Abstract
Chemistry is a science emphasizing both theory and experimentation. After learning the theoretical knowledge, experimental operation can help students understand chemical concepts and transform them into practical knowledge. Considering the safety issue and the lack of teaching time and experimental equipment, some teachers often choose to demonstrate an experiment instead of letting students conduct it by themselves. This may affect their learning motivation as well as the construction of chemical concepts and hands-on skills. This study combined the augmented reality (AR) technology with the operating principle of the Daniell cell to develop a virtual experiment for the application in high school chemistry courses. Students can conduct the virtual experiment using mobile devices by selecting the required equipment and materials from a deck of cards to set up the experimental environment. In the virtual experiment, students can use the galvanometer to measure the current after mounting the salt bridge on the beakers containing zinc sulfate and copper sulfate solutions. They can also see the change of molecular structures and movement of electrons and ions during the redox reactions to understand the important concepts and knowledge. An empirical research has been performed, and the analytical results show that both the virtual experiment and the real experiment could improve students’ learning achievement, but the former was more effective for the low-achievement students because they could explore autonomously to enhance cognition by observing the submicroscopic view of the redox reactions. The post-test results show that the average score of the low-achievement students in the experimental group (41.60) was significantly higher than that of the control group (27.67). Questionnaire results reveal that most students were satisfied with the learning contents, user interface, learning motivation, system reality, and practicality of the virtual experiment, and the average satisfaction score was 3.98 out of 5. The application of virtual experiments is not limited by time or space. Students only need to download the application (APP) software and print out the AR cards to practice at home, so it is suitable for large-scale promotion in rural areas.
1. Introduction
Chemistry is a science studying the composition, structure, properties, behavior of substances, and the energy changes during a reaction. Its research objects involve the relationships between substances and energy transformation. Chemistry is the basis for the development of many applied sciences, and it is a science emphasizing theory and experimentation. Therefore, the experimental operation can enhance learning motivation and the ability of experimental design. Through the process of “learning by doing”, students can find solutions by careful observation and thinking. They can use experimental results to verify the principles of chemical reactions and transform theoretical knowledge into problem-solving skills for future applications.
Experimental operation is an important skill in science education [1], since theoretical knowledge can often be delivered and absorbed from teaching resources, such as teachers, books, and the Internet, but practical experiences are difficult to acquire or to be replaced. Experiments are the solid manifestations of scientific activities because many abstract scientific concepts must be proved by experiments for obtaining the public support and approval. Based on the theoretical knowledge, experimental operation can enhance the understanding of chemical concepts and transform them into practical experiences. Therefore, it is an indispensable part of science education [2].
The main goal of secondary education in Taiwan is focused on preparation for higher education because an average of more than 80% of high school students go to college (93.04% for general high schools and 79.53% for vocational high schools) [3]. However, the entrance exams are mainly multiple-choice questions, causing students to memorize fragmented knowledge, and thus not suitable for the development of scientific ethics, processing skills, and affective objectives. Under the academic pressure, preparing for the comprehensive tests becomes an important part of teaching objective. Considering the safety issue, as well as the lack of teaching time and experimental equipment, some teachers often choose to demonstrate an experiment rather than allowing students to conduct it by themselves. However, a demonstration in chemistry may be defined as a pedagogical event whose objective is to illustrate a scientific concept [4]. One of the main problems existing in teaching chemistry experiments through demonstration by showing the procedure and results is that it may limit the opportunity for students to think, imagine, analyze, and innovate [5].
Even if students are able to perform the experiment, the procedure is often simplified as fixed steps to follow, rather than allowing them to explore autonomously. This way not only ignores the experimental spirit, but also affects students’ learning motivation and the development of scientific concepts and hands-on skills [6]. Dillon [7] stated that there exists a gap between the learning outcomes set by the teachers before conducting an experiment and the actual outcomes received by students at the end of the experiment, because they fail to comprehend the conceptual and procedural parts of the laboratory activities. Therefore, the use of demonstration or following the steps passively during the experiment can only enable students to practice skills at a lower level.
1.1. Virtual Reality
Virtual reality (VR) uses 3D visual effects to simulate real-life situations, allowing us to interact with virtual objects and avatars in the 3D world through our sense organs and special equipment, such as the head-mounted display (HMD) and data gloves. Virtual reality is an integrated technology of computer software and hardware, including simulation and modeling, artificial intelligence (AI), context awareness, 3D display, and network parallel processing. When the user changes his or her position and orientation, the computer can perform complex calculation in real time to create the corresponding 3D scene as if situated in the real world. The user can see, hear, and even feel in the virtual world and interact with other people connecting through the network.
Virtual reality has the ability to simulate the real life with 3D display, and it can present abstract concepts, principles, and experimental processes in a realistic manner. It provides learners with close-to-real situations to help them understand the abstract concepts and knowledge [8]. In traditional teaching models, learners can only absorb knowledge passively in the classroom, not being able to explore real situations actively. When virtual reality is applied in teaching, it can provide immersive human–machine interface and enable learners to experience virtual situations through interactive operation and autonomous exploration. Therefore, learners can obtain more complete knowledge and concepts, including the hidden information that cannot be seen with the naked eye [9,10]. Recently, virtual reality has attracted more attention in the research areas of science, engineering, national defense, education, and entertainment, because a virtual situation can achieve realistic effects while avoiding the danger and cost of being in the real environments. Many studies show that the applications of virtual reality in science education can achieve higher learning effectiveness, especially in conceptual cognition, because it can enhance learning interest and motivation [11,12,13].
1.2. Augmented Reality
Augmented reality (AR) is a technology to impose the virtual information, e.g., videos and images, onto a live-view digital display. For example, when a set of images appear in front of the camera, the AR system can detect the images to generate the corresponding information and attach it to the real objects or environments. Compared with virtual reality, augmented reality integrates virtual information into the real world to enhance the user’s perception and increase the performance of tasks through the interaction with real situations and virtual objects. Azuma et al. [14] proposed the three features of augmented reality: (1) interacting with real and virtual environments, (2) providing real-time feedback, and (3) being in the 3D space. Augmented reality can also attach virtual objects to the preset locations in the real world so that the information in the virtual world can be obtained to provide a more realistic feeling and different ways of interaction.
In general, the implementation of augmented reality is divided into (1) traditional augmented reality requiring a marker for positioning, e.g., the Magic Book [15], (2) identification through image or GPS detection [16,17], and (3) hybrid augmented reality combining markers, image, and/or GPS detection. The applications of augmented reality have become more and more popular, for example: doctors can use augmented reality to perform surgical training, and the car navigation system can also use augmented reality to provide driving direction. The application of augmented reality in the study of historical sites can transform hidden spatial information into explicit visual effects to provide a new meaning for archaeology and tourism [18,19].
In recent years, many scholars have used augmented reality in science education in a variety of studies. Kikuo et al. [20] believe that augmented reality can improve learning effectiveness and overcome the difficulties in many traditional teaching methods. Shelton and Hedley [21] applied augmented reality in teaching the solar system, allowing students to develop a scientific perspective by constructing the virtual scene of rotating planets. They found the interactive operation of 3D models could help students develop the concepts of a planet’s rotation and revolution. Kaufmann and Schmalstieg [22] developed an AR system for applications in mathematics and geometry education, and they discovered that it could improve the spatial abilities and maximize the transfer of concepts. Augmented reality can display learning contents in real time, making it easier for students to obtain knowledge and avoid misconceptions [23,24].
Through the immediate interaction with augmented reality, new teaching strategies can often be implemented such that even an inexperienced user can learn in an easy and smooth way [25,26,27]. Plunkett [28] used the AR technology to demonstrate the projection of virtual information onto a real-world object by using a smart phone, a free HP Reveal application (APP) and AR notecards, which can be applied in the classroom and in the laboratory to guide students during the equipment setup and operation. The contents of AR notecards were based on organic chemical reactions, and each card showed only a reagent for the substrate. The opinions of students in this study showed that the AR notecards provided a reasonable supplement to other study methods.
1.3. Theoretical Framework
Chemistry is a science with microscopic and abstract concepts, and the high school chemistry curriculum is in the stage of development from descriptive knowledge to inferential knowledge. The issues discussed often involve the properties and structures of substances and connection of different domain knowledge, and thus the conversion of visual thinking into abstract thinking is often required. Many students consider chemistry a challenging subject because it is difficult to imagine the abstract concepts without seeing the real objects [29,30]. Some others think the abstract concepts are difficult, which is mainly due to the lack of experimental manipulation and visual assistance for providing the submicroscopic views of chemical reactions [31,32].
1.3.1. Johnstone’s Triangle
Johnstone [33] proposed the triangle model to represent the three levels of chemistry knowledge and he pointed out how to interrelate each new concept or fact in all three domains: (1) macroscopic and tangible subjects: providing observable chemical phenomena appear to the senses, color, smell, density, etc., (2) submicroscopic explanation: showing the invisible 3D world of atoms, molecules, ions and their dynamic motions, interactions, and kinetics, and (3) symbol representation: including formulae, equations, molarity, mathematical manipulation, and graphs (Figure 1). Because most chemical instructions are performed at the symbolic level, students cannot transform the symbols into other levels of representations directly. In addition, teachers often move from one level to another and students may not be able to connect different types of representations in their minds, and thus they have difficulty understanding some abstract concepts. They have to imagine the submicroscopic view of the invisible 3D world because it is outside the range of their experiences. Therefore, a virtual experiment can provide a simultaneous demonstration of all three levels of a chemical concept along with visualization of the submicroscopic explanation.
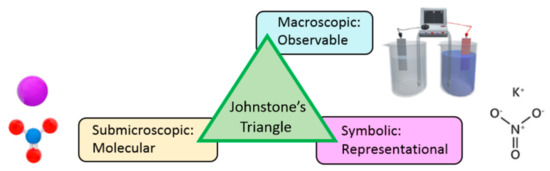
Figure 1.
Johnstone’s triangle, representing the three levels of chemistry knowledge.
The macroscopic and submicroscopic views are two important aspects for observing the changes in substances. To find the causes of chemical reactions, we must understand how the microstructures of reactants and products change, because there is a scientific and logical reasoning between them, e.g., activity, chemical bonds, and electron transfer. The changes in submicroscopic views are closely related to the corresponding changes in macroscopic views such as material properties and chemical energy. Teachers often find it difficult for students to develop an abstract chemical concept that is complex and requires spatial ability to understand it. Therefore, the application of augmented reality can make up for the lack of students’ ability in this situation. It provides an interface for learners to interact with the 3D models of atoms and molecules to enhance the understanding of chemical structures according to the learner’s point of view and the pace of learning.
Laboratory-based chemistry courses provide the practice for students to go beyond surface-level thinking, because they can use quantitative methods to analyze experimental data and establish connections between observation and explanation. Jiménez [34] used augmented reality and virtual reality technologies in teaching chemistry at high schools and colleges to convey the idea that these technologies can help students learn more actively and independently. The selected chemistry topics included molecular 3D structures, chemical bonding, and intermolecular interactions of simple and complex molecules. The results showed that these technologies had a positive effect on student’s learning and engagement because of the immersion and 3D visual effects.
Augmented reality can superimpose the molecular structure on the reactants and products during a chemical reaction, allowing students to visualize the changes that cannot be seen in the real world, so it can expand students’ learning experience and enhance their thinking ability. The research results of Herga et al. [35] showed that using a virtual chemistry laboratory can provide a visual simulation of microscopic reactions to improve learning effectiveness by increasing the cognition of abstract concepts. According to the literature reviews, this study proposed a virtual experiment based on AR/VR technologies to provide a submicroscopic model for students to understand the changes of molecular structures and movement of electrons and ions during a redox reaction to help them establish the connection between macroscopic and submicroscopic knowledge.
1.3.2. Inquiry-Based Learning
Inquiry is the process in which learners take the initiative to discover problems, collect and analyze data, and infer the meaning of information for constructing scientific knowledge and concepts [36,37]. In the inquiry-based learning process, students are the active constructor of knowledge, and teachers simply instruct students to make assumptions, explore problems, verify answers, and summarize results. Inquiry-based learning is based on the active exploration and the process of discovering and solving problems [38]. Trowbridge and Bybee [39] believed that inquiry behavior is essential for science education because it can cultivate scientific thinking and problem-solving ability and enable students to know how scientists conduct research. Kyza and Georgiou [40] developed an AR platform for location-based mobile learning to engage students in evidence-driven reflective activity to support the authoring of inquiry-based learning.
The learning objective of the high school chemistry curriculum is to enhance the understanding of materials and energy in the real world and construct essential knowledge and concepts to become a citizen with scientific literacy. Therefore, teachers can develop students’ scientific attitudes through inquiry-based experimental activities to increase their abilities in solving problems and adapting to changes. As a result, students can understand the mysteries of nature through the exploration of daily-life problems and establish the habits of independent thinking, finding the truth, protecting the environments, and developing a positive and optimistic attitude.
1.4. Virtual Daniell Cell Experiment
In high school chemistry, “acid and alkali” and “redox” are interesting but difficult learning topics. Researchers suggested that specific methods should be used to help students understand the basic concepts [41]. The basic knowledge of the above learning topics involves electrochemistry, a branch of physical chemistry that studies the relationship between electricity and an identifiable chemical change. In electrochemistry, electricity is a measurable and quantitative phenomenon, and it is considered an outcome of a particular chemical reaction or vice versa [42]. The reaction of the Daniell cell experiment involves electric charges moving between electrodes and an electrolyte as well as the ionic species in a solution. A chemical reaction is called an electrochemical reaction if it is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a chemical reaction, as in a battery. Chemical reactions are called oxidation–reduction (redox) reactions where electrons are transferred between atoms and/or molecules. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte, as in the Daniell cell.
The objective of this study is to develop a virtual experiment for learning how to design a Daniell cell to generate electricity. When conducting the experiment, the copper strip (placed in the solution of copper sulfate) is the anode and the zinc strip (placed in the solution of zinc sulfate) is the cathode. The salt bridge is used as a conducting circuit such that electrons can flow through the galvanometer for detecting the current produced by the redox reaction. Students can explore autonomously during the virtual experiment, from selecting the experimental equipment and materials to connecting the Daniell cell with the galvanometer. They can observe if the pointer on the galvanometer moves to determine if there is current generated by the Daniell cell, and measure whether the mass of the zinc strip and the copper strip has changed to tell if it is an oxidation or a reduction reaction.
The design of virtual experiment is based on the theoretical framework of Johnstone’s triangle and it combines different levels (macroscopic, submicroscopic, and symbolic) of representation: (1) Macroscopic: students can conduct the virtual experiment by selecting the required equipment to set up the experimental environment and observe the macroscopic results, (2) Submicroscopic: students can see the change of molecular structures and movement of electrons and ions during the redox reaction to understand the related concepts and knowledge, and (3) Symbolic: the chemical symbols and structural formulas are shown on the AR cards and in the virtual scene to help students understand the principles of chemical reactions.
To solve the dilemma faced by high school chemistry teachers, this study combined the AR and VR technologies with inquiry-based learning to develop a virtual experiment where students can see the change of molecular structures and movement of electrons and ions during the redox reaction to understand the principle of Daniell cells. Students can conduct the virtual experiment using a mobile device by selecting the required equipment from a deck of AR cards to set up the experimental environment. They can also obtain the important concepts and knowledge through the explorative process and the guidance of learning scaffolds (Figure 2).
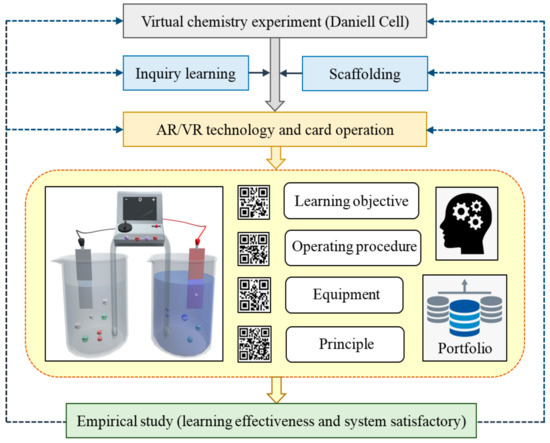
Figure 2.
Research structure of the augmented reality (AR)-based virtual experiment.
The system provides appropriate hints to help students in the experimental process for them to construct scientific concepts along the conceptual framework. It is different from instructing them to conduct the real experiment by following the experimental procedure step by step. Students can acquire the chemical knowledge and concepts of redox reactions in the virtual experiment and stimulate their learning motivation and interest during the inquiry process, which is helpful for improving the learning achievement and hands-on ability.
1.5. Research Questions
In this study, an empirical research was performed to measure the learning effectiveness of the virtual experiment. A questionnaire survey was conducted to investigate the attitudes of students after conducting the virtual experiment. According to the literature review and the research purpose of this study, the research questions are listed as follows:
- (1)
- Is there a significant difference in learning achievement between students conducting the virtual experiment and the real experiment?
- (2)
- What is the students’ response on the learning contents, user interface, learning motivation, system reality, and practicality of the virtual experiment?
2. System Development
The objective of the high school chemistry curriculum is to enhance the understanding of materials and the changes in their structures, properties, and energies during chemical reactions. Teachers can use practical experiments to increase students’ interest and motivation in learning chemistry for them to become familiar with experimental methods and skills. It can also establish scientific attitudes and increase the ability in solving problems and adapting to changes. In the chemistry courses, “acid and alkali” and “redox” are considered interesting but difficult learning topics. Students are susceptible to misconceptions, and thus an effective learning method is needed to help conceptual understanding. This study referred to the high school chemistry textbooks and selected the Daniell cell as the topic for developing the virtual experiment because it is often used in classroom demonstrations.
2.1. Operating Principle
The design of the virtual experiment is implemented by AR and VR technologies based on the operating principle of a redox reaction, which is defined as: “If a substance loses electrons in a chemical reaction, it is called oxidation; if a substance receives electrons, it is called reduction.” Since the activity of zinc is higher than that of copper, zinc atoms lose electrons easily. Therefore, the zinc strip is the negative electrode and the copper strip is the positive electrode. The zinc ion Zn2+ (colorless) formed by the loss of electrons is dissolved in the zinc sulfate solution, so the mass of the zinc strip decreases gradually during the oxidation reaction.
The user can see the symbolic representation as well as the submicroscopic view of the reaction in the virtual experiment, where the electrons emitted by the zinc atoms flow from the zinc strip through the galvanometer to the copper strip. The electrons received by the copper strip combine with the copper ion Cu2+ (blue) in the copper sulfate solution and the copper ion Cu2+ is reduced to the copper atom Cu (red), so the copper strip gradually increases its mass during the reduction reaction. Because the concentration of copper ion Cu2+ is decreasing, the user can see the color of the copper sulfate solution becomes lighter as time goes on (Figure 3).
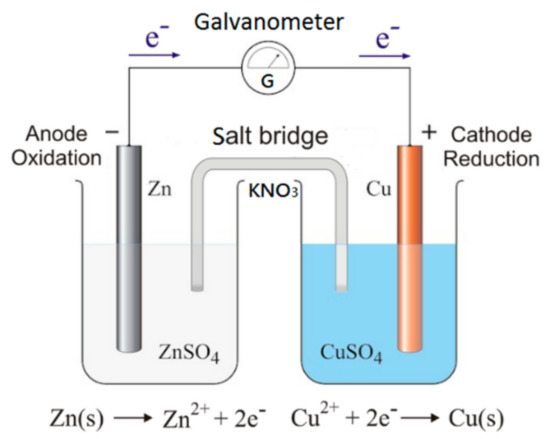
Figure 3.
Operating principle of the Daniell cell’s redox reaction.
When conducting the virtual Daniell cell experiment, the user can determine if there is current and its direction by observing the pointer on the galvanometer when the zinc strip and copper strip are connected to it. The salt bridge is mounted on the beakers to maintain the neutrality of the electrolytic solutions. The salt solution (KNO3) in the salt bridge must be easily dissociated, and it does not react with the electrodes or the electrolytic solutions. The user can see the nitrate ions (anions) move to the zinc sulfate solution (negative electrode), and potassium ions K+ (cations) move to the copper sulfate solution (positive electrode). The user can also observe if the pointer on the galvanometer moves to determine if there is current flowing through and its direction. If the number of salt bridges is increased, the channels for ion communication can also be increased, and thus the current generated by electrochemical cell is increased.
In the design of the virtual experiment, the submicroscopic explanation can display the reaction of molecules and ions to meet students’ expectation by the application of AR and VR technologies in a chemistry education context. It was implemented by integrating the three levels of chemical concepts in Johnstone’s triangle to interrelate each new concept in all three domains such that different levels of knowledge can be linked together to provide more complete concepts for better cognition. The macroscopic, submicroscopic, and symbolic chemistry knowledge in the Daniell cell’s redox reaction are listed in Table 1, which can be used to design the required AR cards, molecular and ion models, and the script of chemical reactions in the virtual experiment.

Table 1.
Three levels of chemistry knowledge for designing the virtual experiment.
2.2. Operating Procedure
The learning objective of the virtual experiment is to design an electrochemical battery using the electrolyte solutions and observe the electrochemical reaction to understand its operating principle. The experimental equipment includes a dropper, an Erlenmeyer flask, a U-shaped tube, tweezers, two beakers, and a galvanometer with black/red connecting wires and alligator clips. The operating procedure of the Daniell cell experiment is described below:
- (1)
- Add 200 mL of zinc sulfate solution and 200 mL of copper sulfate solution (0.1M) to the beakers.
- (2)
- Put the zinc strip in the zinc sulfate solution and the copper strip in the copper sulfate solution.
- (3)
- Connect the zinc strip and the galvanometer with a black alligator clip and connect the copper strip and the galvanometer with a red alligator clip.
- (4)
- Pour 200 mL of potassium nitrate solution (1M) into the U-shaped tube and plug its open ends with cotton. Keep air out of the U-shaped tube to avoid the blocking of current.
- (5)
- Put both ends of the U-shaped tube (salt bridge) into the two beakers.
- (6)
- When the zinc strip and copper strip are connected to the galvanometer, determine if there is current and its direction by observing the pointer on the galvanometer.
- (7)
- After connecting the two beakers with the salt bridge, observe if the pointer on the galvanometer moves to determine if there is current generated by the Daniell cell, and also measure whether the mass of the zinc strip and the copper strip has changed.
2.3. Designing 3D Models
The chemistry laboratory is a place providing equipment, materials, and suitable environments for conducting chemistry experiments. The common experimental equipment includes: iron stands, beakers, measuring cylinders, gas cylinders, test tubes, test tube clamps, glass rods, jars, drip, tubes, flasks, evaporating dishes, alcohol lamps, asbestos nets, etc. A chemical cabinet is usually installed inside the chemical laboratory to store commonly-used experimental materials, including: acid, alkali, salt and various compound solutions, powder, indicator, test paper, etc. In this study, we used 3D modeling software to build the virtual scene of the chemistry laboratory and experimental equipment. In the designing process, 3ds Max was used to create the 3D models, and then the image-processing software was used to draw the textures. After adjusting the brightness and textures, the 3D models were exported to the Unity3D to set up the user interface (Figure 4).
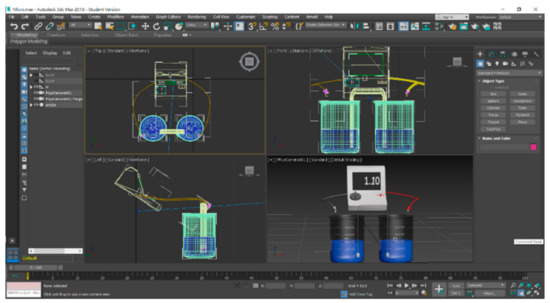
Figure 4.
Using 3ds Max to develop the 3D models of laboratory equipment.
2.4. Designing User Interface
In this study, the user interface of the virtual experiment was designed with the C# programs in the Unity3D to control the experimental process. The Unity3D is a cross-platform 2D/3D game engine developed by Unity Technologies, mainly used to design games for the platforms of Windows, MacOS, Linux, iOS, and Android, etc. In addition to the development of games, the Unity3D is often used in architectural visualization, 3D interactive simulation, and comprehensive creation tools. In this study, the user interface of the virtual laboratory was designed according to the real chemistry laboratory and the operating procedure of the real experiment (Figure 5).
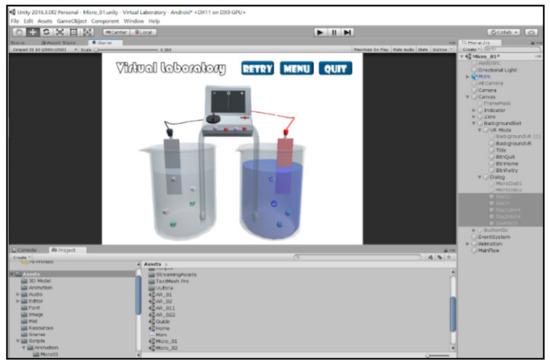
Figure 5.
Designing the user interface of the virtual experiment using the Unity3D.
2.5. Operation of AR Cards
The user can conduct a virtual experiment by selecting the necessary equipment and materials from a deck of AR cards to set up the experimental environment. Therefore, this study first designed the cards for the required laboratory equipment and materials. The front of an AR card shows the picture and name of the laboratory equipment (or material), and the back shows the corresponding quick response (QR) code for identification by the AR system (Figure 6). When the user picks an AR card, the system can identify the experimental equipment (or material) on the card by scanning the QR code to display the 3D model. The user can choose AR cards according to the experimental requirement and scan the QR code to see the 3D model and information of the equipment (or material).

Figure 6.
The AR card showing the 3D model and information of a dropper.
In the design of the virtual experiment, this study used QuickMark QR Code Generator to produce the QR codes for printing on the AR cards, which have to be uploaded to the Vuforia Engine Developer Portal (https://developer.vuforia.com/) to produce the feature images. Using the Android development platform in Unity3D, we can insert multiple feature images to the Vuforia AR software development kit (SDK) in the Unity3D to set up the connection between feature images and the corresponding 3D objects. During operation, the AR system can detect the relative position of the feature image to calculate the position and orientation of the 3D object for displaying in the virtual scene.
In this study, the user interface of virtual experiment is designed through the interaction of AR cards and the triggering of virtual buttons on the touch screen to provide the user with a convenient and more realistic experience. In order to increase the interaction between the user and the 3D object, the Unity3D can attach a virtual button to the 3D object and set up the triggered event. When the user touches the virtual button, the preset event will occur. For example, when the AR system detects the QR code of the alcohol lamp, the 3D model of the alcohol lamp will appear. The user can press the virtual button to light up the alcohol lamp (Figure 7, left). The user can also hold the magnifier card close to the Erlenmeyer flask containing the copper sulfate solution to observe the submicroscopic structure of copper ions and sulfate ions in the copper sulfate solution (Figure 7, right).

Figure 7.
Interaction of 3D objects via the virtual button and QR code identification.
The virtual experiment can be conducted on mobile devices through the operation of AR cards to set up the experimental environment. There are two types of AR cards, namely the laboratory equipment cards and the chemical material cards. In this study, a number of 18 AR cards are required to perform the Daniell cell experiment. Among them, nine cards belong to the laboratory equipment and nine cards belong to the chemical materials. The front of the card shows the picture and name of the laboratory instrument (with a green icon “E”) or chemical material (with a red icon “M”), and the back of the card shows the corresponding QR code. The user can use the AR cards to view the information about the laboratory equipment (or chemical material). Figure 8 shows the pictures and names on the front side, as well as the corresponding QR codes on the back side, for these cards.
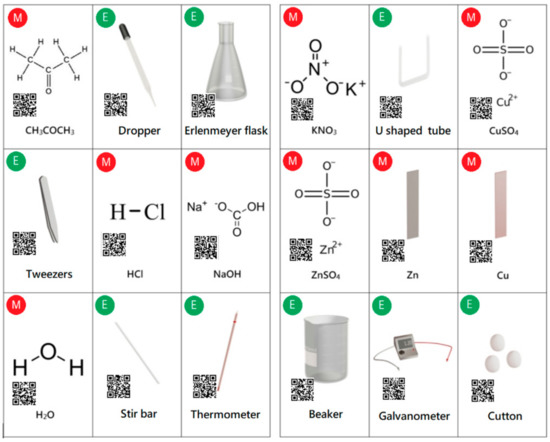
Figure 8.
The pictures, names and quick response (QR) codes of all equipment and materials.
2.6. Explorative Experiment
The virtual experiment developed in this study combined AR and VR technologies and inquiry-based learning with the principle of redox reaction for students to perform on mobile devices using AR cards to enhance cognition of the related knowledge and concepts. The user only needs to download the APP software of the virtual experiment and print out the AR cards of laboratory equipment and materials to conduct the virtual experiment. The operational flowchart of the virtual experiment is shown in Figure 9.
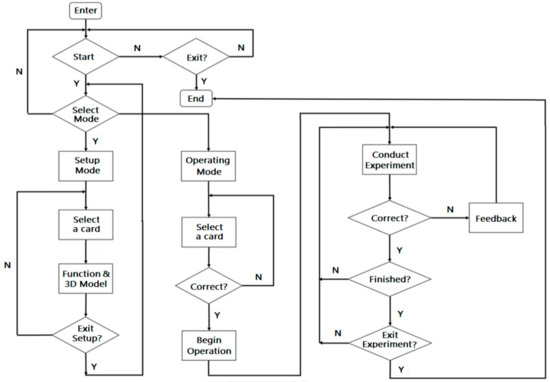
Figure 9.
Operational flowchart of the virtual experiment.
The operation of virtual experiment is divided into the setup stage (using the AR cards to set up the experimental environment) and the operating stage (conducting the experiment on the mobile device). The following examples show how the user can explore autonomously by trying different materials and connections of laboratory equipment (Figure 10). There are several ways to conduct the experiment and the results may also be different. Therefore, students can enhance scientific thinking and learning motivation in the inquiry process.
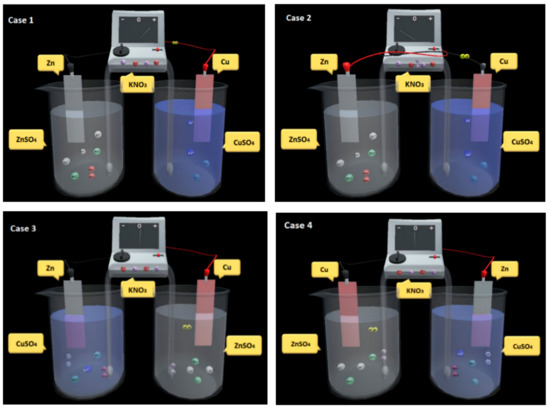
Figure 10.
Trying different materials and connections of equipment.
Example 1.Students can follow the procedure of the experiment manual and connect the red alligator clip with the copper strip and put it into the copper sulfate solution, and then connect the black alligator clip with the zinc strip and put it into the zinc sulfate solution. They can observe the direction of electrons moving in the wire and the movement of galvanometer pointer. They can also measure the change of the copper strip’s mass and observe the change of color in the copper sulfate solution to verify that the reduction has occurred.
Example 2.If they do not want to follow the experimental procedure, they can connect the red alligator clip with the zinc strip and put it into the zinc sulfate solution, and then connect the black alligator clip with the copper strip and put it into the copper sulfate solution. The result shows that the current will flow in the opposite direction, because the anode and cathode are not changed but the black alligator clip and the red alligator clip are switched.
Example 3.Similarly, they can connect the red alligator clip with the copper strip and put it into the zinc sulfate solution, and then connect the black alligator clip with the zinc strip and put it into the copper sulfate solution. The result shows no current detected, because the activity of copper is lower than that of zinc and therefore no reaction will occur in the right beaker, although it does occur in the left beaker where the activity of zinc is higher than that of copper.
Example 4.Alternatively, they can connect the red alligator clip with the zinc strip and put into the copper sulfate solution, and then connect the black alligator clip with the copper strip and put it into the zinc sulfate solution. The result shows no current detected, because the activity of copper is lower than that of zinc and therefore no reaction will occur in the left beaker, although it does occur in the right beaker where the activity of zinc is higher than that of copper.
3. Empirical Research
In this study, empirical research is performed to investigate the learning effectiveness of the virtual experiment and students’ attitude after using the AR system. This study used the quasi-experimental design to compare the difference in learning effectiveness between the virtual experiment and the real experiment. A number of 50 third-grade students from a junior high school in Hsinchu, Taiwan, were used as the research samples. Two classes in the school were selected, containing 26 students in the experimental group (conducting the virtual experiment) and 24 students in the control group (conducting the real experiment). The following describes the research tools, research structure, analytical results, and discussion.
3.1. Research Tools
The research tools include the virtual experiment, the real experiment, the achievement test, and the questionnaire survey, as described in the following:
- Virtual experiment
The experimental group conducted the virtual experiment through the operation of AR cards (Figure 11). It can simulate the redox reaction of the Daniell cell experiment and show the change of molecular structures and movement of electrons and ions during the chemical reactions.

Figure 11.
Experimental group conducting the virtual experiment.
- Real experiment
The control group conducted the real experiment in the chemistry laboratory at school (Figure 12). The teacher first described the operating procedure and then the students followed it step by step to complete the experiment and record the results on their worksheets.

Figure 12.
Control group conducting the real experiment.
- Achievement test
The achievement test was divided into the pre-test and post-test, and the purpose was to explore if the students could improve their learning achievements through the experimental operation and whether there was a significant difference in the learning effectiveness between the experimental group and the control group. There was a total of 25 questions in the achievement test, including two types of questions: multiple-choice questions and two-tier diagnostic questions.
- Questionnaire survey
To verify if the design of the virtual experiment followed the theoretical framework of chemistry education research, the questionnaire was designed to include the survey on the user’s learning experience and whether the system provided the three important elements in Johnstone’s triangle, for example: (1) Has the virtual experiment provided observable chemical phenomena and is it similar to the real experiment? (2) Has the virtual experiment provided simulation in the 3D world, showing the submicroscopic views of atoms, molecules, and ions, as well as their dynamic motions and interactions? Is the submicroscopic simulation realistic? (3) Is the symbolic representation of the chemical reactions and symbols of atom, molecules, and ions shown in the virtual experiment to help the user understand the principle of a redox reaction?
Based on the above consideration, the questionnaire was designed as a user experience survey to see if the system can meet the user’s expectations. There were five categories (learning contents, user interface, system reality, learning motivation, and practicality) containing a total of 15 questions (three questions in each category). In addition, an unstructured question was added to collect the users’ opinions after conducting the virtual experiment. To ensure its reliability, the questionnaire had been revised according to the suggestions of a chemistry expert and the chemistry teacher instructing the experiment. The reliability analysis was performed on the questionnaire results using the statistical software SPSS and the Cronbach’s α = 0.95 for all items, showing a high degree of reliability.
3.2. Research Structure
The research structure of this study is shown in Figure 13, where the dependent variable, the independent variable, and the control variables are described in the following:
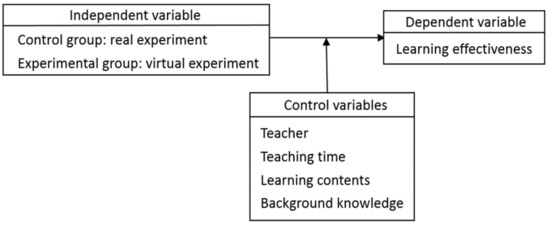
Figure 13.
The research structure and its variables.
- Dependent variable
The experimental group and the control group performed the achievement test before and after experimental operation to investigate the difference in learning effectiveness. The dependent variable is the post-test score, and the covariate is the pre-test score.
- Independent variable
The independent variable is the experimental operation, i.e., the experimental group conducted the virtual experiment while the control group conducted the real experiment.
- Control variables
To reduce the interference of other factors, the teacher, teaching time, learning contents, and students’ background knowledge were listed as the control variables.
The flowchart of the empirical research is shown in Figure 14. The experimental group and the control group took the pre-test (30 min) before conducting the virtual and real experiments, and then they conducted the experiments for the same time (100 min), including the explanation of the experimental procedure. After completing the experiments, both groups took the post-test (30 min), and the experimental group had to fill out the questionnaire (10 min).
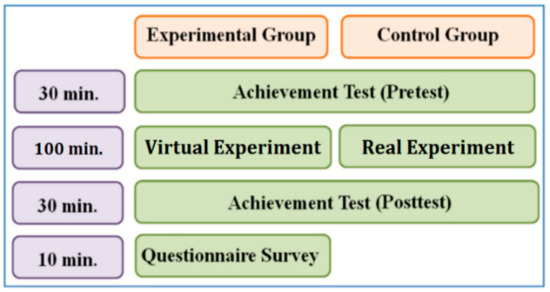
Figure 14.
Flowchart of the empirical research.
3.3. Analytical Results
The statistical software SPSS was used to analyze the achievement test results. The descriptive statistics of the pre-test and post-test scores for the two groups are listed in Table 2.

Table 2.
Descriptive statistics of the pre-test and post-test scores.
This study used the Mann–Whitney U test to check if a significant difference in the initial abilities existed between the two groups. The results in Table 3 show that p = 0.42 > 0.05, indicating no significant difference, and thus their background knowledge was about the same before the experiment.

Table 3.
The Mann–Whitney U test results between the two groups.
To investigate if the students could make significant progress after conducting the experiment, this study used the Mann–Whitney U test to analyze the pre-test and post-test scores of both groups (Table 4). For the experimental group, the asymptotic significance p < 0.001, showing the virtual experiment can effectively improve the learning achievement. For the control group, the asymptotic significance p = 0.014, indicating the real experiment can also improve the learning achievement.

Table 4.
The Mann–Whitney U test results between pre-test and post-test.
In this study, the analysis of covariance (ANCOVA) was used to analyze whether a significant difference exists in the learning effectiveness between the control group and the experimental group. Before performing the ANCOVA, the assumptions for the homogeneity of variance and within-group regression coefficients had to be satisfied.
- Homogeneity of variance
The Levene test was used to check if there is homogeneity of variance between the two groups. The analytical result shows p = 0.57 > 0.05, indicating the variance has not achieved the significant level, and thus the assumption for the homogeneity of variance is satisfied.
- Homogeneity of within-group regression coefficients
Before performing the ANCOVA, the homogeneity of within-group regression coefficients must be satisfied. The analytical result shows p = 0.60 > 0.05, also without achieving the significant level. Therefore, the covariance (pre-test score) and dependent variable (post-test score) were not influenced by the independent variable (experimental operation) and thus the ANCOVA could be performed.
- ANCOVA results
After excluding the effects of the covariance on the dependent variable, the impact of independent variable on the learning effectiveness was calculated as p = 0.70 > 0.05 (Table 5), showing no significant difference between the two groups. As a result, both the virtual experiment and the real experiment can improve students’ learning achievement effectively.

Table 5.
ANCOVA results on learning effectiveness between the two groups.
- Further analysis
To investigate the differences in learning effectiveness between students with different learning achievements, this study further divided each group into high-achievement and low-achievement sub-groups according to the median of the pre-test scores, and then analyzed the results using ANCOVA again. For the high-achievement students, the ANCOVA results show that p = 0.76 > 0.05, indicating no significant difference exists between the two groups (Table 6).

Table 6.
ANCOVA results of the high-achievement students between the two groups.
For the low-achievement students, the results in Table 7 show that p = 0.003 < 0.01, indicating a significant difference in learning effectiveness exists between the two groups. The average score of the experimental group (41.60) is significantly higher than that of the control group (27.67). According to the ANCOVA results, the virtual experiment is more effective than the real experiment for the low-achievement students, because they could conduct exploration autonomously during the virtual experiment to increase the learning motivation and enhance understanding of chemical knowledge and concepts by observing the submicroscopic phenomena in the redox reactions.

Table 7.
ANCOVA results of the low-achievement students between the two groups.
- Questionnaire results
The questionnaire adopts a five-point Likert scale [43] (strongly agree: 5 points; agree: 4 points; neutral: 3 points; disagree: 2 points; strongly disagree: 1 point) to measure students’ attitudes after conducting the virtual experiment. The percentage in each selected item and the average score, denoted by S, were computed for every question (Table 8).

Table 8.
Statistical results of questionnaire survey.
The statistic results in Table 9 show that more than a half of students were satisfied with the learning contents (S = 3.85), user interface (S = 4.11), reality (S = 3.86), learning motivation (S = 3.90), and practicality (S = 4.10) of the virtual experiment. The standard deviations for the categories were between 0.78 and 0.83. The overall average score of the questionnaire survey was 3.98. The internal consistency reliability for each category was calculated as learning contents: α = 0.83, user interface: α = 0.89, reality: α = 0.84, learning motivation: α = 0.89, and practicality: α = 0.85.

Table 9.
Average, standard deviation, and Cronbach’s alpha for each category.
In the category of learning contents, an average of 63% of users agreed or strongly agreed that the virtual experiment could increase their understanding in the experimental process. The results correspond with the answers to the unstructured question, for example: “The virtual experiment is impressive and it is easier for me to understand the chemical concepts”, “Using the virtual experiment, I can observe the submicroscopic motion of electrons and ions”, and “I can see the experimental results more clearly and it helps me better understand the Daniell cell’s principle”.
In the category of user interface, an average of 73% of users agreed or strongly agreed that the interface design of the virtual experiment satisfied their needs during the operating process. The results correspond with the answers to the unstructured question, for example: “Compared with the real experiment, the virtual experiment is more convenient and time-saving”, “It can avoid errors and reduce the experimental waste”, and “Using the tablet PC and VR cards to conduct the virtual experiment is simpler and more fun”.
In the category of system reality, an average of 64% of users agreed or strongly agreed that the simulation of chemical reactions and the process of conducting the virtual experiment were realistic. The results correspond with the answers to the unstructured question, for example: “The virtual experiment is helpful to spatial cognition”, “I can observe the submicroscopic phenomena such as the changes of chemical bonds and molecular structures”, and “I can observe the movement of electrons and ions to understand the concept of a redox reaction”.
In the category of learning motivation, an average of 65% of users agreed or strongly agreed that conducting the virtual experiment could increase their interest and learning motivation. The results correspond with the answers to the unstructured question, for example: students were “interested and engaged” in conducting the virtual experiment and it could inspire them to “have different ideas about learning chemistry”. They thought they could “learn more from trial and error”.
In the category of practicality, an average of 70% of users agreed or strongly agreed with the application of virtual experiments and its extension to other courses. The results correspond with the answers to the unstructured question, for example: “I can learn more than I do from the textbooks to enhance the understanding about the battery”, “I can see clearly the invisible phenomena in the submicroscopic world to understand its principle”, and “It is an option for those who have difficulty conducting chemistry experiments due to the lack of experimental equipment”.
3.4. Discussion
Based on the analytical results, both the virtual experiment and the real experiment could improve students’ achievement significantly in learning the principle of the redox reaction. A further investigation revealed that the virtual experiment was more effective than the real experiment for the low-achievement students. According to the theoretical framework of Johnstone’s triangle, the macroscopic and submicroscopic views are two important aspects for observing the changes in substances. To understand the cause and effect of a chemical reaction, the students have to know how the microstructures of reactants and products change, since there is a scientific and logical reasoning between them. The changes in submicroscopic views such as chemical bonds and electron transfer are closely related to the changes in macroscopic views such as material properties and chemical energy. Therefore, the application of augmented reality can make up for the lack of students’ ability in this situation. It provides an interface for the low-achievement students to visualize the 3D models of atoms and molecules, as well as their reactions, to enhance the understanding of chemical structures according to the learner’s point of view and their pace of learning.
Because the high-achievement students could understand the abstract concepts easily with or without the submicroscopic explanation, no significant difference was found in the learning effectiveness between the two groups. However, it is difficult for the low-achievement students to develop an abstract concept that is invisible in the real world, so they require the submicroscopic explanation to provide the visual aids to integrate the macroscopic and symbolic knowledge into a more complete concept. The finding is similar to that obtained by Lee and Wong [9], indicating the low spatial ability learners’ performance appeared to be more positively affected by the VR-based learning environment because it can provide more complete concepts by displaying information that cannot be seen with the naked eye [5,6]. Similar results can also be found in [25], which showed that the AR and VR technologies have a positive effect on student’s learning motivation and engagement because of the immersion and 3D visual effects.
According to the questionnaire results, the students were satisfied with the learning experience of the virtual experiment, and the overall satisfactory score was close to 4. They had higher satisfaction on user interface and practicality because the operation of virtual experiment is simple and smooth, and it is suitable for self-learning. Students thought the virtual experiment can provide more knowledge about chemistry than the real experiment, so this learning model can be applied to other courses to make learning more flexible. The remaining three categories also received average scores higher than 3.85, and the results with higher scores include: students considered the inquiry-based learning in the virtual experiment useful and very interesting; they thought the simulation of submicroscopic electrons and ions is realistic; the virtual experiment could provide timely assistance and guidance in the operation. These results are similar to the finding in [20], where the students became familiar with the geometrical structures and properties of fullerenes after conducting the virtual experiment, and it improved their learning motivation and effectiveness. In addition, the system provided scaffolds based on the theoretical framework to help students for constructing the scientific concepts, and they could explore autonomously in the inquiry process to stimulate their learning motivation and interest, which was helpful for improving the learning achievement.
In addition to the findings from the analytical results, the advantages of this system include:
- It provides an explorative learning environment for students to choose the required equipment to conduct an experiment. An error during the operation steps can be corrected by the learning scaffolds, enabling students to understand the reason causing the error.
- It provides submicroscopic models for students to understand the changes in atomic bonds and molecular structures during a chemical reaction, which is helpful for establishing the connection between the macroscopic and submicroscopic knowledge and the cognition of basic concepts.
- The system can record the experimental process and learning portfolio in the system database for the teacher to trace a student’s inquiry behavior during the virtual experiment to analyze the relationship between the inquiry process and the learning effectiveness.
- Conducting the virtual experiment is not dangerous and its application is not limited by time or space. Students only need to download the APP software and print out the AR cards to practice at home, so it is favorable for large-scale promotion in rural areas.
- The virtual experiment provides an option for teachers and students with difficulty in conducting chemistry experiments due to the lack of experimental equipment or teaching time.
Considering the system reliability and validity issues, the major tasks of image-based AR include scanning the image targets, rendering digital data into meaningful graphics, and scaling it to fit the perspective of the visual field. When using mobile devices, augmented reality must work with limited processing power, small amount of memory, and little storage. There are some factors affecting its ability to track multiple image targets: (1) the device camera’s field of view, (2) environmental lighting, including the presence of spectral reflections on the targets, (3) the device camera’s resolution setting, (4) the quality of the image targets, and (5) the device’s processing power. In this study, the AR system is unable to track more than 5 or 6 AR cards at the same time because adding more image targets for tracking simultaneously will increase the workload of the CPU, which can decrease the camera’s capture frame rate and the validity. Therefore, it is better to use a mobile device with more processing power to maintain the reliability and validity for the AR application.
4. Conclusions
The virtual experiment in this study was developed using AR and VR technologies and inquiry-based learning for students to obtain chemical knowledge and concepts during the explorative process, and it is helpful for enhancing their scientific thinking and learning effectiveness. The earlier research [21,22,23,24] pointed out that students consider chemistry a challenging subject because it is difficult to imagine the abstract concepts without seeing the atoms, molecules, and ions in the invisible 3D world, and therefore it is hard to understand their dynamic motions, interactions, and kinetics. The virtual experiment allows students to observe the change of molecular structures and movement of electrons and ions during the redox reaction, which is helpful for understanding the abstract concepts and principles in the redox reaction, especially for the low-achievement students.
The reason that students may think the microscopic concepts are difficult is mainly due to the lack of experimental manipulation and visual assistance for providing the submicroscopic views of chemical reactions. The virtual experiment developed in this study allows students to select the required equipment and set up the experimental environment via AR and VR operation. In addition, they can also obtain the important concepts and knowledge through the explorative operation under the guidance of learning scaffolds. Following the theoretical framework of Johnstone’s triangle, different levels of knowledge can be linked together to provide more complete concepts for better cognition. The questionnaire results also revealed that most students considered the inquiry-based learning in the virtual experiment very interesting, and it could help them understand the abstract concepts of chemical reactions. An empirical research was performed to investigate the learning effectiveness of the virtual experiment, and the results show that:
- (1)
- The virtual experiment was more effective for the low-achievement students.
Both groups made a significant progress on learning effectiveness, indicating the real and virtual experiments were both effective in improving learning achievement. For high-achievement students, there was no significant difference between the two groups because most of them could understand the abstract concepts of the redox reaction. The low-achievement students had difficulty imagining the submicroscopic reactions, and thus it was required to provide visual aids to help them understand the abstract concepts. Hence, the virtual experiment can achieve better learning results by showing the submicroscopic changes in the redox reaction in real time. The results confirm that augmented reality visualizes the abstract concepts and enhances the thinking ability in spatial cognition for the low-achievement students so as to achieve better learning effectiveness.
- (2)
- Most students were satisfied with the user interface, learning contents, learning motivation, system reality, and practicality of the virtual experiment.
The questionnaire results also revealed that most students considered the inquiry-based learning in the virtual experiment very interesting, and it can help them understand the abstract concepts of chemical reactions. According to the questionnaire results, the experimental group students were mainly satisfied with the operation of virtual experiment, with an overall average score equal to 3.98. Among the five categories of questions, the user interface and practicality of the virtual experiment had higher degrees of satisfaction (average score > 4), and the remaining three categories also had average scores higher than 3.85, showing that students were also satisfied with the learning contents, system reality, and learning motivation of the virtual experiment.
Limitation and Future Works
This study is focused on learning the concepts of a redox reaction; the results obtained from the virtual experiment may not be suitable for excessive inference to different learning units in chemistry education. However, increasing the reality, flexibility, and applicability remains the major issues in the development of the virtual experiment for science education. According to the feedback of the experimental group students, the design of a virtual experiment and simulation of chemical reactions still had room for improvement. As for the flexibility issue, it is suggested to increase the freedom of exploration by adding more equipment and chemical materials to enhance users’ learning interest and motivation, but the problem of scanning a larger number of AR cards simultaneously has to be solved before a more complex application can be implemented.
The learning model of virtual experiments can be applied to other courses, and our future works include the extension of the AR virtual experiment to:
- Electric circuits: students can build an electric circuit to measure the voltage and current using small light bulbs, batteries, and wires for the cases of parallel and serial connection.
- Optical experiment: students can use the convex and concave lens to observe the images formed on the screen by adjusting the distance of an object inside or outside the focal length.
- Genetic experiment: students can predict the phenotypes (dominant or recession) according to the parents’ genotypes after performing the genetic experiment using the AR cards.
Author Contributions
Investigation and formal analysis: Y.-J.L.; methodology and investigation: W.T.; writing—review and editing: K.-L.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (MOST), Taiwan under the grant numbers 109-2511-H-007-001-MY2 and 109-2511-H-007-006-MY3.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no competing interests regarding the publication of this paper.
References
- Lederman, N.G.; Lederman, J.S. Research on teaching and learning of nature of science. In Handbook of Research on Science Education; Lederman, N.G., Abell, S.K., Eds.; Routledge: New York, NY, USA, 2014; Volume II, pp. 600–620. [Google Scholar]
- Hamidu, M.Y.; Ibrahim, A.I.; Mohammed, A. The use of laboratory method in teaching secondary school students: A key to improving the quality of education. Int. J. Sci. Eng. Res. 2014, 5, 81–86. [Google Scholar]
- Department of Statistic, Ministry of Education, Taiwan. Proportion of High School Students Attending College in Taiwan. 2020. Available online: https://stats.moe.gov.tw/qframe.aspx?qno=MwAxAA2 (accessed on 10 January 2021).
- Taylor, C. The Art and Science of Lecture Demonstration; Adam Hillger: Bristol, UK, 1988. [Google Scholar]
- Ibrahim, N.H.; Surif, J.; Hui, K.P.; Yaakub, S. Typical teaching method applied in chemistry experiment. Procedia Soc. Behav. Sci. 2014, 116, 4946–4954. [Google Scholar] [CrossRef]
- Chiu, Y.J. What can college students learn from inclined plane experiments: A perspective into the inquiry-based learning. In Proceedings of the 56th National Conference on Science Education of National Science Teachers Association (NSTA), Boston, MA, USA, 27–30 March 2008. [Google Scholar]
- Dillon, J. A Review of the Research on Practical Work in School Science; King’s College: London, UK, 2008. [Google Scholar]
- Wang, T.-L.; Tseng, Y.-K. The Comparative Effectiveness of Physical, Virtual, and Virtual-Physical Manipulatives on Third-Grade Students’ Science Achievement and Conceptual Understanding of Evaporation and Condensation. Int. J. Sci. Math. Educ. 2018, 16, 203–219. [Google Scholar] [CrossRef]
- Sala, N. Applications of Virtual Reality Technologies in Architecture and in Engineering. Int. J. Space Technol. Manag. Innov. 2013, 3, 78–88. [Google Scholar] [CrossRef]
- Merchant, Z.; Goetz, E.T.; Cifuentes, L.; Keeney-Kennicutt, W.; Davis, T.J. Effectiveness of virtual reality-based instruction on students’ learning outcomes in K-12 and higher education: A meta-analysis. Comput. Educ. 2014, 70, 29–40. [Google Scholar] [CrossRef]
- Ayman, F.A. Comparing the use of virtual and conventional light microscopy in practical sessions: Virtual reality in Tabuk University. J. Taibah Univ. Med Sci. 2017, 12, 183–186. [Google Scholar]
- Bakas, C.; Mikropoulos, T. Design of virtual environments for the comprehension of planetary phenomena based on students’ ideas. Int. J. Sci. Educ. 2003, 25, 949–967. [Google Scholar] [CrossRef]
- Lee, E.A.; Wong, K.W. Learning with desktop virtual reality: Low spatial ability learners are more positively affected. J. Comput. Educ. 2014, 79, 49–58. [Google Scholar] [CrossRef]
- Azuma, R.; Baillot, Y.; Behringer, R.; Feiner, S.; Julier, S.; MacIntyre, B. Recent advances in augmented reality. IEEE Comput. Graph. Appl. 2001, 21, 34–47. [Google Scholar] [CrossRef]
- Ren, P.X. AR 3D magic book: A healthy interactive reading device based on AR and portable projection. In Proceedings of the 2020 International Conference on Computers, Information Processing and Advanced Education, Ottawa, ON, Canada, 16–18 October 2020. [Google Scholar]
- Chiang, H.C.; Yang, J.H.H.; Huang, S.J.; Su, Y.S. The effects of 5E learning strategies by image-based augmented reality of mobile learning for elementary students. Int. J. Mob. Learn. Organ. 2015, 9, 301–314. [Google Scholar] [CrossRef]
- Chatzopoulos, D.; Bermejo, C.; Huang, Z.; Hui, P. Mobile augmented reality survey: From where we are to where we go. IEEE Access 2017, 5, 6917–6950. [Google Scholar] [CrossRef]
- Ellenberger, K. Virtual and augmented reality in public archaeology teaching. Adv. Archaeol. Pract. 2017, 5, 305–309. [Google Scholar] [CrossRef]
- Özkul, E.; Kumlu, S.T. Augmented reality applications in tourism. Int. J. Contemp. Tour. Res. 2019, 3, 107–122. [Google Scholar] [CrossRef]
- Kikuo, A.; Hideaki, K.; Tomotsugu, K. Augmented instructions—A fusion of augmented reality and printed learning materials. In Proceedings of the Fifth IEEE International Conference on Advanced Learning Technologies, Kaohsiung, Taiwan, 5–8 July 2005. [Google Scholar]
- Shelton, B.E.; Hedley, N.R. Using augmented reality for teaching earth-sun relationships to undergraduate geography students. In Proceedings of the First IEEE International Augmented Reality Toolkit Workshop, Darmstadt, Germany, 29 September 2002. [Google Scholar]
- Kaufmann, H.; Schmalstieg, D. Mathematics and geometry education with collaborative augmented reality. Comput. Graph. 2003, 27, 339–345. [Google Scholar] [CrossRef]
- Woods, E.; Billinghurst, M.; Looser, J.; Aldridge, G.; Brown, D.; Garrie, B.; Nelles, C. Augmenting the Science Centre and Museum Experience; Graphite: Singapore, 2004. [Google Scholar]
- Squires, D.R. Immersive learning experiences: Technology enhanced instruction, adaptive learning, augmented reality, and m-learning in informal learning environments. J. Educ. Technol. 2019, 15, 15–21. [Google Scholar]
- Radu, I. Augmented reality in education: A meta-review and cross-media analysis. J. Pers. Ubiquitous Comput. 2014, 18, 1533–1543. [Google Scholar] [CrossRef]
- Tarng, W.; Liu, C.; Lee, C.-Y.; Lin, C.-M.; Lu, Y. A virtual laboratory for learning fullerene production and nanostructure analysis. Comput. Appl. Eng. Educ. 2018, 27, 472–484. [Google Scholar] [CrossRef]
- Koçak, Ö.; Yilmaz, R.M.; Küçük, S.; Göktas, Y. The educational potential of augmented reality technology: Experiences of instructional designers and practitioners. J. Educ. Future 2019, 5, 17–36. [Google Scholar] [CrossRef]
- Plunkett, K.N. A Simple and Practical Method for incorporating augmented reality into the classroom and laboratory. J. Chem. Educ. 2019, 96, 2628–2631. [Google Scholar] [CrossRef]
- Ayas, A.; Demirbas, A. Turkish secondary students’ conceptions of introductory chemistry concepts. J. Chem. Educ. 1997, 74, 518–521. [Google Scholar] [CrossRef]
- Nakhleh, M.B. Why some students don’t learn chemistry: Chemical misconceptions. J. Chem. Educ. 1992, 69, 191–196. [Google Scholar] [CrossRef]
- Kabapinar, F.; Adik, F. Secondary students’ understanding of the relationship between physical change and chemical bonding. Ank. Univ. J. Fac. Educ. Sci. 2005, 381, 123–147. [Google Scholar] [CrossRef]
- Yang, K.Y.; Heh, J.S. The impact of Internet virtual physics laboratory instruction on the achievement in physics, science process skills and computer attitudes of 10th grade students. J. Sci. Educ. Technol. 2007, 16, 451–461. [Google Scholar] [CrossRef]
- Johnstone, A.H. Why is science difficult to learn? Things are seldom what they seem. J. Comput. Assist. Learn. 1991, 7, 75–83. [Google Scholar] [CrossRef]
- Jiménez, Z.A. Teaching and learning chemistry via augmented and immersive virtual reality. In Technology Integration in Chemistry Education and Research (TICER); American Chemical Society: Washington, DC, USA, 2020; Chapter 3; pp. 31–52. [Google Scholar]
- Herga, N.R.; Glažar, S.A.; Dinevski, D. Dynamic visualization in the virtual laboratory enhances the fundamental understanding of chemical concepts. J. Balt. Sci. Educ. 2015, 14, 351–365. [Google Scholar]
- Wells, G. Action, Talk, and Text: Learning and Teaching through Inquiry; Practitioner Inquiry Series; Teachers College Press: New York, NY, USA, 2001. [Google Scholar]
- Ellis, A.K. Teaching and Learning Elementary Social Studies; Allyn and Bacon: Boston, MA, USA, 2002. [Google Scholar]
- Looi, C.-K. Interactive Learning Environments for Promoting Inquiry Learning. J. Educ. Technol. Syst. 1998, 27, 3–22. [Google Scholar] [CrossRef]
- Trowbridge, L.W.; Bybee, R.W. Becoming a Secondary School Science Teacher; Merrill Publishing Company: Princeton, NC, USA, 1990. [Google Scholar]
- Kyza, E.A.; Georgiou, Y. Scaffolding augmented reality inquiry learning: The design and investigation of the Trace Readers location-based, augmented reality platform. Interact. Learn. Environ. 2018, 27, 211–225. [Google Scholar] [CrossRef]
- Sırakaya, M.; Cakmak, E.K. The Effect of Augmented Reality Use on Achievement, Misconception and Course Engagement. Contemp. Educ. Technol. 2018, 9, 297–314. [Google Scholar] [CrossRef]
- Masterton, W.L.; Hurley, C.N. Chemistry: Principles and Reactions, Cengage Learning; Cengage: Boston, MA, USA, 2008; ISBN 0-495-12671-3. [Google Scholar]
- Allen, I.E.; Seaman, C.A. Likert scales and data analyses. Qual. Prog. 2007, 40, 64–65. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).