Puerarin Improves Dexamethasone-Impaired Wound Healing In Vitro and In Vivo by Enhancing Keratinocyte Proliferation and Migration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Histological and Immunohistochemical Examinations
2.3. Cell Culture
2.4. Cell Viability
2.5. Scratch Wound Healing Assay
2.6. Cell Proliferation Assay
2.7. Cell Cycle Analysis
2.8. Ki67 Proliferation Assay
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
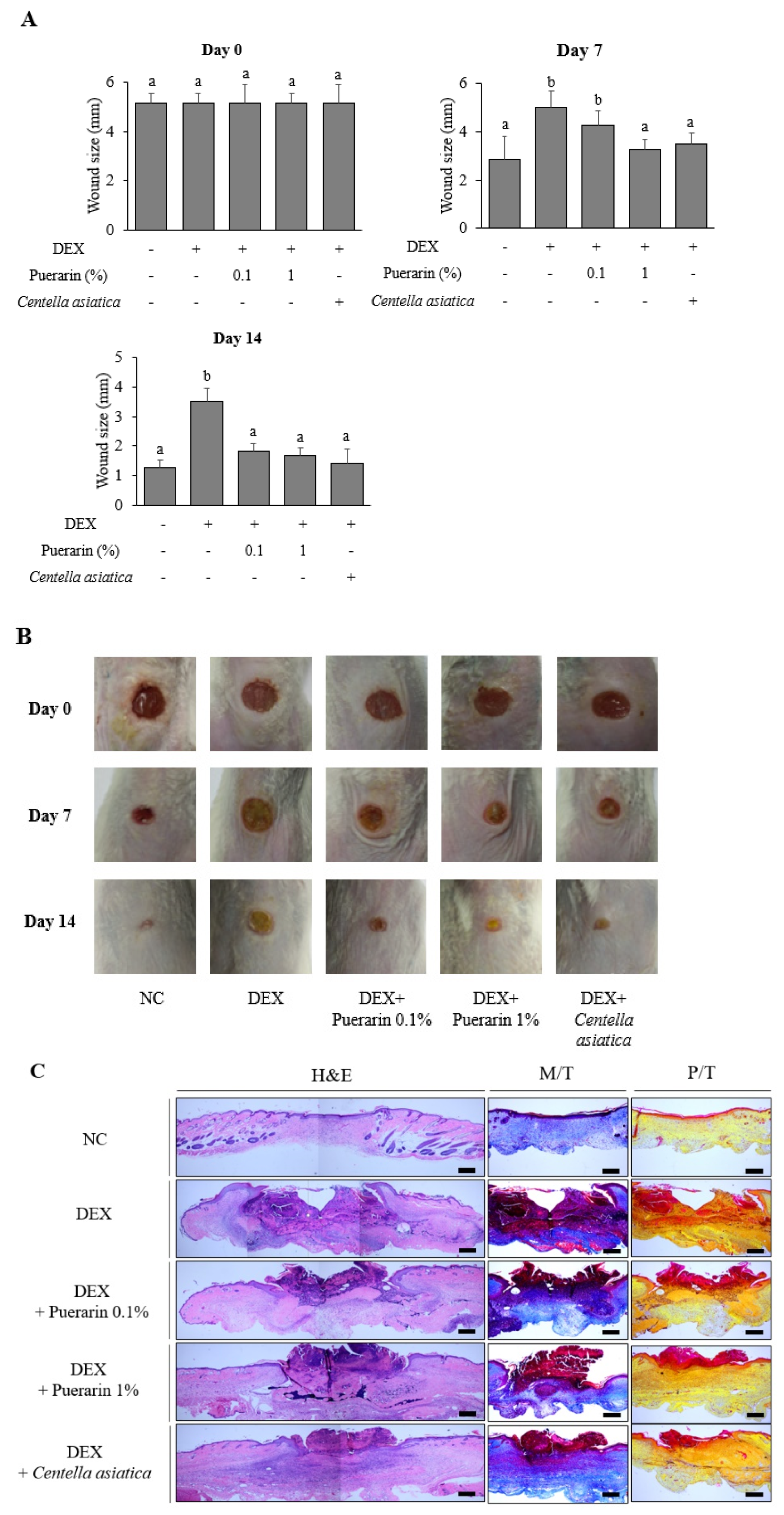
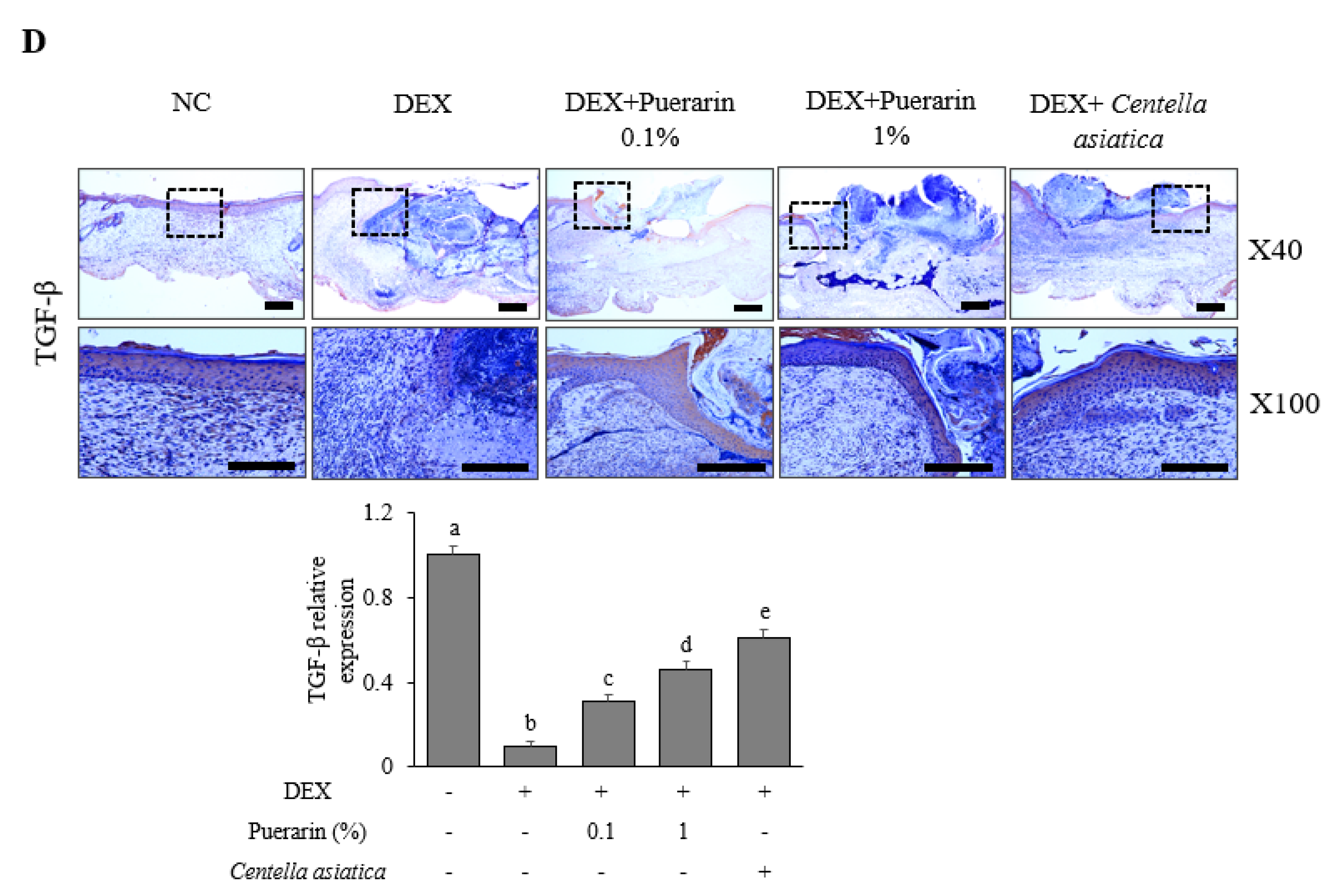
3.1. Effects of Puerarin on Cutaneous Wound Healing in DEX-Treated Mice
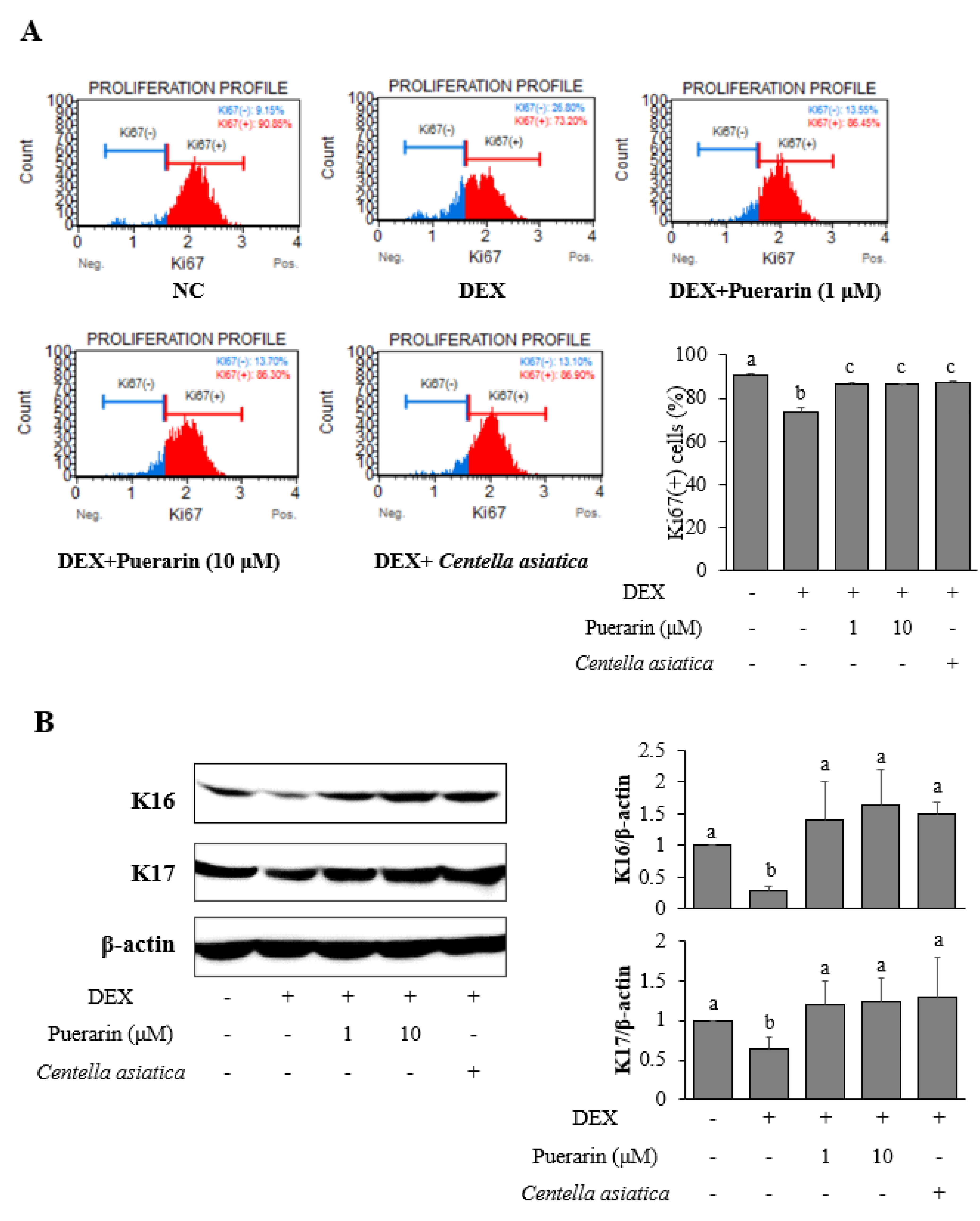
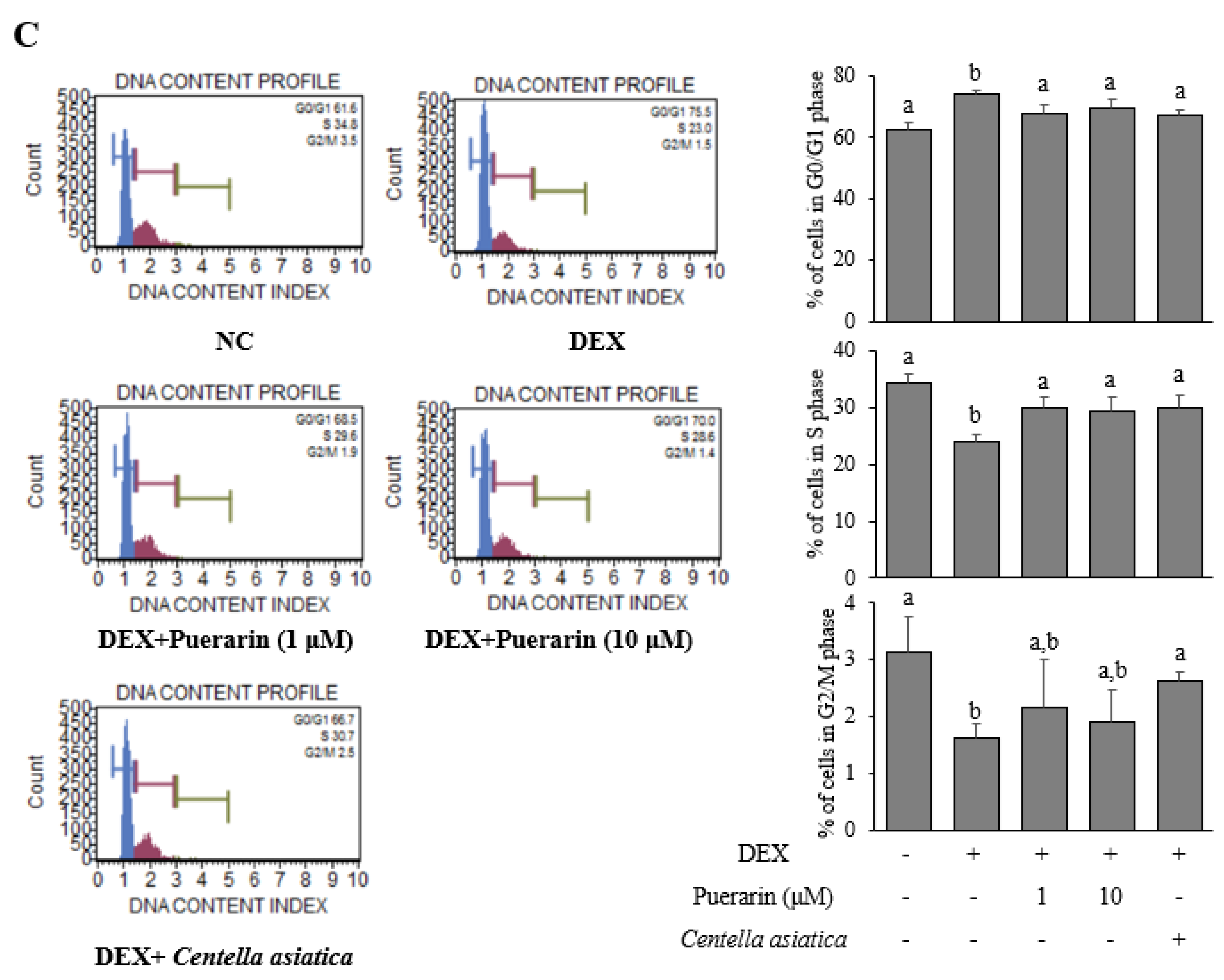
3.2. Effects of Puerarin on the Proliferation in DEX-Treated HaCaT Cells
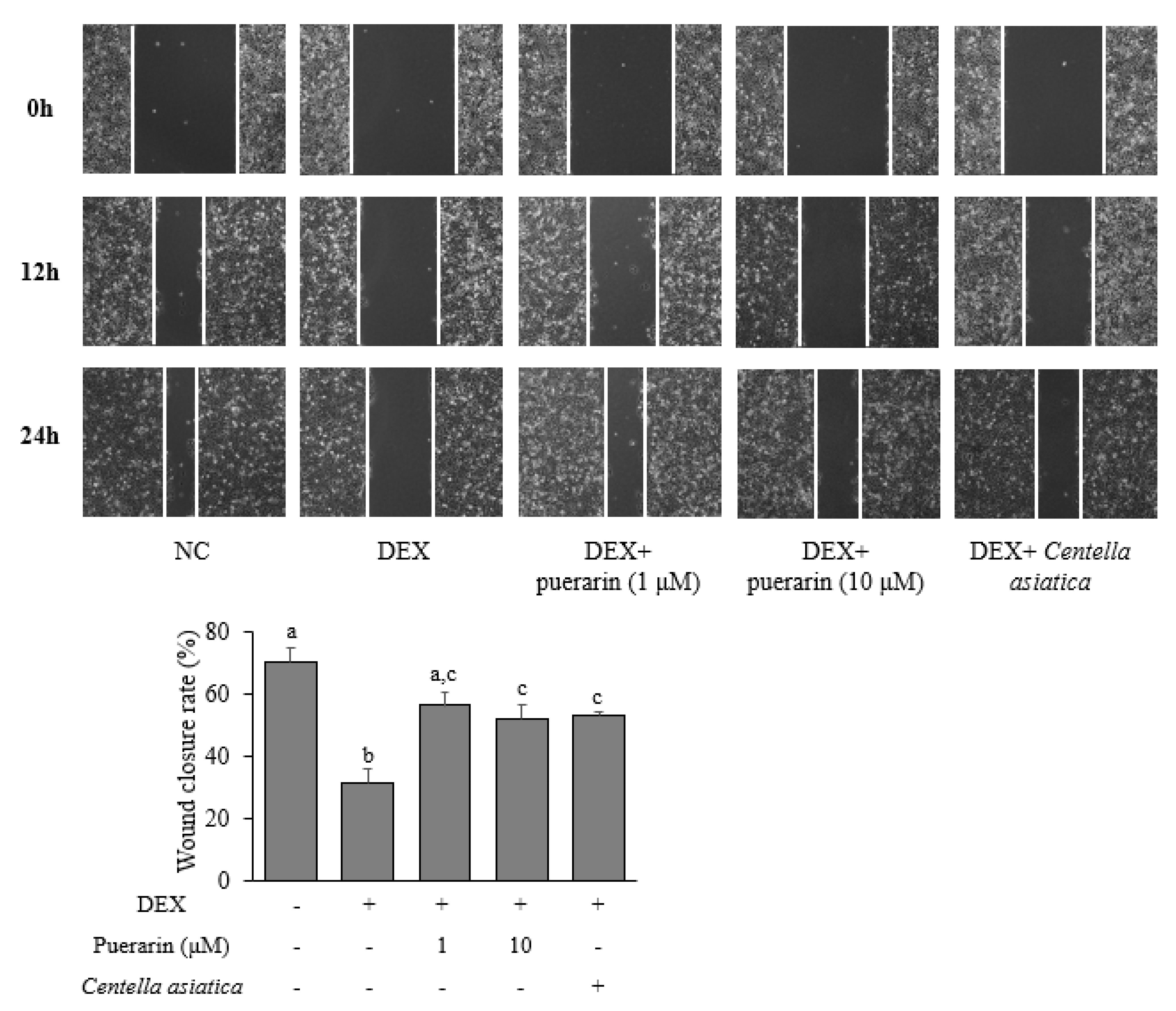
3.3. Effects of Puerarin on the Migration in DEX-Treated HaCaT Cells
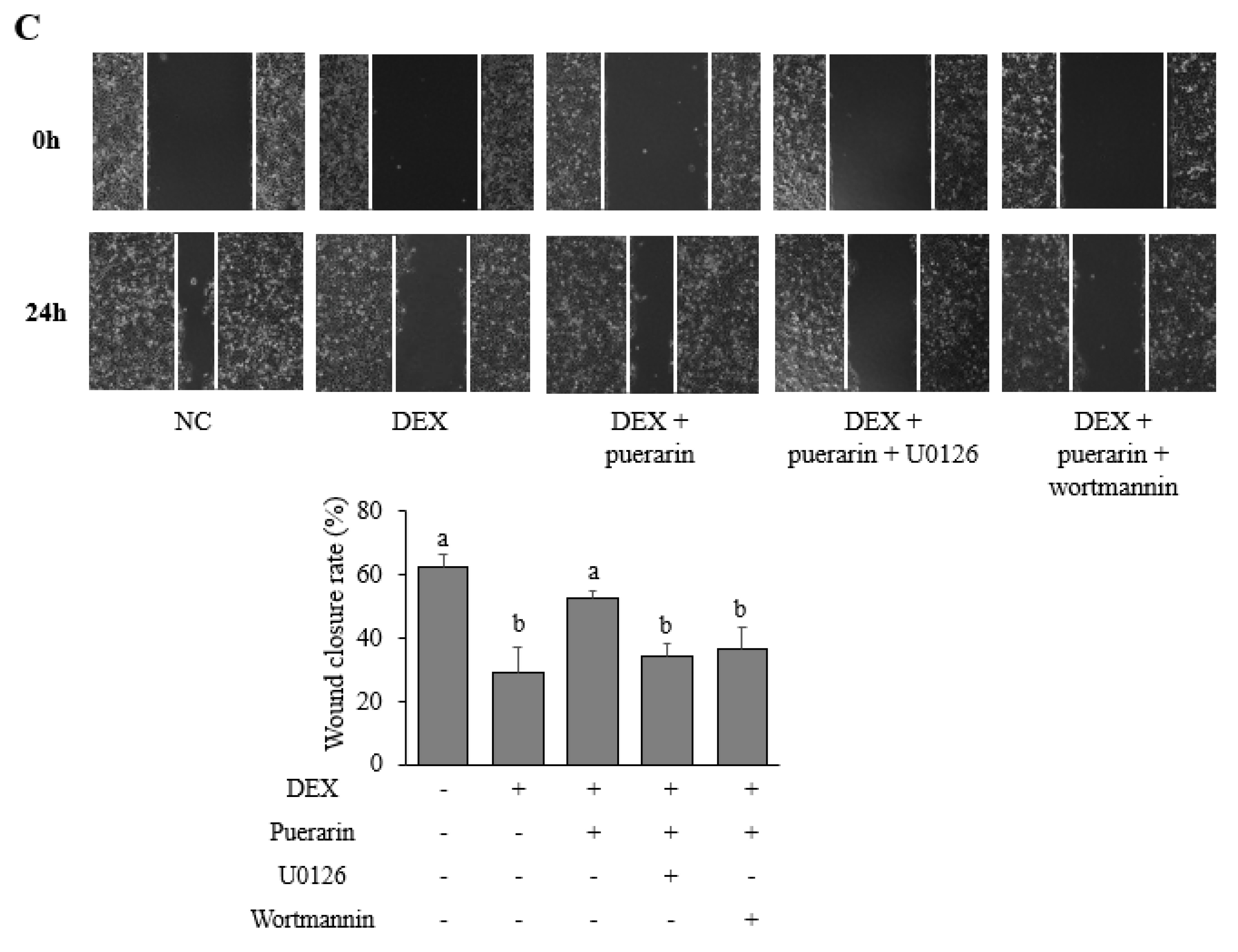
3.4. Effects of Puerarin on Signaling Pathways in DEX-Treated HaCaT Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizuno, K.; Morizane, S.; Takiguchi, T.; Iwatsuki, K. Dexamethasone but not tacrolimus suppresses TNF-alpha-induced thymic stromal lymphopoietin expression in lesional keratinocytes of atopic dermatitis model. J. Dermatol. Sci. 2015, 80, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hengge, U.R.; Ruzicka, T.; Schwartz, R.A.; Cork, M.J. Adverse effects of topical glucocorticosteroids. J. Am. Acad. Dermatol. 2006, 54, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkoli, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell. Res. 2005, 304, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A. Glucocorticoids Inhibit Wound Healing: Novel Mechanism of Action. J. Investig. Dermatol. 2017, 137, 1012–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, H.S.; Han, G.E.; Won, J.; Cho, Y.; Woo, H.; Lee, J.H. Pueraria montana var. lobata root extract inhibits photoaging on skin through Nrf2 pathway. J. Microbiol. Biotechnol. 2019, 29, 518–526. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Intharuksa, A.; Sasaki, Y. A Promising View of Kudzu Plant, Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep: Flavonoid Phytochemical Compounds, Taxonomic Data, Traditional Uses and Potential Biological Activities for Future Cosmetic Application. Cosmetics 2020, 7, 12. [Google Scholar]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef]
- Zhang, S.; Ou, Q.; Xin, P.; Yuan, Q.; Wang, Y.; Wu, J. Polydopamine/puerarin nanoparticle-incorporated hybrid hydrogels for enhanced wound healing. Biomater. Sci. 2019, 7, 4230–4236. [Google Scholar] [CrossRef]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Yoon, S.W.; Lee, S.J.; Park, J.H.; Yoon, M.S.; Kim, B.; Lee, H.M. Potential Skin Regeneration Activity and Chemical Composition of Absolute from Pueraria thunbergiana Flower. Nat. Prod. Commun. 2015, 10, 2009–2012. [Google Scholar] [CrossRef]
- Reppert, A.; Yousef, G.G.; Rogers, R.B.; Lila, M.A. Isolation of radiolabeled isoflavones from kudzu (Pueraria lobata) root cultures. J. Agric. Food Chem. 2008, 56, 7860–7865. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.W.; Kang, A.N.; Kang, S.Y.; Park, Y.-K.; Song, M.Y. The root extract of Pueraria lobata and its main compound, puerarin, prevent obesity by increasing the energy metabolism in skeletal muscle. Nutrients 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, Y.D.; Lee, Y.M.; Kim, D.K. The suppressive effect of puerarin on atopic dermatitis-like skin lesions through regulation of inflammatory mediators in vitro and in vivo. Biochem. Biophys. Res. Commun. 2018, 498, 707–714. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Shen, Y.; Yang, D.; Liu, X.; Sun-Chi, A.C.; Xu, H. Puerarin induces angiogenesis in myocardium of rat with myocardial infarction. Biol. Pharm. Bull. 2006, 29, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.E.; Son, Y.K.; Min, B.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch. Pharm. Res. 2012, 35, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.; Onger, M.E. The effect of oral puerarin administration on wound healing in diabetic rat model. Ann. Med. Res. 2018, 25, 536–539. [Google Scholar] [CrossRef]
- Somboonwong, J.; Kankaisre, M.; Tantisira, B.; Tantisira, M.H. Wound healing activities of different extracts of Centella asiatica in incision and burn wound models: An experimental animal study. BMC Complement. Altern. Med. 2012, 12, 103. [Google Scholar] [CrossRef] [Green Version]
- Azis, H.; Taher, M.; Ahmed, A.; Sulaiman, W.; Susanti, D.; Chowdhury, S.; Zakaria, Z. In vitro and In vivo wound healing studies of methanolic fraction of Centella asiatica extract. S. Afr. J. Bot. 2017, 108, 163–174. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Sringarm, K.; Sommano, S.; Jantrawut, P. Depigmented Centella asiatica Extraction by Pretreated with Supercritical Carbon Dioxide Fluid for Wound Healing Application. Processes 2020, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Shetty, B.S.; Udupa, S.L.; Udupa, A.L.; Somayaji, S.N. Effect of Centella asiatica L (Umbelliferae) on normal and dexamethasone-suppressed wound healing in Wistar Albino rats. Int. J. Low. Extrem. Wounds 2006, 5, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.F.; Cassini-Vieira, P.; da Silva, M.F.; Barcelos, L. Skin wound healing model-excisional wounding and assessment of lesion area. Bio-Protocol 2015, 5, e1661. [Google Scholar] [CrossRef]
- Suvik, A.; Effendy, A. The use of modified Masson’s trichrome staining in collagen evaluation in wound healing study. Mal. J. Vet. Res. 2012, 3, 39–47. [Google Scholar]
- Cho, A.-R.; Hong, S.-U.; Yoon, Y.-J. Gagamyeonryunggobon-dan (Jiājiǎnyánlínggùběn-dān) induces hair regrowth effect from activating hair follicle. J. Korean Orient. Otolaryngol. Dermatol. 2016, 29, 65–80. [Google Scholar]
- Su, L.; Fu, L.; Li, X.; Zhang, Y.; Li, Z.; Wu, X.; Li, Y.; Bai, X.; Hu, D. Loss of CAR promotes migration and proliferation of HaCaT cells and accelerates wound healing in rats via Src-p38 MAPK pathway. Sci. Rep. 2016, 6, 19735. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.S.; Ahmad, M.S.; Riaz, H.; Raza, S.A.; Hussain, S.; Qureshi, O.S.; Maria, P.; Hamzah, Z.; Javed, O. Role of Flavonoids as Wound Healing Agent. In Phytochemicals-Source of Antioxidants and Role in Disease Prevention; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: London, UK, 2018; pp. 95–102. [Google Scholar]
- Öz, B.E.; İşcan, G.S.; Akkol, E.K.; Süntar, İ.; Acıkara, Ö.B. Isoflavonoids as wound healing agents from Ononidis Radix. J. Ethnopharmacol. 2018, 211, 384–393. [Google Scholar]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Dańczak-Pazdrowska, A.; Brzezińska, M. Centella asiatica in dermatology: An overview. Phytother. Res. 2014, 28, 1117–1124. [Google Scholar] [CrossRef]
- Singkhorn, S.; Tantisira, M.H.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Induction of keratinocyte migration by ECa 233 is mediated through FAK/Akt, ERK, and p38 MAPK signaling. Phytother. Res. 2018, 32, 1397–1403. [Google Scholar] [CrossRef]
- Davison-Kotler, E.; Marshall, W.S.; Garcia-Gareta, E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Chicharro-Alcantara, D.; Rubio-Zaragoza, M.; Damia-Gimenez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Pelaez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-beta family in wound healing, burns and scarring: A review. Int. J. Burns Trauma 2012, 2, 18–28. [Google Scholar]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Wicke, C.; Halliday, B.; Allen, D.; Roche, N.S.; Scheuenstuhl, H.; Spencer, M.M.; Roberts, A.B.; Hunt, T.K. Effects of steroids and retinoids on wound healing. Arch. Surg. 2000, 135, 1265–1270. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.; Keen, J.; Halligan, D.; O’Callaghan, A.; Andrew, R.; Livingstone, D.; Abernethie, A.; Maltese, G.; Walker, B.; Hadoke, P. Species-specific regulation of angiogenesis by glucocorticoids reveals contrasting effects on inflammatory and angiogenic pathways. PLoS ONE 2018, 13, e0192746. [Google Scholar] [CrossRef] [Green Version]
- Pastar, I.; Stojadinovic, O.; Tomic-Canic, M. Role of keratinocytes in healing of chronic wounds. Surg. Technol. Int. 2008, 17, 105–112. [Google Scholar]
- Hopf, H.W.; Kelly, M.; Shapshak, D. Oxygen and the basic mechanisms of wound healing. In Physiology and Medicine of Hyperbaric Oxygen Therapy; Neuman, T.S., Thom, S.R., Eds.; Saunders: Philadelphia, PA, USA, 2008; pp. 203–228. [Google Scholar]
- Zeng, X.; Feng, Q.; Zhao, F.; Sun, C.; Zhou, T.; Yang, J.; Zhan, X. Puerarin inhibits TRPM3/miR-204 to promote MC3T3-E1 cells proliferation, differentiation and mineralization. Phytother. Res. 2018, 32, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Hu, Y.; Yang, G.; Li, P. Puerarin suppresses cell growth and migration in HPV-positive cervical cancer cells by inhibiting the PI3K/mTOR signaling pathway. Exp. Ther. Med. 2019, 18, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.M.; Wu, M.; Yuan, S.F.; Geng, J.H.; Kong, X.D.; Shen, J.L. Effects of puerarin on proliferation, apoptosis, migration and invasion of hormone-independent prostate cancer cells. Tumor 2018, 38, 680–688. [Google Scholar]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [Green Version]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Yang, Z.; Chen, Y.; Chen, Y.; Huang, Z.; You, B.; Peng, Y.; Chen, J. Estrogen accelerates cutaneous wound healing by promoting proliferation of epidermal keratinocytes via Erk/Akt signaling pathway. Cell. Physiol. Biochem. 2016, 38, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Liu, J.; Liu, G.; Zhou, W.; Liu, Y.; Hu, L.; Xiong, L.; Ye, S.; Wu, Y. Icariin promotes wound healing by enhancing the migration and proliferation of keratinocytes via the AKT and ERK signaling pathway. Int. J. Mol. Med. 2018, 42, 831–838. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.T.H.; Ahn, S.-H.; Choi, M.-J.; Yang, I.-J.; Shin, H.-M. Puerarin Improves Dexamethasone-Impaired Wound Healing In Vitro and In Vivo by Enhancing Keratinocyte Proliferation and Migration. Appl. Sci. 2021, 11, 9343. https://doi.org/10.3390/app11199343
Nguyen LTH, Ahn S-H, Choi M-J, Yang I-J, Shin H-M. Puerarin Improves Dexamethasone-Impaired Wound Healing In Vitro and In Vivo by Enhancing Keratinocyte Proliferation and Migration. Applied Sciences. 2021; 11(19):9343. https://doi.org/10.3390/app11199343
Chicago/Turabian StyleNguyen, Ly Thi Huong, Sang-Hyun Ahn, Min-Jin Choi, In-Jun Yang, and Heung-Mook Shin. 2021. "Puerarin Improves Dexamethasone-Impaired Wound Healing In Vitro and In Vivo by Enhancing Keratinocyte Proliferation and Migration" Applied Sciences 11, no. 19: 9343. https://doi.org/10.3390/app11199343
APA StyleNguyen, L. T. H., Ahn, S.-H., Choi, M.-J., Yang, I.-J., & Shin, H.-M. (2021). Puerarin Improves Dexamethasone-Impaired Wound Healing In Vitro and In Vivo by Enhancing Keratinocyte Proliferation and Migration. Applied Sciences, 11(19), 9343. https://doi.org/10.3390/app11199343






