Distribution Trends of Cadmium and Lead in Timberline Coniferous Forests in the Eastern Tibetan Plateau
Abstract
:1. Introduction
2. Materials and Methods
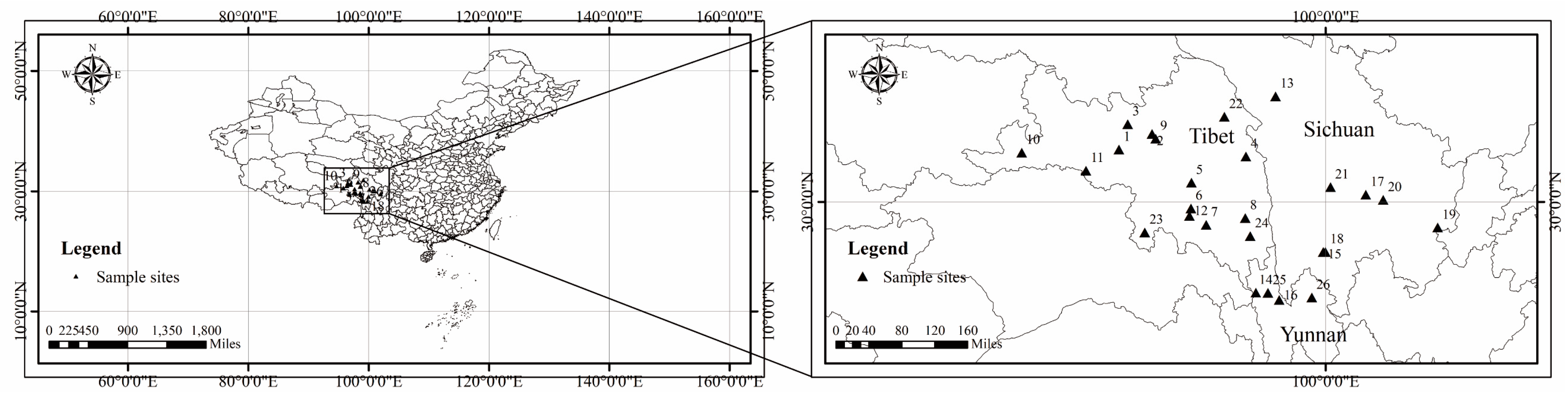
2.1. Site Description
2.2. Sampling
2.3. Element Analysis
3. Result
3.1. Mean Concentrations of Pb and Cd in Needles and Twigs
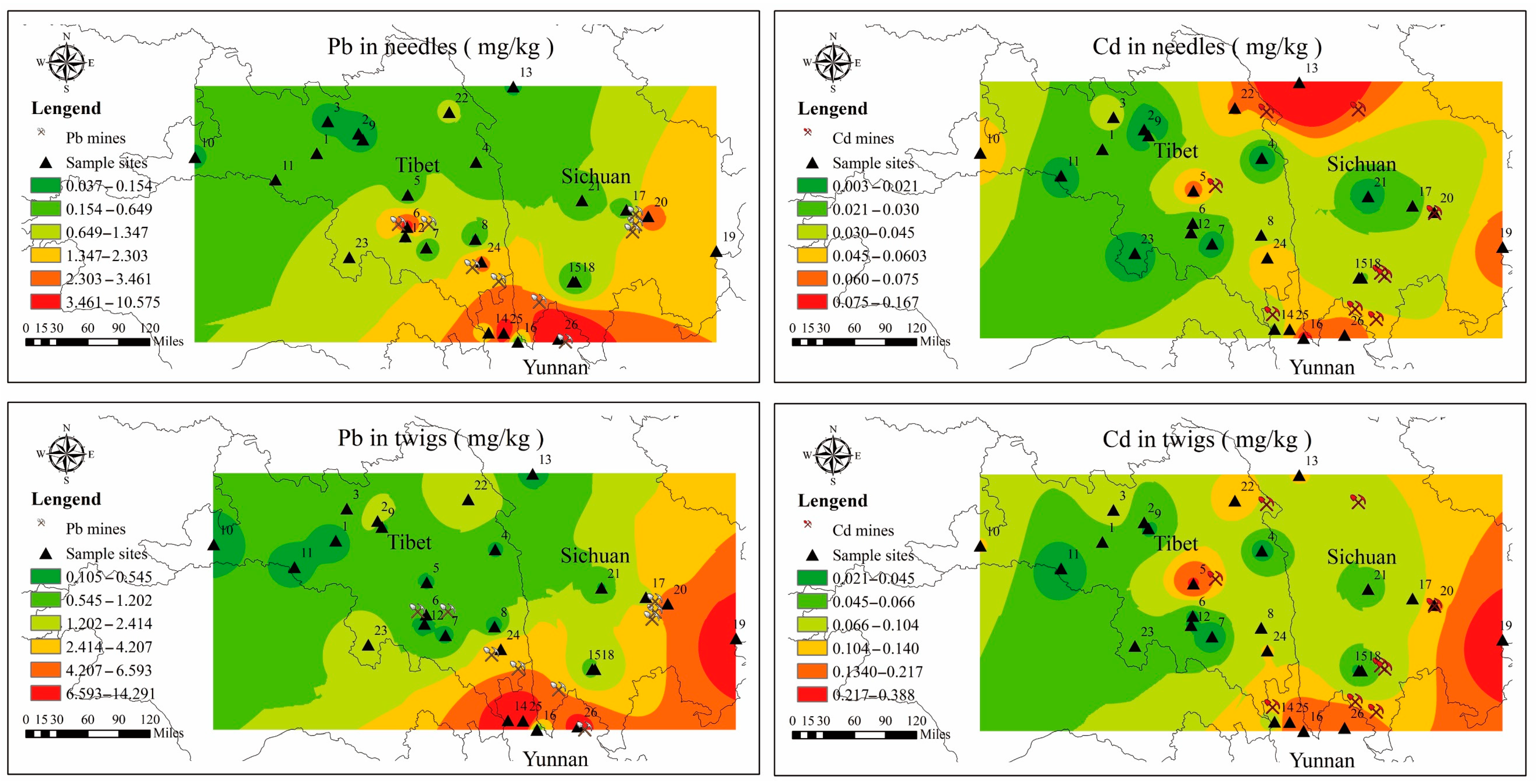
3.2. Spatial Distribution Maps of Pb and Cd in Needles and Twigs
4. Discussion
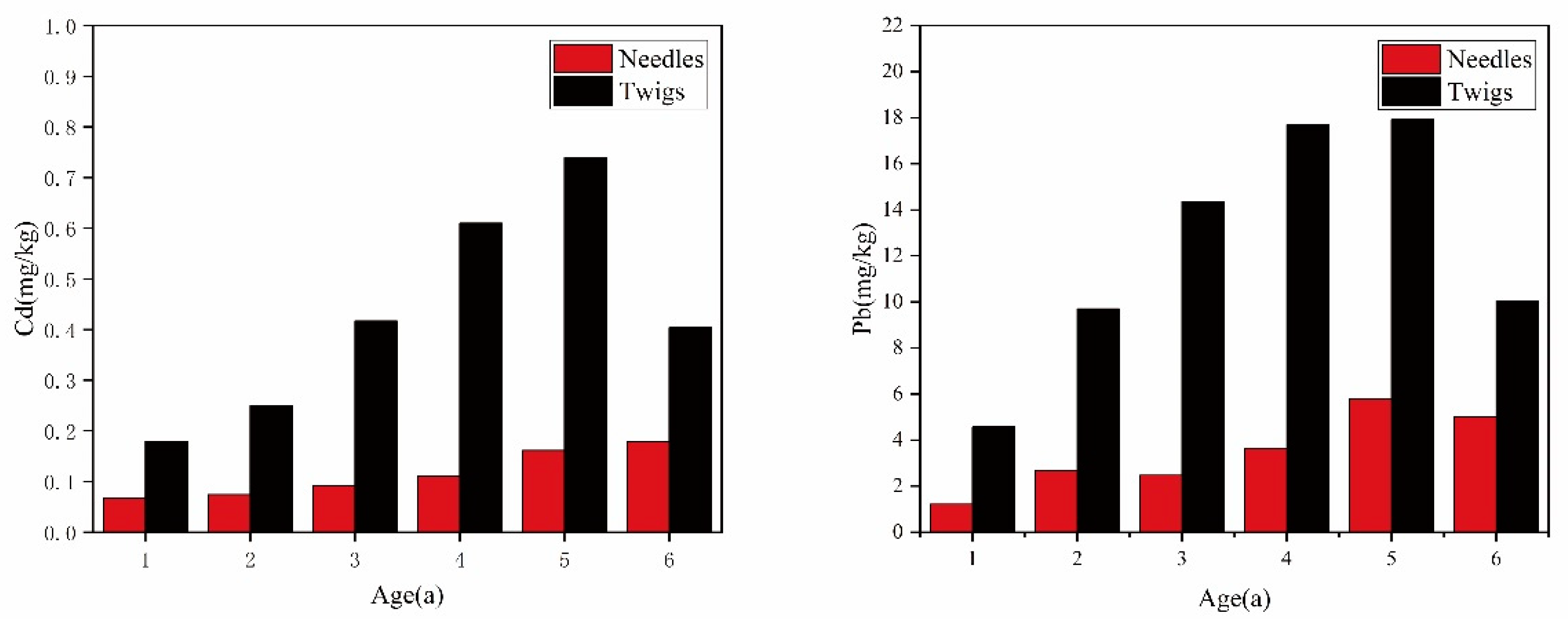
4.1. Concentration of Pb and Cd in Needles and Twigs
4.2. Comparison of Pb and Cd between Needles and Twigs
4.3. Sources of Pb and Cd between Needles and Twigs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, F.; Wen, D.; Kuang, Y.; Li, J.; Li, J.; Zuo, W. Concentrations of heavy metals and polycyclic aromatic hydrocarbons in needles of Masson pine Pinus massonianal growing nearby different industrial sources. J. Environ. Sci. 2010, 22, 1006–1013. [Google Scholar]
- Qin, J.; Li, Z.; Lou, M. Status, Sources of Pollution and Control Measures of Chinese Children Lead Poisoning Guangdong. Trace Elem. Sci. 2010, 17, 1–13. [Google Scholar]
- Kim, N.-S.; Sakong, J.; Choi, J.-W.; Hong, Y.-S.; Moon, J.-D.; Lee, B.-K. Blood lead levels of residents living around 350 abandoned metal mines in Korea. Environ. Monit. Assess 2012, 184, 4139–4149. [Google Scholar]
- Niisoe, T.; Harada, K.H.; Hitomi, T.; Watanabe, T.; Hung, N.; Ishikawa, H.; Wang, Z.; Koizumi, A. Environmental ecological modeling of human blood lead levels in East Asia. Environ. Sci. Technol. 2011, 45, 2856–2862. [Google Scholar]
- Migon, C.; Journel, B.; Nicolas, E. Measurement of trace metal wet, dry and total atmospheric fluxes over the Ligurian Sea. Atmos. Environ. 1997, 31, 889–896. [Google Scholar]
- Bur, T.; Probst, A.; Bianco, A.; Gandois, L.; Crouau, Y. Determining cadmium critical concentrations in natural soils by assessing Collembola mortality, reproduction and growth. Ecotoxicol. Environ. Saf. 2010, 73, 415–422. [Google Scholar]
- Lovett, G.M.; Lindberg, S.E. Dry deposition and canopy exchange in a mixed oak forest as determined by analysis of throughfall. J. Appl. Ecol. 1984, 21, 1013–1027. [Google Scholar] [CrossRef]
- Kuang, Y.W.; Wen, D.Z.; Zhou, G.Y.; Liu, S.Z. Distribution of elements in needles of Pinus massoniana (Lamb) was uneven and affected by needle age. Environ. Pollut. 2007, 145, 146–153. [Google Scholar]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar]
- Markert, B. Plants as Biomonitors: Indicators for Heavy Metals in the Terrestrial Environment; Vch Weinheim: Weinheim, Germany, 1993. [Google Scholar]
- Kord, B.; Mataji, A.; Babaie, S. Pine (Pinus eldarica Medw.) needles as indicator for heavy metals pollution. Int. J. Environ. Sci. Technol. 2010, 7, 79–84. [Google Scholar]
- Aboal, J.; Ferna´ndez, J.; Carballeira, A. Oak leaves and pine needles as biomonitors of airborne trace elements pollution. Environ. Exp. Bot. 2004, 51, 215–225. [Google Scholar] [CrossRef]
- Rossbach, M.; Jayasekera, R. Air pollution monitoring at the Environmental Specimen Bank of Germany: Spruce and pine shoots as bioindicators. Fresenius J. Anal Chem. 1996, 354, 511–514. [Google Scholar]
- Al-Alawi, M.t.M.; Mandiwana, K.L. The use of Aleppo pine needles as a biomonitor of heavy metals in the atmosphere. J. Hazard Mater. 2007, 148, 43–46. [Google Scholar] [PubMed]
- Trimbacher, C.; Weiss, P. Norway spruce: Anovel method using surface characteristics and heavy metal concentrations of needles for a large-scale monitoring survey in Austria. Water Air. Soil Pollut. 2004, 152, 363–386. [Google Scholar] [CrossRef]
- Gratton, W.; Nkongolo, K.; Spiers, G. Heavy metal accumulation in soil and jack pine (Pinus banksiana) needles in Sudbury, Ontario, Canada. Bull. Environ. Contam. Toxicol. 2000, 64, 550–557. [Google Scholar] [PubMed]
- Sun, S.Q.; Wu, Y.H.; Zhou, J.; Yu, D.; Luo, J.; Bing, H.J. Comparison of element concentrations in fir and rhododendron leaves and twigs along an altitudinal gradient. Environ. Toxicol. Chem. 2011, 30, 2608–2619. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Wen, T.; Li, W.; Zhao, Y.; Li, L. Elemental composition of PM2.5 and PM10 at Mount Gongga in China during 2006. Atmos. Res. 2009, 93, 801–810. [Google Scholar]
- Steinnes, E.; Friedland, A.J. Metal contamination of natural surface soils from long-range atmospheric transport: Existing and missing knowledge. Environ. Rev. 2005, 14, 169–186. [Google Scholar] [CrossRef]
- Luo, J.; She, J.; Wu, Y.; Yu, D.; Chen, Y.; Zhou, P. Cadmium distribution in a timberline forest in the Hengduan mountains in the Eastern Tibetan Plateau. Anal. Lett. 2013, 46, 394–405. [Google Scholar] [CrossRef]
- Ceburnis, D.; Steinnes, E. Conifer needles as biomonitors of atmospheric heavy metal deposition: Comparison with mosses and precipitation, role of the canopy. Atmos. Environ. 2000, 34, 4265–4271. [Google Scholar]
- Holoubek, I.; Korínek, P.; Seda, Z.; Schneiderová, E.; Holoubková, I.; Pacl, A.; Tríska, J.; Cudlın, P.; Cáslavsk, J. The use of mosses and pine needles to detect persistent organic pollutants at local and regional scales. Environ. Pollut. 2000, 109, 283–292. [Google Scholar] [PubMed]
- Pouyat, R.; McDonnell, M. Heavy metal accumulations in forest soils along an urban–rural gradient in southeastern New York, USA. Water Air Siol Pollut. 1991, 57, 797–807. [Google Scholar] [CrossRef]
- Oral, E.V.; Dolak, I.; Temel, H.; Ziyadanogullari, B. Preconcentration and determination of copper and cadmium ions with 1,6-bis (2-carboxy aldehyde phenoxy) butane functionalized Amberlite XAD-16 by flame atomic absorption spectrometry. J. Hazard. Mater. 2011, 186, 724–730. [Google Scholar] [CrossRef]
- Aragay, G.; Merkoçi, A. Nanomaterials application in electrochemical detection of heavy metals. Electrochim. Acta 2012, 84, 49. [Google Scholar] [CrossRef]
- Lee, S.; Jiseop, O.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [PubMed]
- Cai, S.; Pan, H.; Gonzalez-Vila, Á.; Guo, T.; Gillan, D.C.; Wattiez, R.; Caucheteur, C. Selective detection of cadmium ions using plasmonic optical fiber gratings functionalized with bacteria. Opt. Exp. 2020, 28, 19740–19749. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, R.; Borguet, F.; DeKimpe, J. Trace elements in medicine. Speciation: The new frontier. Anal. Chim. Acta 1993, 283, 183–189. [Google Scholar]
- Crompton, T.R. A review of the analysis of organometallic compounds in the environment. Environ. Int. 1998, 14, 417–463. [Google Scholar]
- Tang, R.; Luo, J.; Yang, P.; She, J.; Chen, Y.; Gonga, Y.; Zhou, J. Trace metals of needles and litter in timberline forests in the Eastern of Tibetan Plateau, China. Ecol. Indic. 2014, 45, 669–676. [Google Scholar]
- Gandois, L.; Probst, A. Localisation and mobility of trace metal in silver fir needles. Chemosphere 2012, 87, 204–210. [Google Scholar]
- Xiao, C.; Qin, D.; Yao, T. The global pollution revealed by Pb, Cd in the surface snow of Antarctic, Arctic and QingHai- Xizang plateau. Chin. Sci. Bull. 1999, 44, 2558–2563. [Google Scholar]
- Li, Y.; Yao, T.; Wang, N. Atmosphere pollution revealed by Cadmium in the Guliya Ice core, Qinghai- Tibet Plateau. Environ. Chem. 2000, 19, 176–180. [Google Scholar]
- Duan, K.; Yao, T.; Li, Y. Lead pollution in the river water of Shuanghu Region, center of Tibetan Plateau. J. Glaciol. Geocryol. 2000, 22, 282–283. [Google Scholar]
- Jozef, M.P.; Elisabeth, G.P. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar]
- Loewen, M.; Kang, S.; Armstrong, D.; Zhang, Q.; Tomy, G.; Wang, F. Atmospheric transport of mercury to the Tibetan Plateau. Environ. Sci. Technol. 2007, 41, 7632–7638. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil-plant transfer of trace elements–an environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Klaminder, J.; Bindler, R.; Emteryd, O.; Renberg, I. Uptake and recycling of lead by boreal forest plants: Quantitative estimates from a site in northern Sweden. Geochim. Cosmochim. Acta 2005, 69, 2485–2496. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Breckle, S.-W.; Kahle, H. Effects of toxic heavy metals (Cd, Pb) on growth and mineral nutrition of beech (Fagus sylvatica L). Vegetation 1992, 101, 43–53. [Google Scholar] [CrossRef]
- Begum, B.A.; Kim, E.; Biswas, S.K.; Hopke, P.K. Investigation of sources of atmospheric aerosol at urban and semi-urban areas in Bangladesh. Atmos. Environ. 2004, 38, 3025–3038. [Google Scholar] [CrossRef]
- Salam, A.; Bauer, H.; Kassin, K.; Mohammad, U.S.; Puxbaum, H. Aerosol chemical characteristics of a mega-city in Southeast Asia (DhakaeBangladesh). Atmos. Environ. 2003, 37, 2517–2528. [Google Scholar] [CrossRef]
- Hovmand, M.F.; Kemp, K.; Kystol, J.; Johnsen, I.; Riis-Nielsen, T.; Pacyna, J. Atmospheric heavy metal deposition accumulated in rural forest soils of southern Scandinavia. Environ. Pollut. 2008, 155, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bin, H.; Zhou, J.; Luo, J.; Yu, D.; Sun, S.; Li, W. Atmospheric deposition of Cd accumulated in the montane soil, Gongga Mt, China. J. Soils Sediments 2011, 11, 940–946. [Google Scholar] [CrossRef]
- Unger, H.J.; Prinz, D. Bodenbelastung an Straßen mit Schwermetallen und organischen Fremdstoffen; Erich Schmidt Verlag: Berlin, Germany, 1997; Volume 23, p. 97. [Google Scholar]
- Schroeder, W.H.; Munthe, J. Atmospheric mercury-an overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]

| Heavy Element Concentrations (mg kg−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Needles (n = 30) | Twigs (n = 19) | ||||||||
| Min | Max | Mean | SD | Min | Max | Mean | SD | ||
| Cd | 0.003 | 0.167 | 0.034 | 0.070 | Cd | 0.021 | 0.388 | 0.101 | 0.084 |
| Pb | 0.037 | 10.576 | 1.291 | 2.371 | Pb | 0.102 | 14.311 | 2.551 | 3.711 |
| Concentrations (mg/kg) | |||||
|---|---|---|---|---|---|
| n | Site | Pb | Cd | ||
| 1 | 126 | Tibetan Plateau | 0.92 | 0.05 | Tang et al. (2014) |
| 2 | 50 | Remote from a source in Norway | 0.4 | 0.04 | Trimbacher and Weiss (2001) |
| 3 | 25 | Steel works in Norway | 0.6 | 0.06 | Trimbacher and Weiss (2001) |
| 4 | 27 | Metallurgical industry in Norway | 0.8 | 0.11 | Trimbacher and Weiss (2001) |
| 5 | 11 | Highways in Norway | 1.1 | 0.08 | Trimbacher and Weiss (2001) |
| 6 | 11 | South France | 0.2 | 0.09 | L. Gandois and A. Probst (2012) |
| 7 | 48 | Lithuania | 0.77 | 0.09 | D. Ceburnis, E. Steinnes (1999) |
| 8 | 7 | Southern Norway | 0.89 | 0.173 | Berthelsen et al. (1995) |
| 9 | 7 | Central Norway | 0.03 | 0.011 | Berthelsen et al. (1995) |
| 10 | 26 | Tibet Plateau | 1.291 | 0.034 | Present study |
| Parameters | Mann–Whitney U | Wilcoxon W | Z | p | n | Twigs/Needles |
|---|---|---|---|---|---|---|
| Cd | 144 | 495 | −3.511 | p < 0.001 | 26 | 1.9 |
| Pb | 50 | 199 | −2.544 | p < 0.05 | 26 | 2.9 |
| Component Matrix (70.642% of Total Variance Explained) | ||||||
|---|---|---|---|---|---|---|
| Element | Component Matrix | Rotated Component Matrix | ||||
| 1(42%) | 2(16%) | 3(12%) | 1(35%) | 2(19%) | 3(17%) | |
| V | 0.885 | −0.233 | −0.175 | 0.903 | 0.186 | 0.136 |
| Cr | 0.775 | −0.298 | 0.368 | 0.701 | −0.105 | 0.567 |
| Co | 0.902 | −0.105 | −0.171 | 0.857 | 0.3 | 0.172 |
| Ni | 0.499 | −0.475 | 0.018 | 0.64 | −0.233 | 0.109 |
| Cu | 0.682 | −0.406 | −0.339 | 0.853 | 0.021 | −0.126 |
| Zn | 0.63 | 0.048 | 0.116 | 0.481 | 0.23 | 0.358 |
| As | 0.926 | 0.006 | −0.228 | 0.84 | 0.425 | 0.155 |
| Mo | 0.413 | 0.073 | 0.841 | 0.101 | −0.087 | 0.93 |
| Sb | 0.424 | 0.588 | −0.24 | 0.147 | 0.746 | 0.072 |
| Pb | 0.591 | 0.661 | 0.199 | 0.141 | 0.713 | 0.546 |
| Cd | 0.424 | 0.474 | 0.272 | 0.068 | 0.465 | 0.508 |
| Hg | 0.222 | 0.621 | −0.426 | 0.008 | 0.767 | −0.166 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Luo, J.; Peng, P.; Li, W.; Yang, D.; Shi, W.; Xu, Q.; Lai, X. Distribution Trends of Cadmium and Lead in Timberline Coniferous Forests in the Eastern Tibetan Plateau. Appl. Sci. 2021, 11, 753. https://doi.org/10.3390/app11020753
Jia L, Luo J, Peng P, Li W, Yang D, Shi W, Xu Q, Lai X. Distribution Trends of Cadmium and Lead in Timberline Coniferous Forests in the Eastern Tibetan Plateau. Applied Sciences. 2021; 11(2):753. https://doi.org/10.3390/app11020753
Chicago/Turabian StyleJia, Longyu, Ji Luo, Peihao Peng, Wei Li, Danli Yang, Wenbo Shi, Qian Xu, and Xiyi Lai. 2021. "Distribution Trends of Cadmium and Lead in Timberline Coniferous Forests in the Eastern Tibetan Plateau" Applied Sciences 11, no. 2: 753. https://doi.org/10.3390/app11020753
APA StyleJia, L., Luo, J., Peng, P., Li, W., Yang, D., Shi, W., Xu, Q., & Lai, X. (2021). Distribution Trends of Cadmium and Lead in Timberline Coniferous Forests in the Eastern Tibetan Plateau. Applied Sciences, 11(2), 753. https://doi.org/10.3390/app11020753




