Platinum Based Nanoparticles Produced by a Pulsed Spark Discharge as a Promising Material for Gas Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Platinum-Based Nanoparticles
2.2. Ink Formulation
2.3. Aerosol Jet Printing
2.4. Characterization of Materials
3. Results and Discussion
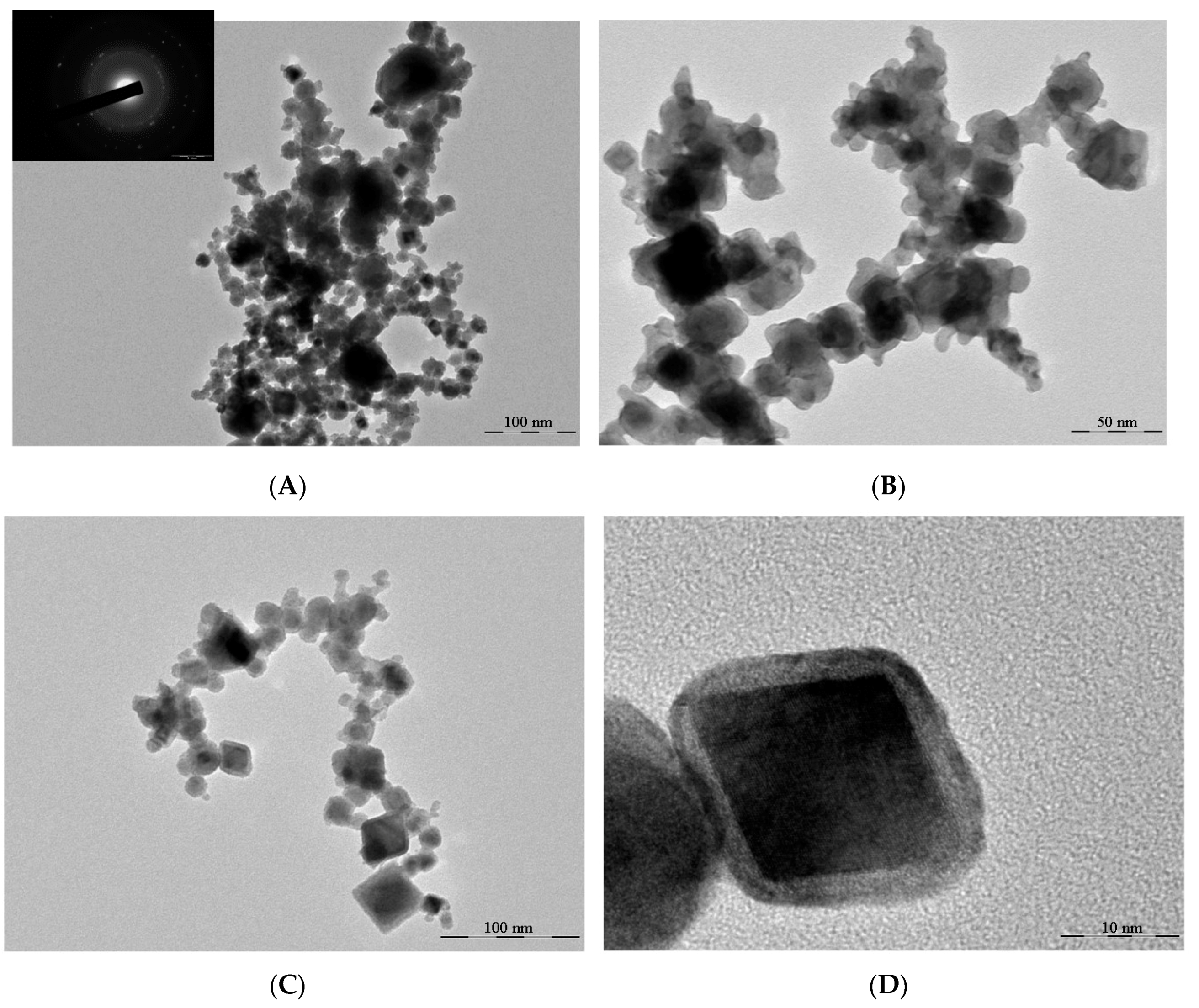
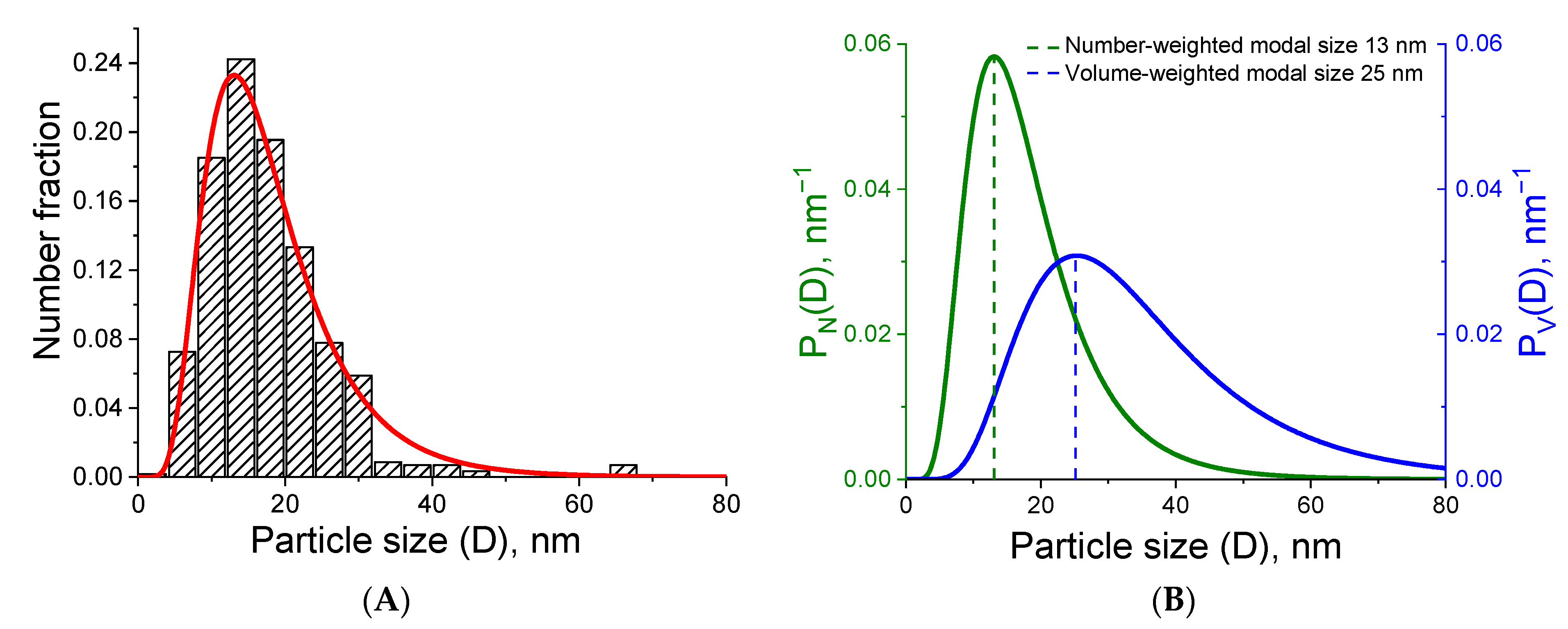
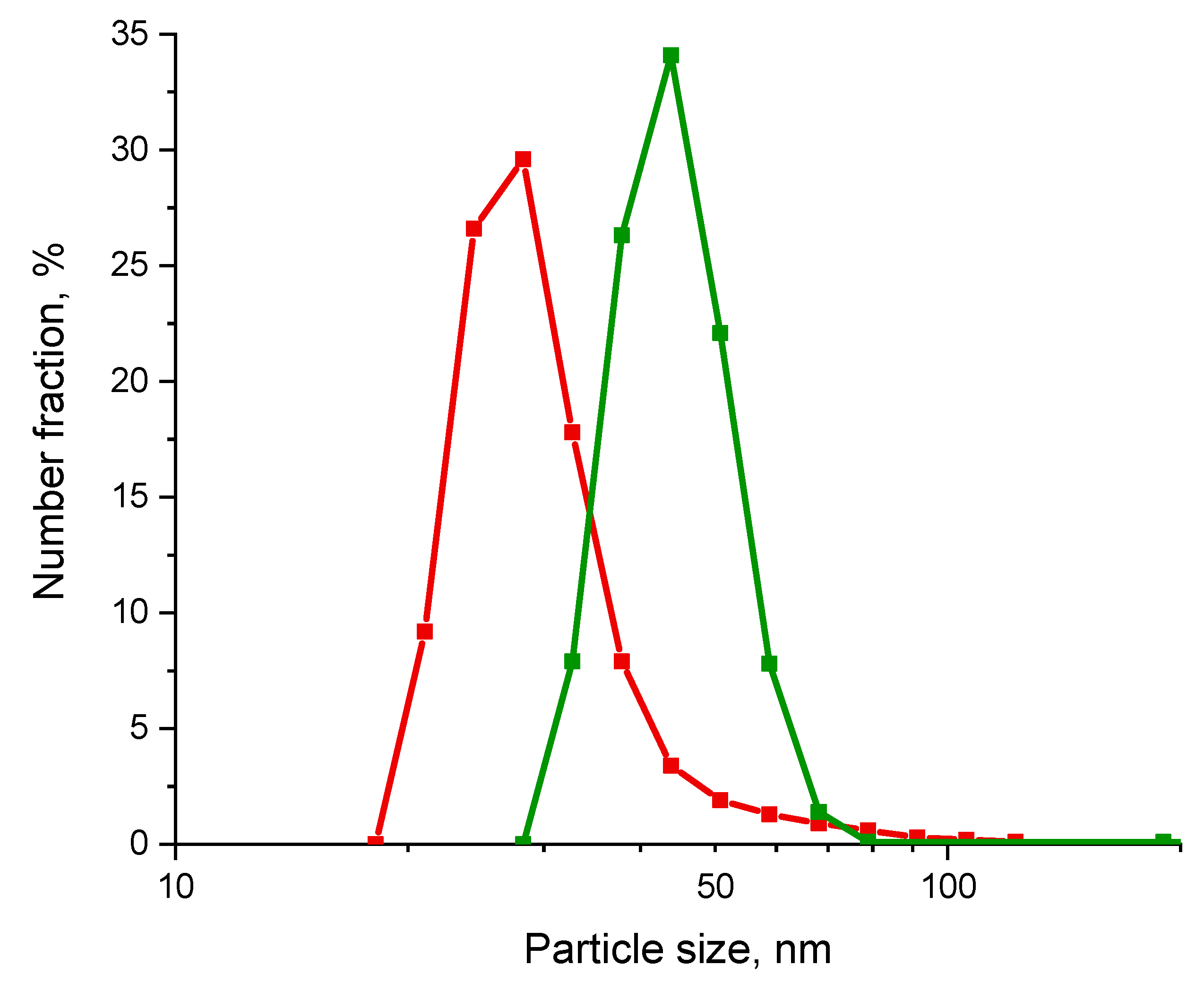
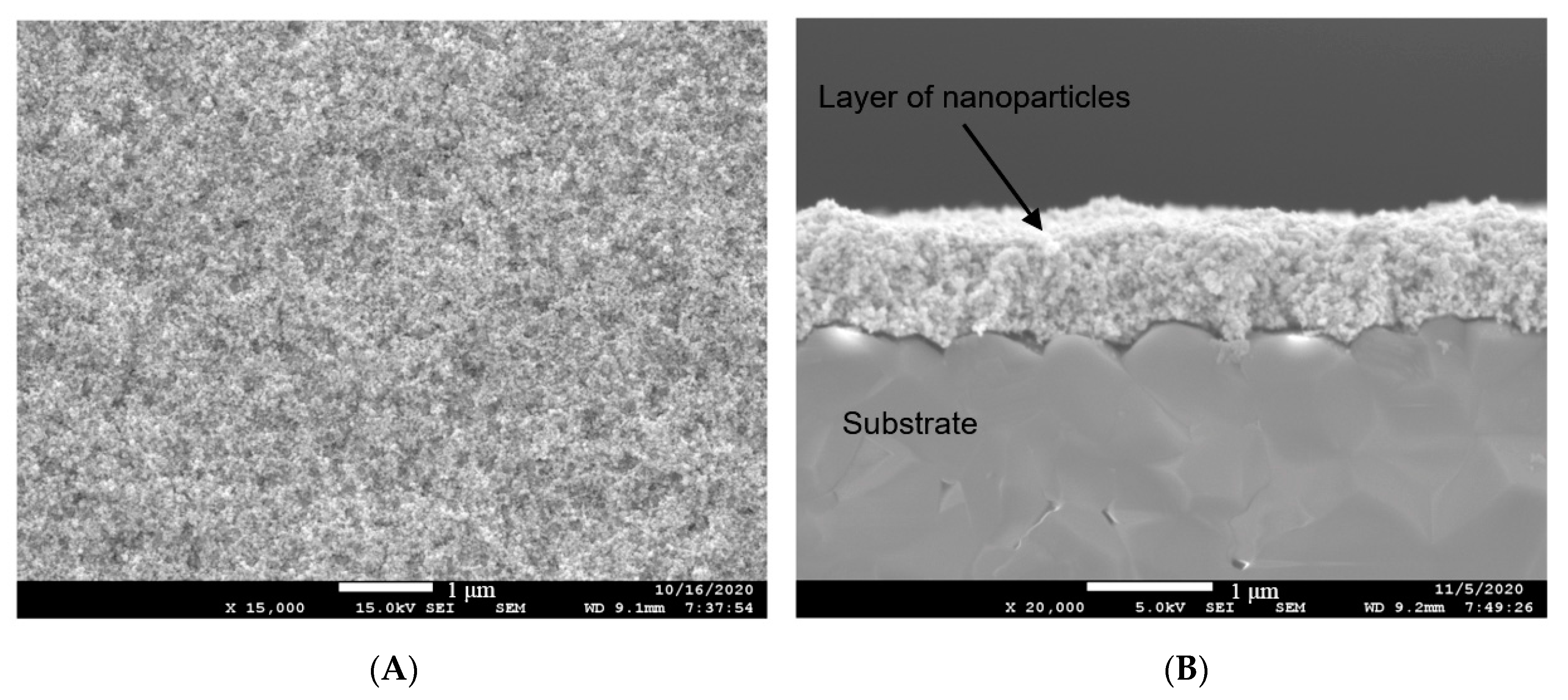
3.1. Results of Characterization of Materials
3.1.1. Platinum-Based Powder and Sintered Material
3.1.2. Platinum-Based Functional Ink
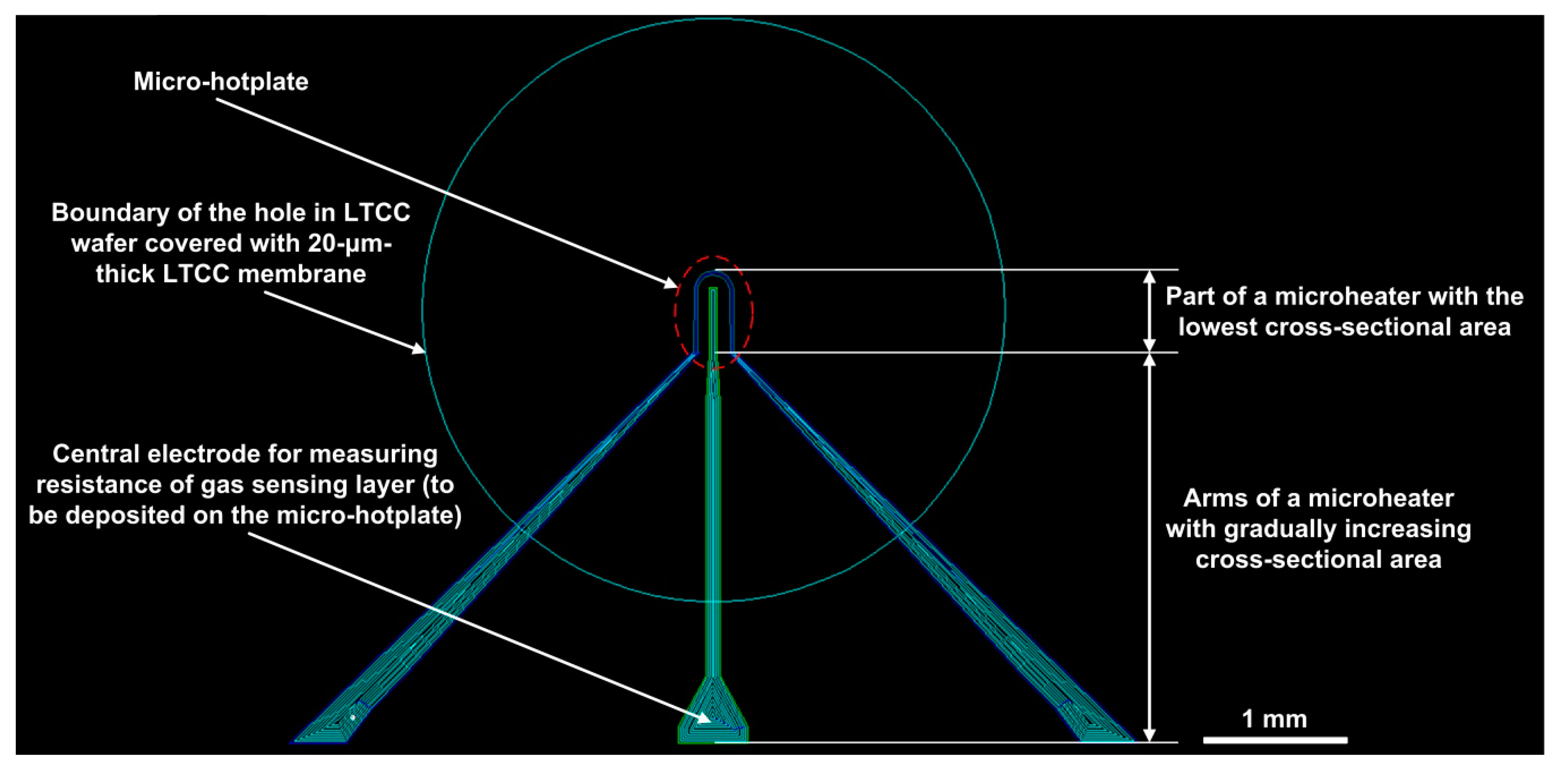
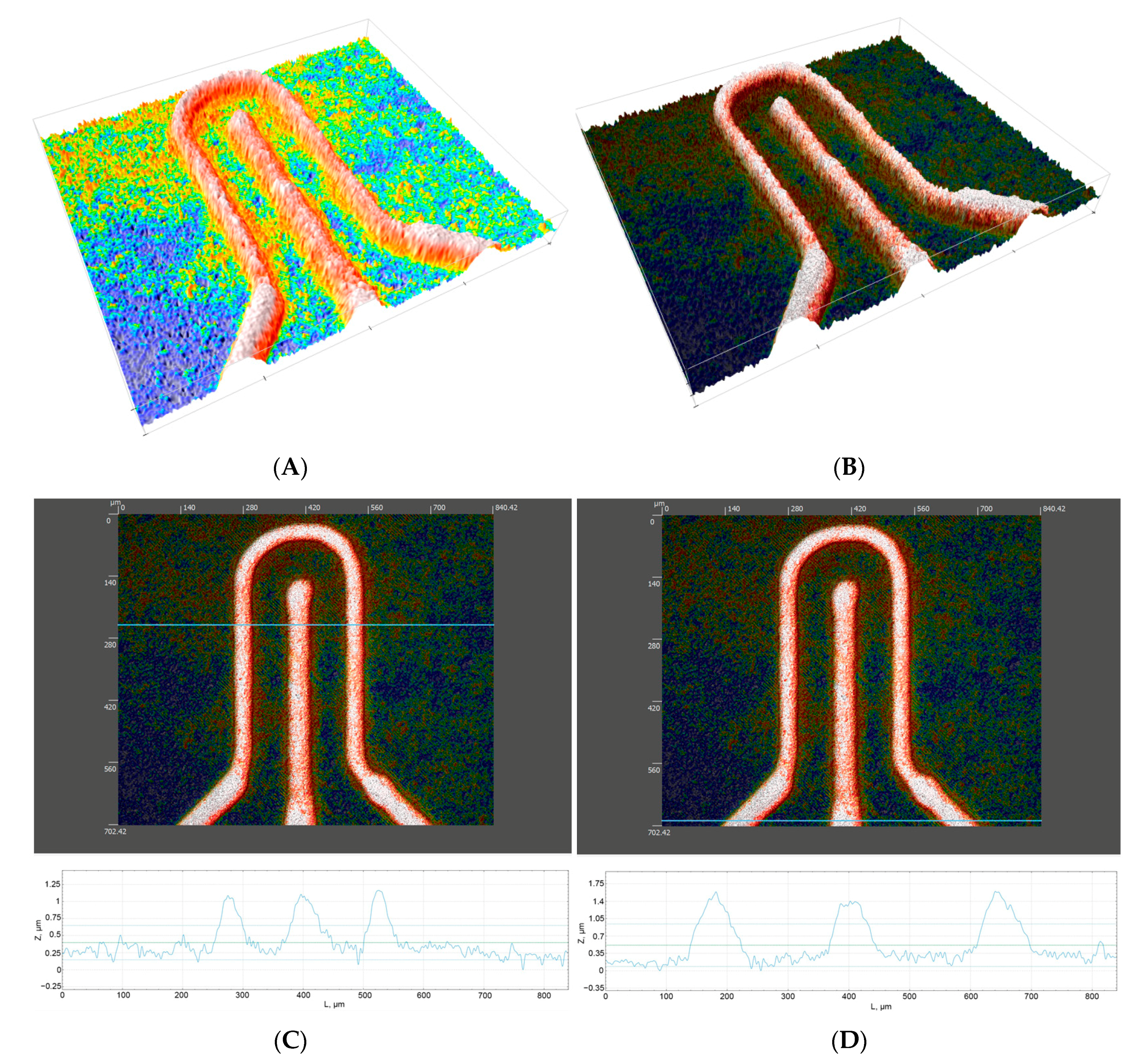
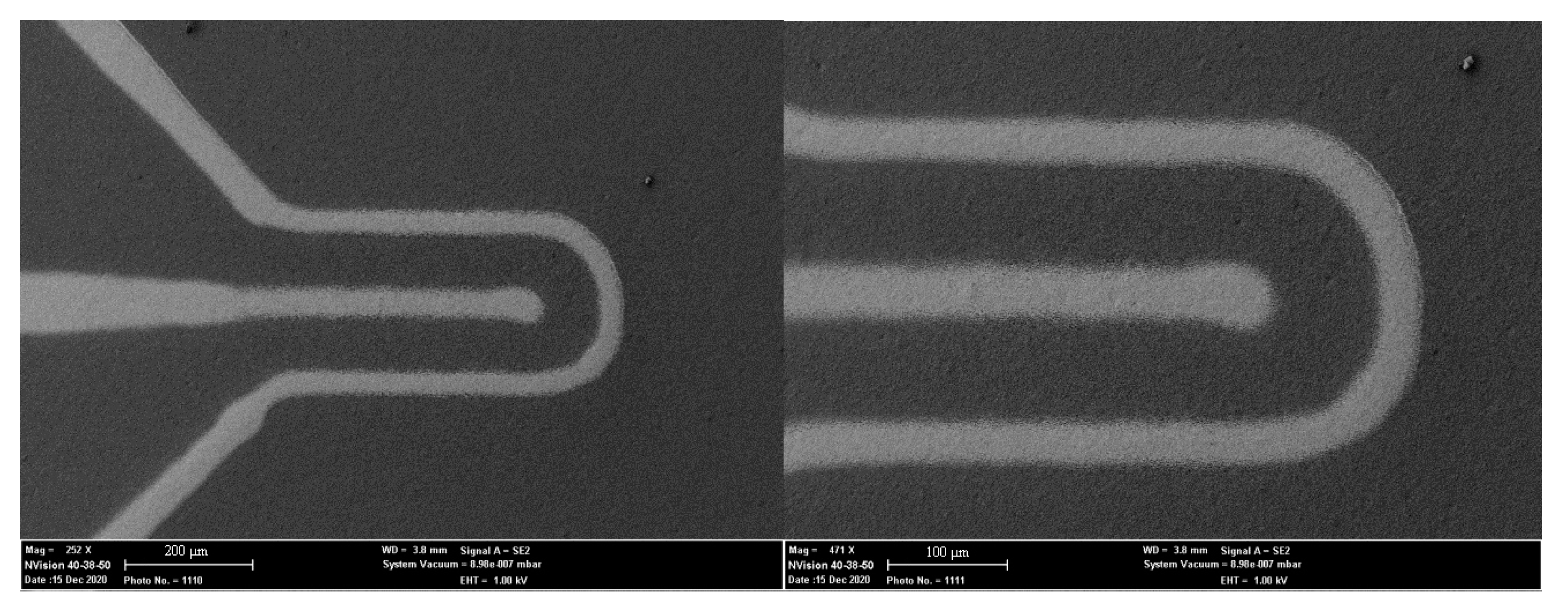
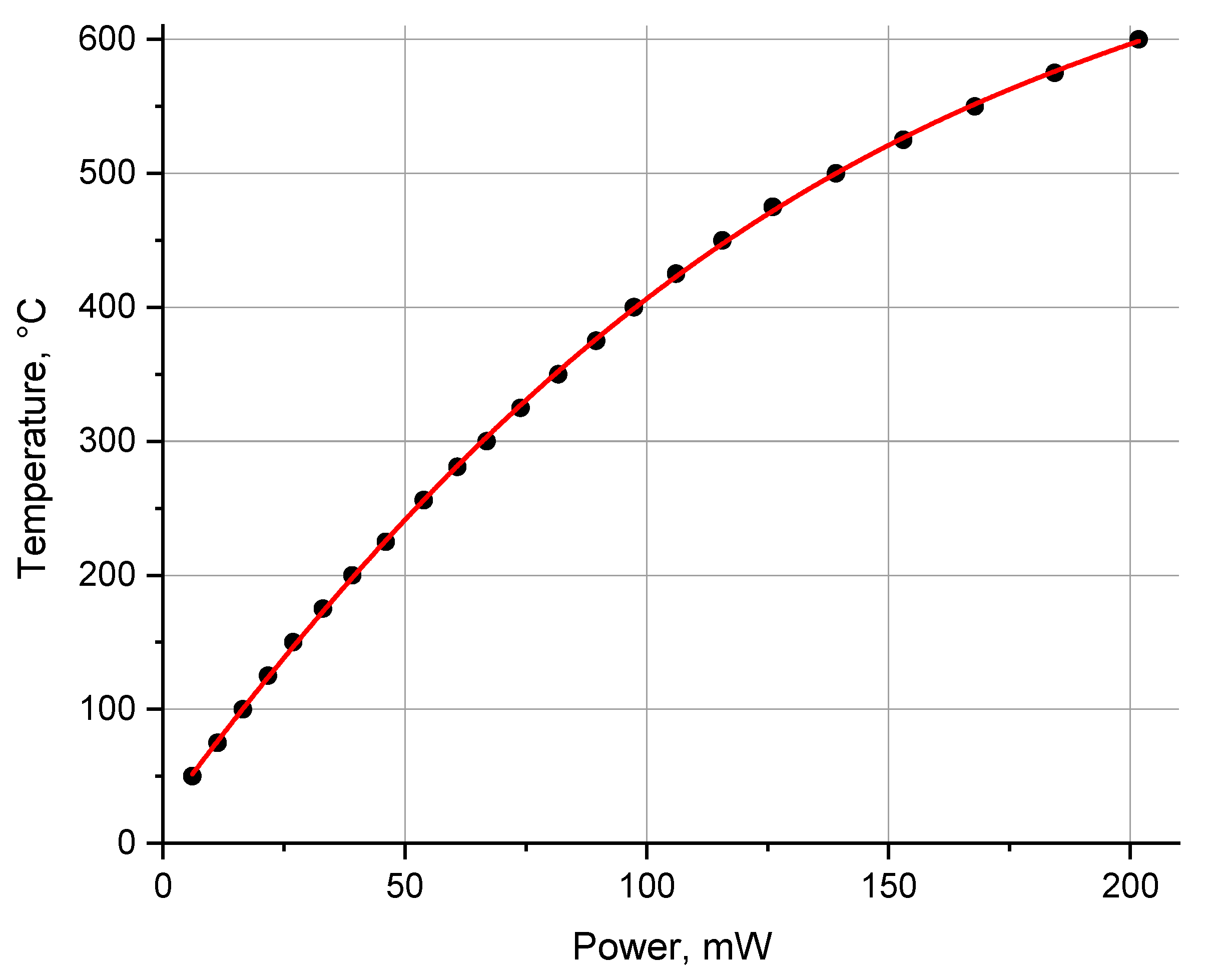
3.2. Topography and Performance of Microheaters
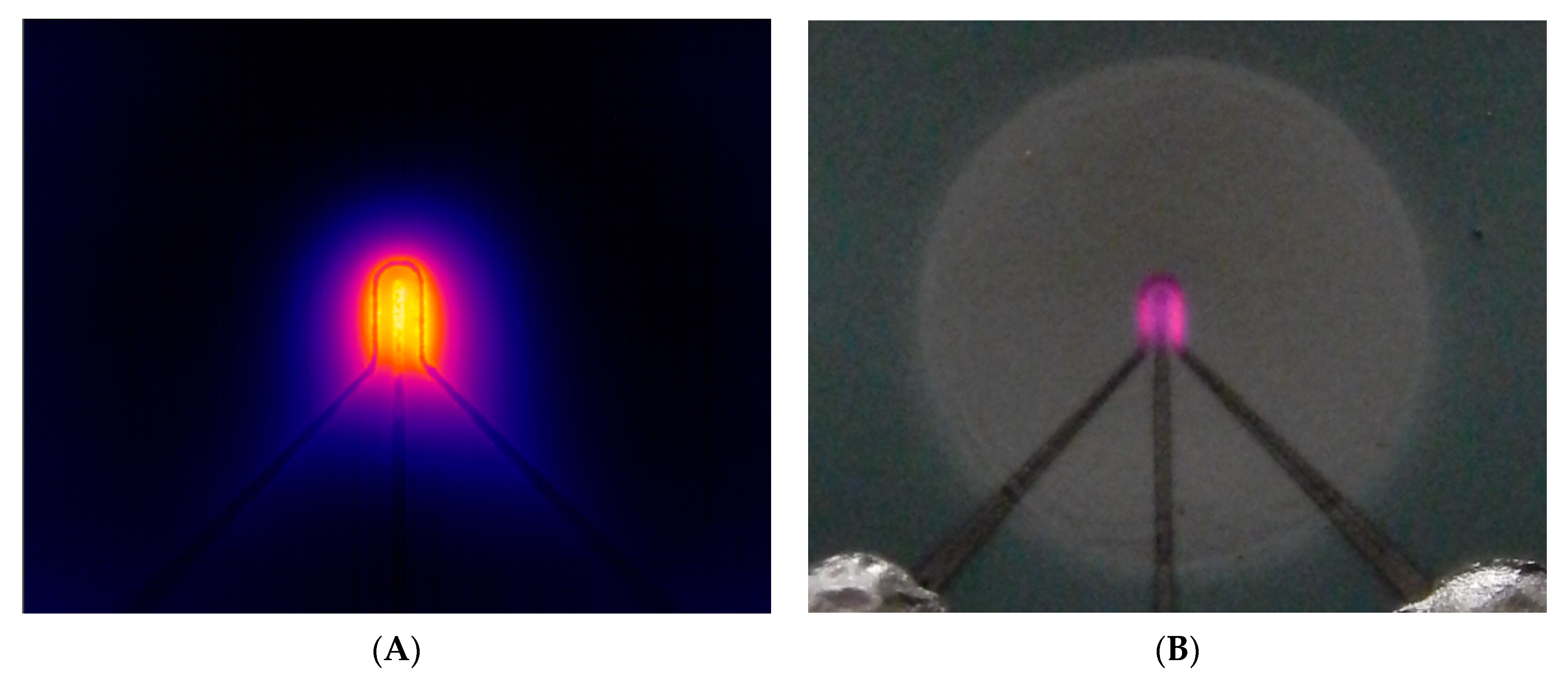
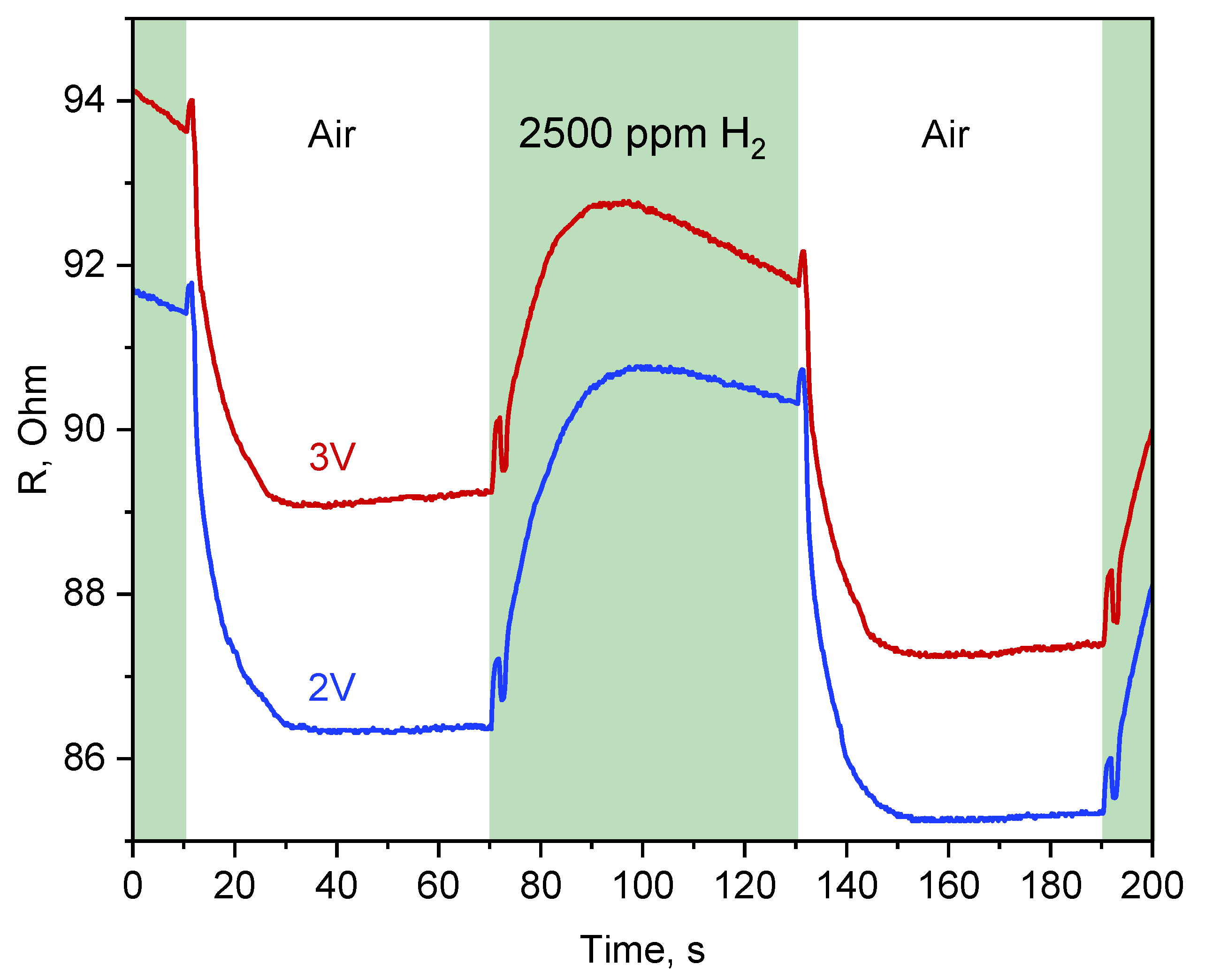
3.3. Catalytic Activity of Synthesized Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Powder | Sintered Material | |
|---|---|---|
| Elemental composition (ICP-AES) | Pt: 92.7 wt. % | Pt: 99.7 wt. % |
| Sn: 0.077 wt. % | Sn: 0.086 wt. % | |
| Cu: 0.074 wt. % | Cu: 0.072 wt. % | |
| Ag: 0.033 wt. % | Ag: 0.037 wt. % | |
| Pd: 0.022 wt. % | Pd: 0.023 wt. % | |
| Fe: 0.014 wt. % | Fe: 0.020 wt. % | |
| Zn: 0.004 wt. % | Zn: 0.014 wt. % | |
| Ca: 0.004 wt. % | Ca: 0.005 wt. % | |
| Phase composition (XRD, XPS) |
| Crystalline metallic platinum (Fm3m crystal structure)–more than 99 wt. %. |
| Particle size parameters determined from TEM images Crystalline phase identified from SAED patterns |
| – |
| Grain size (SEM) | – | 100–400 nm |
| Resistivity | – | 1.2·10−7 Ω·m (1.1 times greater than that of the bulk Pt due to the insignificant residual porosity) |
References
- Gänzler, A.M.; Casapu, M.; Vernoux, P.; Loridant, S.; Cadete Santos Aires, F.J.; Epicier, T.; Grunwaldt, J.D. Tuning the structure of platinum particles on ceria in situ for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chem. Int. Ed. 2017, 56, 13078–13082. [Google Scholar] [CrossRef]
- Yoon, K.; Yang, Y.; Lu, P.; Wan, D.; Peng, H.C.; Stamm Masias, K.; Xia, Y. A highly reactive and sinter-resistant catalytic system based on platinum nanoparticles embedded in the inner surfaces of CeO2 hollow fibers. Angew. Chem. 2012, 124, 9681–9684. [Google Scholar] [CrossRef]
- Ioroi, T.; Siroma, Z.; Yamazaki, S.I.; Yasuda, K. Electrocatalysts for PEM fuel cells. Adv. Energy Mater. 2019, 9, 1801284. [Google Scholar] [CrossRef]
- Li, K.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Platinum nanoparticles partially-embedded into carbon sphere surfaces: A low metal-loading anode catalyst with superior performance for direct methanol fuel cells. J. Mater. Chem. A 2017, 5, 19857–19865. [Google Scholar] [CrossRef]
- Spirjakin, D.; Baranov, A.M.; Somov, A.; Sleptsov, V. Investigation of heating profiles and optimization of power consumption of gas sensors for wireless sensor networks. Sens. Actuators A Phys. 2016, 247, 247–253. [Google Scholar] [CrossRef]
- Harley-Trochimczyk, A.; Pham, T.; Chang, J.; Chen, E.; Worsley, M.A.; Zettl, A.; Maboudian, R. Platinum Nanoparticle Loading of Boron Nitride Aerogel and Its Use as a Novel Material for Low-Power Catalytic Gas Sensing. Adv. Funct. Mater. 2016, 26, 433–439. [Google Scholar] [CrossRef]
- Shlenkevitch, D.; Stolyarova, S.; Blank, T.; Brouk, I.; Nemirovsky, Y. Novel Miniature and Selective Combustion-Type CMOS Gas Sensor for Gas-Mixture Analysis—Part 1: Emphasis on Chemical Aspects. Micromachines 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Comert, B.; Akin, N.; Donmez, M.; Saglam, S.; Ozcelik, S. Titanium dioxide thin films as methane gas sensors. IEEE Sensors J. 2016, 16, 8890–8896. [Google Scholar] [CrossRef]
- Wan, H.; Yin, H.; Lin, L.; Zeng, X.; Mason, A.J. Miniaturized planar room temperature ionic liquid electrochemical gas sensor for rapid multiple gas pollutants monitoring. Sens. Actuators B Chem. 2018, 255, 638–646. [Google Scholar] [CrossRef]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon nanotube-based chemiresistive sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef]
- Fritsch, M.; Mosch, S.; Vinnichenko, M.; Trofimenko, N.; Kusnezoff, M.; Fuchs, F.M.; Oblov, K. Printed Miniaturized Platinum Heater on Ultra-Thin Ceramic Membrane for Mox Gas Sensors. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2020; p. 2125. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Zhang, C. Research on explosive burning characteristics of pulverized coal. Proc. AISTech. 2017, 1, 617. [Google Scholar] [CrossRef]
- Khan, S.; Briand, D. All-printed low-power metal oxide gas sensors on polymeric substrates. Flex. Print. Electron. 2019, 4, 015002. [Google Scholar] [CrossRef]
- Chen, S.; Yang, Q.; Wang, H.; Zhang, S.; Li, J.; Wang, Y.; Song, L. Initial reaction mechanism of platinum nanoparticle in methanol–water system and the anomalous catalytic effect of water. Nano Lett. 2015, 15, 5961–5968. [Google Scholar] [CrossRef] [PubMed]
- Boita, J.; Nicolao, L.; Alves, M.C.; Morais, J. Observing Pt nanoparticle formation at the atomic level during polyol synthesis. Phys. Chem. Chem. Phys. 2014, 16, 17640–17647. [Google Scholar] [CrossRef]
- Sharma, R.; Wang, Y.; Li, F.; Chamier, J.; Andersen, S.M. Synthesis of a Pt/C Electrocatalyst from a User-Friendly Pt Precursor (Ammonium Hexachloroplatinate) through Microwave-Assisted Polyol Synthesis. ACS Appl. Energy Mater. 2019, 2, 6875–6882. [Google Scholar] [CrossRef]
- Cheong, S.; Watt, J.; Ingham, B.; Toney, M.F.; Tilley, R.D. In situ and ex situ studies of platinum nanocrystals: Growth and evolution in solution. J. Am. Chem. Soc. 2009, 131, 14590–14595. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Shi, C.; Ji, Y.; Graham, U.M.; Jacobs, G.; Crocker, M.; Zhang, Z.; Toops, T.J. NOx storage and reduction properties of model ceria-based lean NOx trap catalysts. Appl. Catal. B Environ. 2012, 119, 183–196. [Google Scholar] [CrossRef]
- Filatov, E.; Lagunova, V.; Potemkin, D.; Kuratieva, N.; Zadesenets, A.; Plyusnin, P.; Korenev, S. Tetraammineplatinum (II) and Tetraamminepalladium (II) Chromates as Precursors of Metal Oxide Catalysts. Chem. A Eur. J. 2020. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.H.; Kim, J.H. A comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Schmidt-Ott, A. (Ed.) Spark Ablation: Building Blocks for Nanotechnology; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Schwyn, S.; Garwin, E.; Schmidt-Ott, A. Aerosol generation by spark discharge. J. Aerosol Sci. 1988, 19, 639–642. [Google Scholar] [CrossRef]
- Meuller, B.O.; Messing, M.E.; Engberg, D.L.; Jansson, A.M.; Johansson, L.I.; Norlén, S.M.; Deppert, K. Review of spark discharge generators for production of nanoparticle aerosols. Aerosol Sci. Technol. 2012, 46, 1256–1270. [Google Scholar] [CrossRef]
- Pfeiffer, T.V.; Feng, J.; Schmidt-Ott, A. New developments in spark production of nanoparticles. Adv. Powder Technol. 2014, 25, 56–70. [Google Scholar] [CrossRef]
- Němec, T.; Šonský, J.; Gruber, J.; de Prado, E.; Kupčík, J.; Klementová, M. Platinum and platinum oxide nanoparticles generated by unipolar spark discharge. J. Aerosol Sci. 2020, 141, 105502. [Google Scholar] [CrossRef]
- Vasiliev, A.A.; Varfolomeev, A.E.; Volkov, I.A.; Simonenko, N.P.; Arsenov, P.V.; Vlasov, I.S.; Maeder, T. Reducing humidity response of gas sensors for medical applications: Use of spark discharge synthesis of metal oxide nanoparticles. Sensors 2018, 18, 2600. [Google Scholar] [CrossRef]
- Mylnikov, D.; Efimov, A.; Ivanov, V. Measuring and optimization of energy transfer to the interelectrode gaps during the synthesis of nanoparticles in a spark discharge. Aerosol Sci. Technol. 2019, 53, 1393–1403. [Google Scholar] [CrossRef]
- Tabrizi, N.S.; Ullmann, M.; Vons, V.A.; Lafont, U.; Schmidt-Ott, A. Generation of nanoparticles by spark discharge. J. Nanoparticle Res. 2009, 11, 315. [Google Scholar] [CrossRef]
- Shchukin, E.D.; Pertsov, A.V.; Amelina, E.A.; Zelenev, A.S. Colloid and Surface Chemistry; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Eckstein, R.; Lemmer, U. Aerosol Jet Printed Electronic Devices and Systems. Ph.D. Thesis, Karlsruher Institute of Technology, Karlsruhe, Germany, 2016. [Google Scholar]
- Hutchings, I.M.; Martin, G.D. (Eds.) Inkjet Technology for Digital Fabrication; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Smith, P.J.; Shin, D.H. Inkjet-Based Micromanufacturing; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Suganuma, K. Introduction to Printed Electronics; Springer: Berlin, Germany, 2014; Volume 74. [Google Scholar]
- Ono, L.K.; Yuan, B.; Heinrich, H.; Cuenya, B.R. Formation and thermal stability of platinum oxides on size-selected platinum nanoparticles: Support effects. J. Phys. Chem. C 2010, 114, 22119–22133. [Google Scholar] [CrossRef]
- Lebedinskii, Y.Y.; Chernikova, A.G.; Markeev, A.M.; Kuzmichev, D.S. Effect of dielectric stoichiometry and interface chemical state on band alignment between tantalum oxide and platinum. Appl. Phys. Lett. 2015, 107, 142904. [Google Scholar] [CrossRef]
- Zevin, L.S.; Kimmel, G. Quantitative X-Ray Diffractometry; Springer: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- SGX Sensortech. Available online: https://www.sgxsensortech.com/products-services/industrial-safety/metal-oxide-sensors/ (accessed on 30 November 2020).
- Deja, R.; Peters, R.; Blum, L.; Stolten, D. Study of the catalytic combustion of lean hydrogen-air mixtures in a monolith reactor. Int. J. Hydrog. Energy 2018, 43, 17520–17530. [Google Scholar] [CrossRef]
- L’vov, B.V.; Galwey, A.K. Catalytic oxidation of hydrogen on platinum. J. Therm. Anal. Calorim. 2013, 112, 815–822. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, T.; Wang, H.; Tang, C.; Zhang, L. A Novel Fabricating Process of Catalytic Gas Sensor Based on Droplet Generating Technology. Micromachines 2019, 10, 71. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkov, I.A.; Simonenko, N.P.; Efimov, A.A.; Simonenko, T.L.; Vlasov, I.S.; Borisov, V.I.; Arsenov, P.V.; Lebedinskii, Y.Y.; Markeev, A.M.; Lizunova, A.A.; et al. Platinum Based Nanoparticles Produced by a Pulsed Spark Discharge as a Promising Material for Gas Sensors. Appl. Sci. 2021, 11, 526. https://doi.org/10.3390/app11020526
Volkov IA, Simonenko NP, Efimov AA, Simonenko TL, Vlasov IS, Borisov VI, Arsenov PV, Lebedinskii YY, Markeev AM, Lizunova AA, et al. Platinum Based Nanoparticles Produced by a Pulsed Spark Discharge as a Promising Material for Gas Sensors. Applied Sciences. 2021; 11(2):526. https://doi.org/10.3390/app11020526
Chicago/Turabian StyleVolkov, Ivan A., Nikolay P. Simonenko, Alexey A. Efimov, Tatiana L. Simonenko, Ivan S. Vlasov, Vladislav I. Borisov, Pavel V. Arsenov, Yuri Yu. Lebedinskii, Andrey M. Markeev, Anna A. Lizunova, and et al. 2021. "Platinum Based Nanoparticles Produced by a Pulsed Spark Discharge as a Promising Material for Gas Sensors" Applied Sciences 11, no. 2: 526. https://doi.org/10.3390/app11020526
APA StyleVolkov, I. A., Simonenko, N. P., Efimov, A. A., Simonenko, T. L., Vlasov, I. S., Borisov, V. I., Arsenov, P. V., Lebedinskii, Y. Y., Markeev, A. M., Lizunova, A. A., Mokrushin, A. S., Simonenko, E. P., Buslov, V. A., Varfolomeev, A. E., Liu, Z., Vasiliev, A. A., & Ivanov, V. V. (2021). Platinum Based Nanoparticles Produced by a Pulsed Spark Discharge as a Promising Material for Gas Sensors. Applied Sciences, 11(2), 526. https://doi.org/10.3390/app11020526







