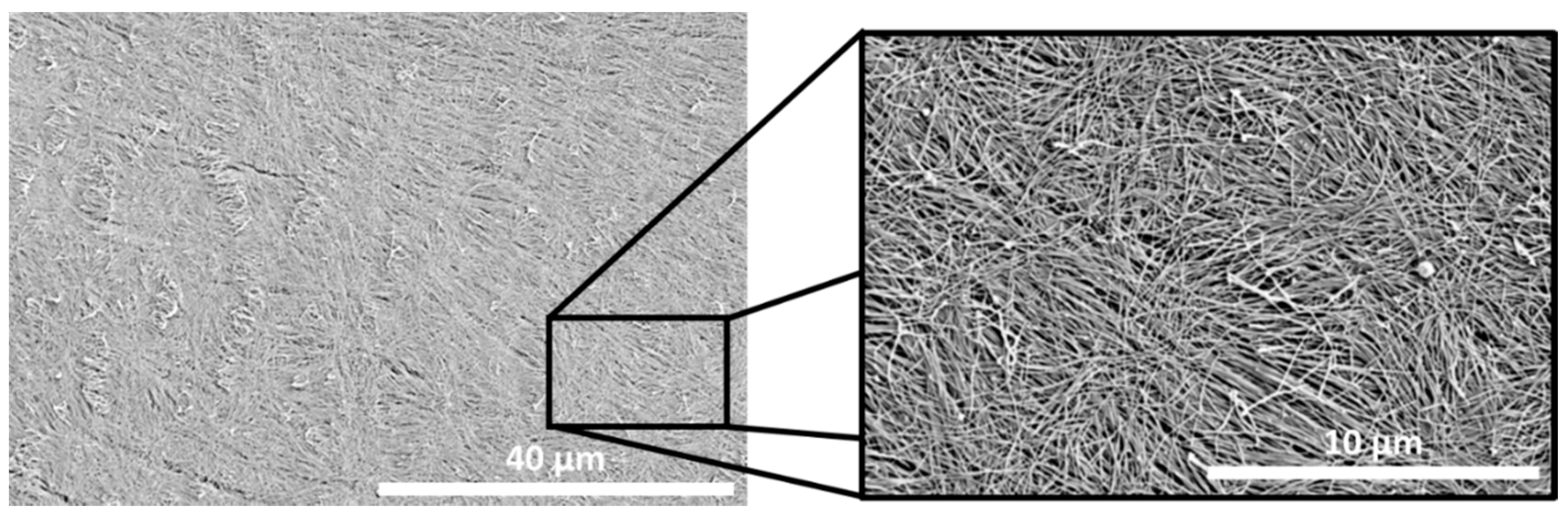
2. Nanofabrication Techniques to Design Instructive Cell Substrates
| Fabrication Method | Feature Dimension (nm) | Cell Type | Reference |
|---|---|---|---|
| Electron beam lithography and solvent casting | Width: 20 nm–1 μm Depth: 5–350 nm | Rat dermal fibroblasts | [60] |
| Electron beam lithography and hot embossing | Diameter: 35–120 nm | Primary human fibroblasts | [61] |
| Diameter: 120 nm Interval: 300 nm Height: 100 nm | Human osteoprogenitor and mesenchymal stem cells | [62] | |
| Diameter: 120 nm Interval: 300 nm Depth: 100 nm | Human mesenchymal stem cells | [63] | |
| Diameter: 35–120 nm Interval: 100–300 nm | Human fibroblasts and rat epitenon | [64] | |
| Photolithography and soft lithography | Diameter: 1 μm Interval: 600 nm Height: 800 nm | Mouse 3T3 fibroblasts | [65] |
| Interference lithography and deep reactive ion etching | Pitch: 230 nm Height: 50–600 nm | Human foreskin fibroblasts | [66] |
| Interference lithography and nanoimprint lithography | Width: 200 nm Interval: 700 nm | Human osteoblasts | [67] |
| Nanoimprint lithography | Pitch: 420–800 nm Height: 0–350 nm | Murine preosteoblasts | [68] |
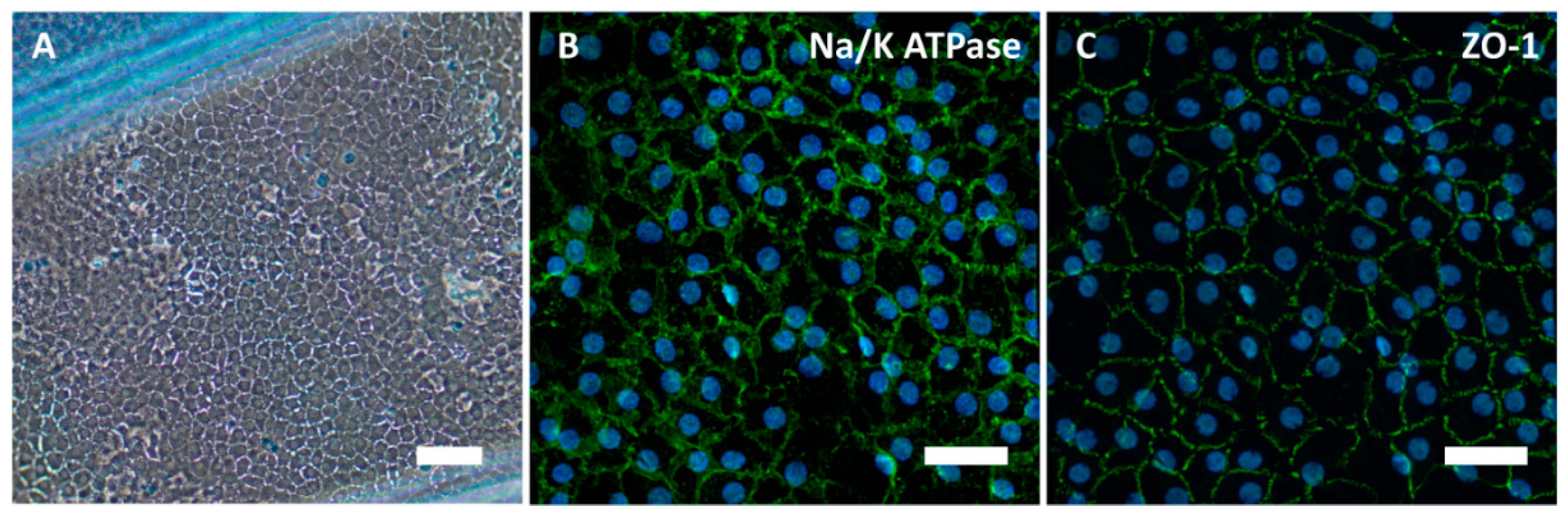
3. The Impact of Bio-Instructive Nanosubstrates for Culture on Corneal Endothelial Cells
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, S.; Alió, J.L.; Pérez-Santonja, J.J. Refractive Index Change in Bovine and Human Corneal Stroma before and after LASIK: A Study of Untreated and Re-Treated Corneas Implicating Stromal Hydration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Wilson, S.E. Descemet’s Membrane Development, Structure, Function and Regeneration. Exp. Eye Res. 2020, 197, 108090. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Raghunathan, V.K.; Li, J.Y.; Murphy, C.J.; Thomasy, S.M. Biomechanical Relationships between the Corneal Endothelium and Descemet’s Membrane. Exp. Eye Res. 2016, 152, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Lwigale, P.Y. Corneal Development: Different Cells from a Common Progenitor, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 134, pp. 43–59. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative Capacity of Corneal Endothelial Cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W. Corneal Endothelial Dysfunction: Evolving Understanding and Treatment Options. Prog. Retin. Eye Res. 2020. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Amano, S. Corneal Endothelial Regeneration and Tissue Engineering. Prog. Retin. Eye Res. 2013, 35, 1–17. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Beuerman, R.W.; Colman, A.; Tan, D.T.; Mehta, J.S. Human Corneal Endothelial Cell Expansion for Corneal Endothelium Transplantation: An Overview. Transplantation 2011, 91, 811–819. [Google Scholar] [CrossRef]
- Roy, O.; Leclerc, V.B.; Bourget, J.M.; Thériault, M.; Proulx, S. Understanding the Process of Corneal Endothelial Morphological Change in Vitro. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1228–1237. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Chng, Z.; Ang, H.P.; Cheng, T.Y.D.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.P.; Tan, D.T.; Yam, G.H.F.; et al. Propagation of Human Corneal Endothelial Cells: A Novel Dual Media Approach. Cell Transpl. 2015, 24, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Frausto, R.F.; Swamy, V.S.; Peh, G.S.L.; Boere, P.M.; Hanser, E.M.; Chung, D.D.; George, B.L.; Morselli, M.; Kao, L.; Azimov, R.; et al. Phenotypic and Functional Characterization of Corneal Endothelial Cells during in Vitro Expansion. Sci. Rep. 2020, 10, 7402. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, M.D.; Bohrer, L.R.; Aldrich, B.T.; Greiner, M.A.; Mullins, R.F.; Worthington, K.S.; Tucker, B.A.; Wiley, L.A. Feeder-Free Differentiation of Cells Exhibiting Characteristics of Corneal Endothelium from Human Induced Pluripotent Stem Cells. Biol. Open 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Afshari, N.A. Generation of Human Corneal Endothelial Cells via in Vitro Ocular Lineage Restriction of Pluripotent Stem Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6878–6884. [Google Scholar] [CrossRef] [PubMed]
- McCabe, K.L.; Kunzevitzky, N.J.; Chiswell, B.P.; Xia, X.; Goldberg, J.L.; Lanza, R. Efficient Generation of Human Embryonic Stem Cell-Derived Corneal Endothelial Cells by Directed Differentiation. PLoS ONE 2015, 10, e0145266. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Mirbagheri, M.; Adibnia, V.; Hughes, B.R.; Waldman, S.D.; Banquy, X.; Hwang, D.K. Advanced Cell Culture Platforms: A Growing Quest for Emulating Natural Tissues. Mater. Horiz. 2019, 6, 45–71. [Google Scholar] [CrossRef]
- Kulangara, K.; Leong, K.W. Substrate Topography Shapes Cell Function. Soft Matter 2009, 5, 4072–4076. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering Substrate Topography at the Micro- and Nanoscale to Control Cell Function. Angew. Chem. Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3d Bioprinting. Int. J. Mol. Sci. 2020, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, F.; Labib, M.; Ahmadi, M.; Chen, H.; Das, J.; Sargent, E.H.; Kelley, S.O.; Ahmed, S.U. Nanostructured Architectures Promote the Mesenchymal-Epithelial Transition for Invasive Cells. ACS Nano 2020, 14, 5324–5336. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kwiatkowska, B.; Lu, H.; Voglsta, M.; Ueda, E.; Grunze, M.; Sleeman, J.; Levkin, P.A.; Nazarenko, I. Collaborative Action of Surface Chemistry and Topography in the Regulation of Mesenchymal and Epithelial Markers and the Shape of Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 28554–28565. [Google Scholar] [CrossRef] [PubMed]
- Barui, A.; Chowdhury, F.; Pandit, A.; Datta, P. Biomaterials Rerouting Mesenchymal Stem Cell Trajectory towards Epithelial Lineage by Engineering Cellular Niche. Biomaterials 2018, 156, 28–44. [Google Scholar] [CrossRef]
- Downing, T.L.; Soto, J.; Morez, C.; Houssin, T.; Fritz, A.; Yuan, F.; Chu, J.; Patel, S.; Schaffer, D.V.; Li, S. Biophysical Regulation of Epigenetic State and Cell Reprogramming. Nat. Mater. 2013, 12, 1154–1162. [Google Scholar] [CrossRef]
- Yi, B.; Shen, Y.; Tang, H.; Wang, X.; Zhang, Y. Acta Biomaterialia Stiffness of the Aligned Fibers Affects Structural and Functional Integrity of the Oriented Endothelial Cells. Acta Biomater. 2020, 108, 237–249. [Google Scholar] [CrossRef]
- Nasrollahi, S.; Pathak, A. Topographic Confinement of Epithelial Clusters Induces Epithelial-to-Mesenchymal Transition in Compliant Matrices. Sci. Rep. 2016, 6, 18831. [Google Scholar] [CrossRef]
- Saha, S.; Duan, X.; Wu, L.; Lo, P.; Chen, H.; Wang, Q. Electrospun Fibrous Scaffolds Promote Breast Cancer Cell Alignment and Epithelial—Mesenchymal Transition. Langmuir 2012, 28, 2028–2034. [Google Scholar] [CrossRef]
- Xu, X.; Ma, L.; Wu, Y.; Tang, L. Micropillar-Based Culture Platform Induces Epithelial—Mesenchymal Transition in the Alveolar Epithelial Cell Line. J. Biomed. Mater. Res. Part A 2018, 3165–3174. [Google Scholar] [CrossRef]
- Limongi, T.; Tirinato, L.; Pagliari, F.; Giugni, A.; Allione, M.; Perozziello, G.; Candeloro, P.; Di Fabrizio, E. Fabrication and Applications of Micro/Nanostructured Devices for Tissue Engineering. Nano-Micro Lett. 2017, 9, 1. [Google Scholar] [CrossRef]
- Kim, H.N.; Jiao, A.; Hwang, N.S.; Kim, M.S.; Kang, D.H.; Kim, D.H.; Suh, K.Y. Nanotopography-Guided Tissue Engineering and Regenerative Medicine. Adv. Drug Deliv. Rev. 2013, 65, 536–558. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New Approaches to Nanofabrication: Molding, Printing, and Other Techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Patel, A.; Gil, D.; Smith, H.I. Maskless Lithography. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chem. Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Xia, Y.; Rogers, J.A.; Paul, K.E.; Whitesides, G.M. Unconventional Methods for Fabricating and Patterning Nanostructures. Chem. Rev. 1999, 99, 1823–1848. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Guo, L.J. Recent Progress in Nanoimprint Technology and Its Applications. J. Phys. D Appl. Phys. 2004, 37, R123–R141. [Google Scholar] [CrossRef]
- Lantada, A.D.; Piotter, V.; Plewa, K.; Barié, N.; Guttmann, M.; Wissmann, M. Toward Mass Production of Microtextured Microdevices: Linking Rapid Prototyping with Microinjection Molding. Int. J. Adv. Manuf. Technol. 2015, 76, 1011–1020. [Google Scholar] [CrossRef]
- Mondrinos, M.J.; Dembzynski, R.; Lu, L.; Byrapogu, V.K.C.; Wootton, D.M.; Lelkes, P.I.; Zhou, J. Porogen-Based Solid Freeform Fabrication of Polycaprolactone-Calcium Phosphate Scaffolds for Tissue Engineering. Biomaterials 2006, 27, 4399–4408. [Google Scholar] [CrossRef]
- Choi, N.W.; Cabodi, M.; Held, B.; Gleghorn, J.P.; Bonassar, L.J.; Stroock, A.D. Microfluidic Scaffolds for Tissue Engineering. Nat. Mater. 2007, 6, 908–915. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Narayan, R.J. Two-Photon Polymerization for Biological Applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Raimondi, M.T.; Eaton, S.M.; Nava, M.M.; Laganà, M.; Cerullo, G.; Osellame, R. Two-Photon Laser Polymerization: From Fundamentals to Biomedical Application in Tissue Engineering and Regenerative Medicine. J. Appl. Biomater. Funct. Mater. 2012, 10, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, A.; Maassen, J.; Harnisch, E.; Kuhlen, D.; Sauer-Budge, A.; Skazik-Voogt, C.; Engelmann, K. Descemet’s Membrane Biomimetic Microtopography Differentiates Human Mesenchymal Stem Cells Into Corneal Endothelial-Like Cells. Cornea 2019, 38, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Jayne, R.K.; Tamborini, A.; Eyckmans, J.; White, A.E.; Chen, C.S. Studies of 3D Directed Cell Migration Enabled by Direct Laser Writing of Curved Wave Topography. Biofabrication 2019, 11, 21001. [Google Scholar] [CrossRef] [PubMed]
- Prina, E.; Amer, M.H.; Sidney, L.; Tromayer, M.; Moore, J.; Liska, R.; Bertolin, M.; Ferrari, S.; Hopkinson, A.; Dua, H.; et al. Bioinspired Precision Engineering of Three-Dimensional Epithelial Stem Cell Microniches. Adv. Biosyst. 2020, 4, 2000016. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Tran, Q.; Ajeti, V.; Freeman, B.T.; Fast, V.G.; et al. Myocardial Tissue Engineering with Cells Derived from Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef]
- Koroleva, A.; Gittard, S.; Schlie, S.; Deiwick, A.; Jockenhoevel, S.; Chichkov, B. Fabrication of Fibrin Scaffolds with Controlled Microscale Architecture by a Two-Photon Polymerization-Micromolding Technique. Biofabrication 2012, 4, 15001. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Malinauskas, M.; Schlie, S.; Chichkov, B.; Gittard, S.; Narayan, R.; Löbler, M.; Sternberg, K.; Schmitz, K.P.; Haverich, A. Three-Dimensional Laser Micro- and Nano-Structuring of Acrylated Poly(Ethylene Glycol) Materials and Evaluation of Their Cytoxicity for Tissue Engineering Applications. Acta Biomater. 2011, 7, 967–974. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Gruene, M.; Pflaum, M.; Koch, L.; Maiorana, F.; Wilhelmi, M.; Haverich, A.; Chichkov, B. Laser Printing of Cells into 3D Scaffolds. Biofabrication 2010, 2, 14104. [Google Scholar] [CrossRef]
- Nair, L.S.; Bhattacharyya, S.; Laurencin, C.T. Development of Novel Tissue Engineering Scaffolds via Electrospinning. Expert Opin. Biol. Ther. 2004, 4, 659–668. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Chen, H.; Samal, P.; Giselbrecht, S.; Baker, M.B.; Moroni, L. Self-Assembly of Electrospun Nanofibers into Gradient Honeycomb Structures. Mater. Des. 2019, 168, 107614. [Google Scholar] [CrossRef]
- Dalton, P.D.; Woodfield, T.B.F.; Mironov, V.; Groll, J. Advances in Hybrid Fabrication toward Hierarchical Tissue Constructs. Adv. Sci. 2020, 7, 1902953. [Google Scholar] [CrossRef]
- Giselbrecht, S.; Gietzelt, T.; Gottwald, E.; Trautmann, C.; Truckenmuller, R.; Weibezahn, K.F.; Welle, A. 3D Tissue Culture Substrates Produced by Microthermoforming of Pre-Processed Polymer Films. Biomed. Microdevices 2006, 8, 191–199. [Google Scholar] [CrossRef]
- Truckenmüller, R.; Giselbrecht, S.; Escalante-Marun, M.; Groenendijk, M.; Papenburg, B.; Rivron, N.; Unadkat, H.; Saile, V.; Subramaniam, V.; Van Den Berg, A.; et al. Fabrication of Cell Container Arrays with Overlaid Surface Topographies. Biomed. Microdevices 2012, 14, 95–107. [Google Scholar] [CrossRef]
- Truckenmüller, R.; Giselbrecht, S.; Rivron, N.; Gottwald, E.; Saile, V.; Van Den Berg, A.; Wessling, M.; Van Blitterswijk, C. Thermoforming of Film-Based Biomedical Microdevices. Adv. Mater. 2011, 23, 1311–1329. [Google Scholar] [CrossRef]
- Loesberg, W.A.; te Riet, J.; van Delft, F.C.; Schon, P.; Figdor, C.G.; Speller, S.; van Loon, J.J.; Walboomers, X.F.; Jansen, J.A. The Threshold at Which Substrate Nanogroove Dimensions May Influence Fibroblast Alignment and Adhesion. Biomaterials 2007, 28, 3944–3951. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Riehle, M.O.; Wilkinson, C.D.W.; Curtis, A.S.G. Investigating Filopodia Sensing Using Arrays of Defined Nano-Pits down to 35 Nm Diameter in Size. Int. J. Biochem. Cell Biol. 2004, 36, 2005–2015. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The Control of Human Mesenchymal Cell Differentiation Using Nanoscale Symmetry and Disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.J.; Gadegaard, N.; Tsimbouri, P.M.; Burgess, K.V.; McNamara, L.E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R.O.C.; Dalby, M.J. Nanoscale Surfaces for the Long-Term Maintenance of Mesenchymal Stem Cell Phenotype and Multipotency. Nat. Mater. 2011, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.S.G.; Gadegaard, N.; Dalby, M.J.; Riehle, M.O.; Wilkinson, C.D.W.; Aitchison, G. Cells React to Nanoscale Order and Symmetry in Their Surroundings. IEEE Trans. Nanobiosci. 2004, 3, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkova-Chevolleau, T.; Stéphanou, A.; Fuard, D.; Ohayon, J.; Schiavone, P.; Tracqui, P. The Motility of Normal and Cancer Cells in Response to the Combined Influence of the Substrate Rigidity and Anisotropic Microstructure. Biomaterials 2008, 29, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Hagvall, S.H.; Wu, B.M.; Dunn, J.C.Y.; Beygui, R.E.; Kim, C.J. Cell Interaction with Three-Dimensional Sharp-Tip Nanotopography. Biomaterials 2007, 28, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Eunhye, K.; Jinwoo, L.; Sungmo, A.; Heonsu, J.; Kyuback, L. Cell Culture over Nanopatterned Surface Fabricated by Holographic Lithography and Nanoimprint Lithography. In Proceedings of the 2008 3rd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Sanya, China, 6–9 January 2008; pp. 725–728. [Google Scholar] [CrossRef]
- Sun, J.; Ding, Y.; Lin, N.J.; Zhou, J.; Ro, H.; Soles, C.L.; Cicerone, M.T.; Lin-Gibson, S. Exploring Cellular Contact Guidance Using Gradient Nanogratings. Biomacromolecules 2010, 11, 3067–3072. [Google Scholar] [CrossRef][Green Version]
- Soh, Y.Q.; Mehta, J.S. Regenerative Therapy for Fuchs Endothelial Corneal Dystrophy. Cornea 2018, 37, 523–527. [Google Scholar] [CrossRef]
- Walckling, M.; Waterstradt, R.; Baltrusch, S. Collagen Remodeling Plays a Pivotal Role in Endothelial Corneal Dystrophies. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1–15. [Google Scholar] [CrossRef]
- Salehi, S.; Czugala, M.; Stafiej, P.; Fathi, M.; Bahners, T.; Gutmann, J.S.; Singer, B.B.; Fuchsluger, T.A. Poly (Glycerol Sebacate)-Poly (ε-Caprolactone) Blend Nanofibrous Scaffold as Intrinsic Bio- and Immunocompatible System for Corneal Repair. Acta Biomater. 2017, 50, 370–380. [Google Scholar] [CrossRef]
- Salehi, S.; Bahners, T.; Gutmann, J.S.; Gao, S.L.; Mäder, E.; Fuchsluger, T.A. Characterization of Structural, Mechanical and Nano-Mechanical Properties of Electrospun PGS/PCL Fibers. RSC Adv. 2014, 4, 16951–16957. [Google Scholar] [CrossRef]
- Saino, E.; Focarete, M.L.; Gualandi, C.; Emanuele, E.; Cornaglia, A.I.; Imbriani, M.; Visai, L. Effect of Electrospun Fiber Diameter and Alignment on Macrophage Activation and Secretion of Proinflammatory Cytokines and Chemokines. Biomacromolecules 2011, 12, 1900–1911. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Walter, P.; Bauer, B.; Rütten, S.; Schaefer, K.; Plange, N.; Gries, T.; Jockenhoevel, S.; Fuest, M. Electro-Spun Membranes as Scaffolds for Human Corneal Endothelial Cells. Curr. Eye Res. 2018, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, C.; Zhu, M.; Yao, Q.; Shao, C.; Lu, W.; Wang, J.; Mo, X.; Gu, P.; Fu, Y.; et al. Electrospun Nanofibrous SF/P(LLA-CL) Membrane: A Potential Substratum for Endothelial Keratoplasty. Int. J. Nanomed. 2015, 10, 3337–3350. [Google Scholar] [CrossRef]
- Chua, J.; Liew, L.; Yim, E. Cultivation of Human Microvascular Endothelial Cells on Topographical Substrates to Mimic the Human Corneal Endothelium. J. Funct. Biomater. 2013, 4, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.K.K.; Goh, K.J.; Ng, Z.J.; Koo, S.; Yim, E.K.F. Functional Reconstruction of Corneal Endothelium Using Nanotopography for Tissue-Engineering Applications. Acta Biomater. 2012, 8, 2941–2952. [Google Scholar] [CrossRef]
- Koo, S.; Muhammad, R.; Peh, G.S.; Mehta, J.S.; Yim, E.K. Micro- and Nanotopography with Extracellular Matrix Coating Modulate Human Corneal Endothelial Cell Behavior. Acta Biomater. 2014, 10, 1975–1984. [Google Scholar] [CrossRef]
- Muhammad, R.; Peh, G.S.L.; Adnan, K.; Law, J.B.K.; Mehta, J.S.; Yim, E.K.F. Micro- and Nano-Topography to Enhance Proliferation and Sustain Functional Markers of Donor-Derived Primary Human Corneal Endothelial Cells. Acta Biomater. 2015, 19, 138–148. [Google Scholar] [CrossRef]
- Rizwan, M.; Peh, G.S.; Adnan, K.; Naso, S.L.; Mendez, A.R.; Mehta, J.S.; Yim, E.K.F. In Vitro Topographical Model of Fuchs Dystrophy for Evaluation of Corneal Endothelial Cell Monolayer Formation. Adv. Healthc. Mater. 2016, 5, 2896–2910. [Google Scholar] [CrossRef]
- Rizwan, M.; Peh, G.S.L.; Ang, H.P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-Crosslinked Bioactive Hydrogels as Nano-Patterned Substrates with Customizable Stiffness and Degradation for Corneal Tissue Engineering Applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Kim, E.Y.; Tripathy, N.; Park, J.Y.; Lee, S.E.; Joo, C.K.; Khang, G. Silk Fibroin Film as an Efficient Carrier for Corneal Endothelial Cells Regeneration. Macromol. Res. 2015, 23, 189–195. [Google Scholar] [CrossRef]
- Nandakumar, A.; Truckenmüller, R.; Ahmed, M.; Damanik, F.; Santos, D.R.; Auffermann, N.; De Boer, J.; Habibovic, P.; Van Blitterswijk, C.; Moroni, L. A Fast Process for Imprinting Micro and Nano Patterns on Electrospun Fiber Meshes at Physiological Temperatures. Small 2013, 9, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Li, D.J.; Pham, L.K.; Wong, B.G.; Hui, E.E. Microfabrication of High-Resolution Porous Membranes for Cell Culture. J. Memb. Sci. 2014, 452, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Apel, P. Track Etching Technique in Membrane Technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Liang, J.; Li, B.; Wu, L. Recent Advances on Porous Interfaces for Biomedical Applications. Soft Matter 2020, 16, 7231–7245. [Google Scholar] [CrossRef] [PubMed]
- Yabu, H. Fabrication of Honeycomb Films by the Breath Figure Technique and Their Applications. Sci. Technol. Adv. Mater. 2018, 19, 802–822. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Xu, Z.; Wei, Y.; Yang, H. Formation of Microporous Polymeric Membranes via Thermally Induced Phase Separation: A Review. Front. Chem. Sci. Eng. 2016, 10, 57–75. [Google Scholar] [CrossRef]
- Honig, F.; Vermeulen, S.; Zadpoor, A.A.; de Boer, J.; Fratila-Apachitei, L.E. Natural Architectures for Tissue Engineering and Regenerative Medicine. J. Funct. Biomater. 2020, 11, 47. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal Structure and Transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Scribante, A.; Poggio, C.; Gallo, S.; Riva, P.; Cuocci, A.; Carbone, M.; Arciola, C.R.; Colombo, M. In Vitro Re-Hardening of Bleached Enamel Using Mineralizing Pastes: Toward Preventing Bacterial Colonization. Materials 2020, 13, 818. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formisano, N.; Sahin, G.; Català, P.; Truckenmüller, R.; Nuijts, R.M.M.A.; Dickman, M.M.; LaPointe, V.L.S.; Giselbrecht, S. Nanoscale Topographies for Corneal Endothelial Regeneration. Appl. Sci. 2021, 11, 827. https://doi.org/10.3390/app11020827
Formisano N, Sahin G, Català P, Truckenmüller R, Nuijts RMMA, Dickman MM, LaPointe VLS, Giselbrecht S. Nanoscale Topographies for Corneal Endothelial Regeneration. Applied Sciences. 2021; 11(2):827. https://doi.org/10.3390/app11020827
Chicago/Turabian StyleFormisano, Nello, Gozde Sahin, Pere Català, Roman Truckenmüller, Rudy M. M. A. Nuijts, Mor M. Dickman, Vanessa L. S. LaPointe, and Stefan Giselbrecht. 2021. "Nanoscale Topographies for Corneal Endothelial Regeneration" Applied Sciences 11, no. 2: 827. https://doi.org/10.3390/app11020827
APA StyleFormisano, N., Sahin, G., Català, P., Truckenmüller, R., Nuijts, R. M. M. A., Dickman, M. M., LaPointe, V. L. S., & Giselbrecht, S. (2021). Nanoscale Topographies for Corneal Endothelial Regeneration. Applied Sciences, 11(2), 827. https://doi.org/10.3390/app11020827





