Methods for Studying Bacterial–Fungal Interactions in the Microenvironments of Soil
Abstract
1. Introduction
2. Fungi, Bacteria and Their Microenvironment
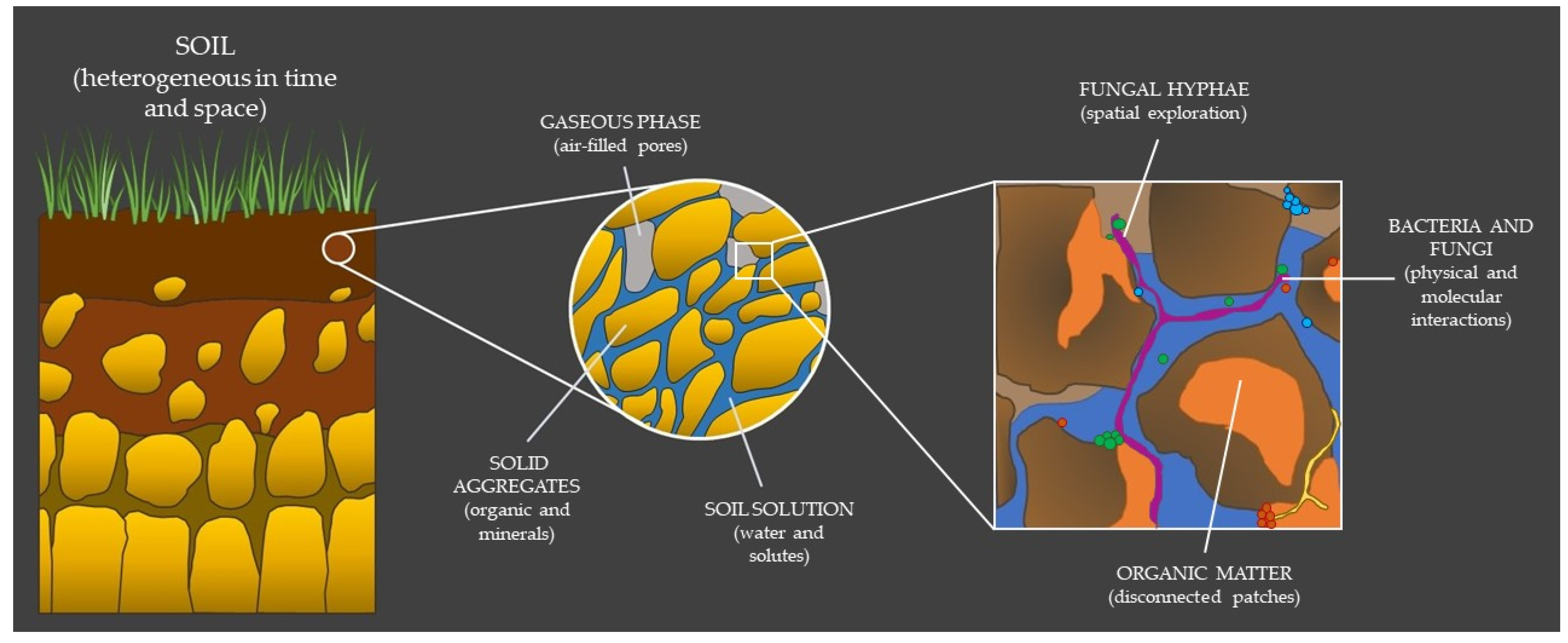
2.1. The Hidden Properties of Soil
2.2. Bacterial–Fungal Interactions: A Harsh Existence
3. Get Your Hands Dirty: Methods for Studying BFI
3.1. Reconstructing the Spatial Heterogeneity of Soil
3.2. Playing Hide and Seek with Bacteria and Fungi: Unravel Their Physical Interactions
3.3. What Are They Doing? Investigating the Molecular Interactions in BFI
4. Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jansson, J.K.; Hofmockel, K.S. The soil microbiome—From metagenomics to metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef]
- Baldrian, P. The known and the unknown in soil microbial ecology. FEMS Microbiol. Ecol. 2019, 95, 1–9. [Google Scholar] [CrossRef]
- Baveye, P.C.; Otten, W.; Kravchenko, A.; Balseiro-Romero, M.; Beckers, É.; Chalhoub, M.; Darnault, C.; Eickhorst, T.; Garnier, P.; Hapca, S.; et al. Emergent properties of microbial activity in heterogeneous soil microenvironments: Different research approaches are slowly converging, yet major challenges remain. Front. Microbiol. 2018, 9, 1929. [Google Scholar] [CrossRef]
- Young, I.M. Interactions and self-organization in the soil-microbe complex. Science 2004, 304, 1634–1637. [Google Scholar] [CrossRef]
- Dexter, A. Advances in characterization of soil structure. Soil Tillage Res. 1988, 11, 199–238. [Google Scholar] [CrossRef]
- Hillel, D. Introduction to Environmental Soil Physics; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Young, I.; Crawford, J.; Rappoldt, C. New methods and models for characterising structural heterogeneity of soil. Soil Tillage Res. 2001, 61, 33–45. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Horn, R.; Smucker, A. Structure formation and its consequences for gas and water transport in unsaturated arable and forest soils. Soil Tillage Res. 2005, 82, 5–14. [Google Scholar] [CrossRef]
- Mueller, C.W.; Koelbl, A.; Hoeschen, C.; Hillion, F.; Heister, K.; Herrmann, A.M.; Kögel-Knabner, I. Submicron scale imaging of soil organic matter dynamics using NanoSIMS—From single particles to intact aggregates. Org. Geochem. 2012, 42, 1476–1488. [Google Scholar] [CrossRef]
- Rawlins, B.G.; Wragg, J.; Reinhard, C.; Atwood, R.C.; Houston, A.; Lark, R.M.; Rudolph, S. Three-dimensional soil organic matter distribution, accessibility and microbial respiration in macroaggregates using osmium staining and synchrotron X-ray computed tomography. SOIL 2016, 2, 659–671. [Google Scholar] [CrossRef]
- Jasinska, E.; Wetzel, H.; Baumgartl, T.; Horn, R. Heterogeneity of physico-chemical properties in structured soils and its consequences. Pedosphere 2006, 16, 284–296. [Google Scholar] [CrossRef]
- Schurgers, G.; Dörsch, P.; Bakken, L.; Leffelaar, P.; Haugen, L. Modelling soil anaerobiosis from water retention characteristics and soil respiration. Soil Biol. Biochem. 2006, 38, 2637–2644. [Google Scholar] [CrossRef]
- Ranjard, L.; Richaume, A. Quantitative and qualitative microscale distribution of bacteria in soil. Res. Microbiol. 2001, 152, 707–716. [Google Scholar] [CrossRef]
- Otten, W.; Gilligan, C.; Watts, C.; Dexter, A.; Hall, D. Continuity of air-filled pores and invasion thresholds for a soil-borne fungal plant pathogen, Rhizoctonia solani. Soil Biol. Biochem. 1999, 31, 1803–1810. [Google Scholar] [CrossRef]
- Otten, W.; Gilligan, C.A. Soil structure and soil-borne diseases: Using epidemiological concepts to scale from fungal spread to plant epidemics. Eur. J. Soil Sci. 2006, 57, 26–37. [Google Scholar] [CrossRef]
- Kaisermann, A.; Maron, P.; Beaumelle, L.; Lata, J. Fungal communities are more sensitive indicators to non-extreme soil moisture variations than bacterial communities. Appl. Soil Ecol. 2015, 86, 158–164. [Google Scholar] [CrossRef]
- Baveye, P.C.; Ebaveye, J.; Egowdy, J. Soil “ecosystem” services and natural capital: Critical appraisal of research on uncertain ground. Front. Environ. Sci. 2016, 4, 1–49. [Google Scholar] [CrossRef]
- Demanèche, S.; Jocteur-Monrozier, L.; Quiquampoix, H.; Simonet, P. Evaluation of biological and physical protection against nuclease degradation of clay-bound plasmid DNA. Appl. Environ. Microbiol. 2001, 67, 293–299. [Google Scholar] [CrossRef]
- Pietramellara, G.; Ascher, J.; Borgogni, F.; Ceccherini, M.T.; Guerri, G.; Nannipieri, P. Extracellular DNA in soil and sediment: Fate and ecological relevance. Biol. Fertil. Soils 2009, 45, 219–235. [Google Scholar] [CrossRef]
- Romanowski, G.; Lorenz, M.G.; Sayler, G.; Wackernagel, W. Persistence of free plasmid DNA in soil monitored by various methods, including a transformation assay. Appl. Environ. Microbiol. 1992, 58, 3012–3019. [Google Scholar] [CrossRef]
- Stotzky, G. Influence of soil mineral colloids on metabolic processes, growth, adhesion, and ecology of microbes and viruses. Geochem. Soil Radionucl. 2015, 17, 305–428. [Google Scholar] [CrossRef]
- Nielsen, K.M.; Calamai, L.; Pietramellara, G. Stabilization of extracellular DNA and proteins by transient binding to various soil components. Soil Biol. 2006, 8, 141–157. [Google Scholar] [CrossRef]
- Konhauser, K. Introduction to Geomicrobiology, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA; Blackwell Publishing: Hoboken, NJ, USA, 2007. [Google Scholar]
- Rappoldt, C.; Crawford, J. The distribution of anoxic volume in a fractal model of soil. Geoderma 1999, 88, 329–347. [Google Scholar] [CrossRef]
- Lombard, N.; Prestat, E.; van Elsas, J.D.; Simonet, P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS Microbiol. Ecol. 2011, 78, 31–49. [Google Scholar] [CrossRef]
- Nunan, N.; Schmidt, H.; Raynaud, X. The ecology of heterogeneity: Soil bacterial communities and C dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190249. [Google Scholar] [CrossRef]
- Strong, D.T.; Wever, H.D.E.; Merckx, R.; Recous, S. Spatial location of carbon decomposition in the soil pore system. Eur. J. Soil Sci. 2004, 55, 739–750. [Google Scholar] [CrossRef]
- Salomé, C.; Nunan, N.; Pouteau, V.; Lerch, T.Z.; Chenu, C. Carbon dynamics in topsoil and in subsoil may be controlled by different regulatory mechanisms. Glob. Change Biol. 2010, 16, 416–426. [Google Scholar] [CrossRef]
- Ruamps, L.S.; Nunan, N.; Pouteau, V.; Leloup, J.; Raynaud, X.; Roy, V.; Chenu, C. Regulation of soil organic C mineralisation at the pore scale. FEMS Microbiol. Ecol. 2013, 86, 26–35. [Google Scholar] [CrossRef]
- Ruamps, L.S.; Nunan, N.; Chenu, C. Microbial biogeography at the soil pore scale. Soil Biol. Biochem. 2011, 43, 280–286. [Google Scholar] [CrossRef]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial–fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.-A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial–fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Clarholm, M. Protozoan grazing of bacteria in soil—Impact and importance. Microb. Ecol. 1981, 7, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Kuikman, P.; van Elsas, J.; Jansen, A.; Burgers, S.; van Veen, J. Population dynamics and activity of bacteria and protozoa in relation to their spatial distribution in soil. Soil Biol. Biochem. 1990, 22, 1063–1073. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Juma, N.G. Influence of texture on habitable pore space and bacterial-protozoan populations in soil. Biol. Fertil. Soils 1992, 12, 221–227. [Google Scholar] [CrossRef]
- Wright, D.A.; Killham, K.; Glover, L.A.; Prosser, J.I. Role of pore size location in determining bacterial activity during predation by protozoa in soil. Appl. Environ. Microbiol. 1995, 61, 3537–3543. [Google Scholar] [CrossRef]
- Wright, D.; Killham, K.; Glover, L.; Prosser, J. The effect of location in soil on protozoal grazing of a genetically modified bacterial inoculum. Geoderma 1993, 56, 633–640. [Google Scholar] [CrossRef]
- Männik, J.; Driessen, R.; Galajda, P.; Keymer, J.E.; Dekker, C. Bacterial growth and motility in sub-micron constrictions. Proc. Natl. Acad. Sci. USA 2009, 106, 14861–14866. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Negassa, W.C.; Guber, A.K.; Hildebrandt, B.; Marsh, T.; Rivers, M.L. Intra-aggregate pore structure influences phylogenetic composition of bacterial community in macroaggregates. Soil Sci. Soc. Am. J. 2014, 78, 1924–1939. [Google Scholar] [CrossRef]
- Dechesne, A.; Wang, G.; Gulez, G.; Or, D.; Smets, B.F. Hydration-controlled bacterial motility and dispersal on surfaces. Proc. Natl. Acad. Sci. USA 2010, 107, 14369–14372. [Google Scholar] [CrossRef]
- Vos, M.; Wolf, A.B.; Jennings, S.J.; Kowalchuk, G.A. Micro-scale determinants of bacterial diversity in soil. FEMS Microbiol. Rev. 2013, 37, 936–954. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, X.; Nunan, N. Spatial ecology of bacteria at the microscale in soil. PLoS ONE 2014, 9, e87217. [Google Scholar] [CrossRef]
- Pennell, K.D. Specific surface area. In Reference Module in Earth Systems and Environmental Sciences; Elias, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–8. [Google Scholar]
- Boswell, G.P.; Jacobs, H.; Ritz, K.; Gadd, G.M.; Davidson, F. The development of fungal networks in complex environments. Bull. Math. Biol. 2007, 69, 605–634. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.D.; Heaton, L.L.M.; Jones, N.S.; Boddy, L. The mycelium as a network. Fungal Kingd. 2017, 5, 335–367. [Google Scholar] [CrossRef]
- Kohlmeier, S.; Smits, T.H.; Ford, R.M.; Keel, C.; Harms, H.; Wick, L.Y. Taking the fungal highway: Mobilization of pollutant-degrading bacteria by fungi. Environ. Sci. Technol. 2005, 39, 4640–4646. [Google Scholar] [CrossRef]
- Warmink, J.A.; Nazir, R.; van Elsas, J.D. Universal and species-specific bacterial ‘fungiphiles’ in the mycospheres of different basidiomycetous fungi. Environ. Microbiol. 2009, 11, 300–312. [Google Scholar] [CrossRef]
- Simon, A.; Hervé, V.; Al-Dourobi, A.; Verrecchia, E.; Junier, P. An in situ inventory of fungi and their associated migrating bacteria in forest soils using fungal highway columns. FEMS Microbiol. Ecol. 2016, 93, fiw217. [Google Scholar] [CrossRef]
- Worrich, A.; Stryhanyuk, H.; Musat, N.; König, S.; Banitz, T.; Centler, F.; Frank, K.; Thullner, M.; Harms, H.; Richnow, H.-H.; et al. Mycelium-mediated transfer of water and nutrients stimulates bacterial activity in dry and oligotrophic environments. Nat. Commun. 2017, 8, 15472. [Google Scholar] [CrossRef]
- Guhr, A.; Borken, W.; Spohn, M.; Matzner, E. Redistribution of soil water by a saprotrophic fungus enhances carbon mineralization. Proc. Natl. Acad. Sci. USA 2015, 112, 14647–14651. [Google Scholar] [CrossRef] [PubMed]
- Pion, M.; Spangenberg, J.E.; Simon, A.; Bindschedler, S.; Flury, C.; Chatelain, A.; Bshary, R.; Job, D.; Junier, P. Bacterial farming by the fungus Morchella crassipes. Proc. R. Soc. B Boil. Sci. 2013, 280, 20132242. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Zhang, M.; Yang, P.; van Elsas, J.D. The interactions of bacteria with fungi in soil. Adv. Appl. Microbiol. 2014, 89, 185–215. [Google Scholar] [CrossRef]
- Berthold, T.; Centler, F.; Hübschmann, T.; Remer, R.; Thullner, M.; Harms, H.; Wick, L.Y. Mycelia as a focal point for horizontal gene transfer among soil bacteria. Sci. Rep. 2016, 6, 36390. [Google Scholar] [CrossRef] [PubMed]
- Nazir, R.; Mazurier, S.; Yang, P.; Lemanceau, P.; van Elsas, J.D. The ecological role of type three secretion systems in the interaction of bacteria with fungi in soil and related habitats is diverse and context-dependent. Front. Microbiol. 2017, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Bruni, E.P.; Harms, H.; Wick, L.Y. Catch me if you can: Dispersal and foraging of Bdellovibrio bacteriovorus 109J along mycelia. ISME J. 2016, 11, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Esser, D.S.; Leveau, J.H.; Meyer, K.M.; Wiegand, K. Spatial scales of interactions among bacteria and between bacteria and the leaf surface. FEMS Microbiol. Ecol. 2015, 91, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gantner, S.; Schmid, M.; Dürr, C.; Schuhegger, R.; Steidle, A.; Hutzler, P.; Langebartels, C.; Eberl, L.; Hartmann, A.; Dazzo, F.B. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol. Ecol. 2006, 56, 188–194. [Google Scholar] [CrossRef]
- Cai, P.; Sun, X.; Wu, Y.; Gao, C.; Mortimer, M.; Holden, P.A.; Redmile-Gordon, M.; Huang, Q. Soil biofilms: Microbial interactions, challenges, and advanced techniques for ex-situ characterization. Soil Ecol. Lett. 2019, 1, 85–93. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; de Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef]
- Nazir, R.; Warmink, J.A.; Boersma, H.; van Elsas, J.D. Mechanisms that promote bacterial fitness in fungal-affected soil microhabitats. FEMS Microbiol. Ecol. 2009, 71, 169–185. [Google Scholar] [CrossRef]
- Bignell, E. The molecular basis of pH sensing, signaling, and homeostasis in fungi. Adv. Appl. Microbiol. 2012, 79, 1–18. [Google Scholar] [PubMed]
- Braunsdorf, C.; Mailänder-Sánchez, D.; Schaller, M. Fungal sensing of host environment. Cell. Microbiol. 2016, 18, 1188–1200. [Google Scholar] [CrossRef]
- Lurthy, T.; Cantat, C.; Jeudy, C.; Declerck, P.; Gallardo, K.; Barraud, C.; Leroy, F.; Ourry, A.; Lemanceau, P.; Salon, C.; et al. Impact of bacterial siderophores on iron status and ionome in pea. Front. Plant Sci. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2011, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Genet. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Jiang, D.; Armour, C.R.; Hu, C.; Mei, M.; Tian, C.; Sharpton, T.J.; Jiang, Y. Microbiome multi-omics network analysis: Statistical considerations, limitations, and opportunities. Front. Genet. 2019, 10, 995. [Google Scholar] [CrossRef]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; Baumbach, J.; Haller, D.; List, M. Network analysis methods for studying microbial communities: A mini review. Comput. Struct. Biotechnol. J. 2021, 19, 2687–2698. [Google Scholar] [CrossRef]
- Weiss, S.; van Treuren, W.; Lozupone, C.; Faust, K.; Friedman, J.; Deng, Y.; Xia, L.C.; Xu, Z.Z.; Ursell, L.; Alm, E.J.; et al. Correlation detection strategies in microbial data sets vary widely in sensitivity and precision. ISME J. 2016, 10, 1669–1681. [Google Scholar] [CrossRef]
- Telagathoti, A.; Probst, M.; Peintner, U. Habitat, snow-cover and soil pH, affect the distribution and diversity of mortierellaceae species and their associations to bacteria. Front. Microbiol. 2021, 12, 669784. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Landmark Papers: No. 6. Eur. J. Soil Sci. 2017, 70, 1. [Google Scholar] [CrossRef]
- Nunan, N. The microbial habitat in soil: Scale, heterogeneity and functional consequences. J. Plant Nutr. Soil Sci. 2017, 180, 425–429. [Google Scholar] [CrossRef]
- Cordero, O.X.; Datta, M.S. Microbial interactions and community assembly at microscales. Curr. Opin. Microbiol. 2016, 31, 227–234. [Google Scholar] [CrossRef]
- Gause, G.F. The Struggle for Existence; Williams and Wilkins Co.: Baltimore, MD, USA, 1934. [Google Scholar]
- Bravo, D.; Cailleau, G.; Bindschedler, S.; Simon, A.; Job, D.; Verrecchia, E.; Junier, P. Isolation of oxalotrophic bacteria able to disperse on fungal mycelium. FEMS Microbiol. Lett. 2013, 348, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Ingham, C.J.; Kalisman, O.; Finkelshtein, A.; Ben-Jacob, E. Mutually facilitated dispersal between the nonmotile fungus Aspergillus fumigatus and the swarming bacterium Paenibacillus vortex. Proc. Natl. Acad. Sci. USA 2011, 108, 19731–19736. [Google Scholar] [CrossRef] [PubMed]
- Krug, L.; Erlacher, A.; Markut, K.; Berg, G.; Cernava, T. The microbiome of alpine snow algae shows a specific inter-kingdom connectivity and algae-bacteria interactions with supportive capacities. ISME J. 2020, 14, 2197–2210. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Vitale, S.; Lima, G.; di Pietro, A.; Turrà, D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Schmidt, R.; de Jager, V.; Zühlke, D.; Wolff, C.; Bernhardt, J.; Cankar, K.; Beekwilder, J.; van Ijcken, W.; Sleutels, F.; de Boer, W.; et al. Fungal volatile compounds induce production of the secondary metabolite sodorifen in Serratia plymuthica PRI-2C. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Stopnišek, N.; Zühlke, D.; Carlier, A.; Barberán, A.; Fierer, N.; Becher, D.; Riedel, K.; Eberl, L.; Weisskopf, L. Molecular mechanisms underlying the close association between soil Burkholderia and fungi. ISME J. 2015, 10, 253–264. [Google Scholar] [CrossRef]
- Aspray, T.J.; Jones, E.E.; Davies, M.W.; Shipman, M.; Bending, G.D. Increased hyphal branching and growth of ectomycorrhizal fungus Lactarius rufus by the helper bacterium Paenibacillus sp. Mycorrhiza 2013, 23, 403–410. [Google Scholar] [CrossRef]
- Dresch, P.; Falbesoner, J.; Ennemoser, C.; Hittorf, M.; Kuhnert, R.; Peintner, U. Emerging from the ice-fungal communities are diverse and dynamic in earliest soil developmental stages of a receding glacier. Environ. Microbiol. 2019, 21, 1864–1880. [Google Scholar] [CrossRef]
- Casar, C.P.; Kruger, B.R.; Flynn, T.M.; Masterson, A.L.; Momper, L.M.; Osburn, M.R. Mineral-hosted biofilm communities in the continental deep subsurface, Deep Mine Microbial Observatory, SD, USA. Geobiology 2020, 18, 508–522. [Google Scholar] [CrossRef]
- Wallander, H.; Nilsson, L.O.; Hagerberg, D.; Bååth, E. Estimation of the biomass and seasonal growth of external mycelium of ectomycorrhizal fungi in the field. New Phytol. 2001, 151, 753–760. [Google Scholar] [CrossRef]
- Harvey, H.; Wildman, R.D.; Mooney, S.J.; Avery, S.V. Soil aggregates by design: Manufactured aggregates with defined microbial composition for interrogating microbial activities in soil microhabitats. Soil Biol. Biochem. 2020, 148, 107870. [Google Scholar] [CrossRef]
- Helliwell, J.; Miller, T.; Whalley, R.; Mooney, S.; Sturrock, C. Quantifying the impact of microbes on soil structural development and behaviour in wet soils. Soil Biol. Biochem. 2014, 74, 138–147. [Google Scholar] [CrossRef]
- Junier, P.; Cailleau, G.; Palmieri, I.; Vallotton, C.; Trautschold, O.C.; Junier, T.; Paul, C.; Bregnard, D.; Palmieri, F.; Estoppey, A.; et al. Democratization of fungal highway columns as a tool to investigate bacteria associated with soil fungi. FEMS Microbiol. Ecol. 2021, 97, fiab003. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of ichip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl. Environ. Microbiol. 2010, 76, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Das, P.S.; Gagnon-Turcotte, G.; Ouazaa, K.; Bouzid, K.; Hosseini, S.N.; Bharucha, E.; Tremblay, D.; Moineau, S.; Messaddeq, Y.; Corbeil, J.; et al. The EcoChip 2: An autonomous sensor platform for multimodal bio-environmental monitoring of the northern habitat. In Proceedings of the 42nd Annual International Conferences of the IEEE Engineering in Medicine and Biology Society, Montreal, QC, Canada, 20–24 July 2020; pp. 4101–4104. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Ma, Z.; Chen, J.; Akbar, J.; Zhang, S.; Che, C.; Zhang, M.; Cerdà, A. A review of preferential water flow in soil science. Can. J. Soil Sci. 2018, 98, 604–618. [Google Scholar] [CrossRef]
- Lilje, O.; Lilje, E.; Marano, A.V.; Gleason, F.H. Three dimensional quantification of biological samples using micro-computer aided tomography (microCT). J. Microbiol. Methods 2013, 92, 33–41. [Google Scholar] [CrossRef]
- Davit, Y.; Iltis, G.; Debenest, G.; Veran-Tissoires, S.; Wildenschild, D.; Gerino, M.; Quintard, M. Imaging biofilm in porous media using X-ray computed microtomography. J. Microsc. 2010, 242, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Iltis, G.C.; Armstrong, R.T.; Jansik, D.P.; Wood, B.D.; Wildenschild, D. Imaging biofilm architecture within porous media using synchrotron-based X-ray computed microtomography. Water Resour. Res. 2011, 47, 1–5. [Google Scholar] [CrossRef]
- Peszynska, M.; Trykozko, A.; Iltis, G.; Schlueter, S.; Wildenschild, D. Biofilm growth in porous media: Experiments, computational modeling at the porescale, and upscaling. Adv. Water Resour. 2016, 95, 288–301. [Google Scholar] [CrossRef]
- Sanderlin, A.B.; Vogt, S.; Grunewald, E.; Bergin, B.A.; Codd, S.L. Biofilm detection in natural unconsolidated porous media using a low-field magnetic resonance system. Environ. Sci. Technol. 2012, 47, 987–992. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, S.K. Probing the water distribution in porous model sands with two immiscible fluids: A nuclear magnetic resonance micro-imaging study. J. Hydrol. 2017, 553, 637–650. [Google Scholar] [CrossRef]
- Vogt, S.J.; Sanderlin, A.B.; Seymour, J.D.; Codd, S.L. Permeability of a growing biofilm in a porous media fluid flow analyzed by magnetic resonance displacement-relaxation correlations. Biotechnol. Bioeng. 2012, 110, 1366–1375. [Google Scholar] [CrossRef]
- Carrel, M.; Beltran, M.A.; Morales, V.L.; Derlon, N.; Morgenroth, E.; Kaufmann, R.; Holzner, M. Biofilm imaging in porous media by laboratory X-ray tomography: Combining a non-destructive contrast agent with propagation-based phase-contrast imaging tools. PLoS ONE 2017, 12, e0180374. [Google Scholar] [CrossRef]
- Downie, H.F.; Valentine, T.; Otten, W.; Spiers, A.; Dupuy, L. Transparent soil microcosms allow 3D spatial quantification of soil microbiological processes in vivo. Plant Signal. Behav. 2014, 9, e970421. [Google Scholar] [CrossRef]
- Downie, H.; Holden, N.; Otten, W.; Spiers, A.; Valentine, T.; Dupuy, L.X. Transparent soil for imaging the rhizosphere. PLoS ONE 2012, 7, e44276. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, F.E.; Braga, R.; Neilson, R.; Macfarlane, S.A.; Dupuy, L.X. New live screening of plant-nematode interactions in the rhizosphere. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Foster, R.C. Microenvironments of soil microorganisms. Biol. Fertil. Soils 1988, 6, 189–203. [Google Scholar] [CrossRef]
- Lamandé, M.; Schjønning, P.; Ferro, N.D.; Morari, F. Soil pore system evaluated from gas measurements and CT images: A conceptual study using artificial, natural and 3D-printed soil cores. Eur. J. Soil Sci. 2021, 72, 769–781. [Google Scholar] [CrossRef]
- Otten, W.; Pajor, R.; Schmidt, S.; Baveye, P.; Hague, R.; Falconer, R.E. Combining X-ray CT and 3D printing technology to produce microcosms with replicable, complex pore geometries. Soil Biol. Biochem. 2012, 51, 53–55. [Google Scholar] [CrossRef]
- Aleklett, K.; Kiers, E.T.; Ohlsson, P.; Shimizu, T.S.; Caldas, V.E.; Hammer, E.C. Build your own soil: Exploring microfluidics to create microbial habitat structures. ISME J. 2018, 12, 312–319. [Google Scholar] [CrossRef]
- Burmeister, A.; Grünberger, A. Microfluidic cultivation and analysis tools for interaction studies of microbial co-cultures. Curr. Opin. Biotechnol. 2020, 62, 106–115. [Google Scholar] [CrossRef]
- Harvey, H.J.; Wildman, R.D.; Mooney, S.J.; Avery, S.V. Challenges and approaches in assessing the interplay between microorganisms and their physical micro-environments. Comput. Struct. Biotechnol. J. 2020, 18, 2860–2866. [Google Scholar] [CrossRef]
- Wessel, A.K.; Hmelo, L.; Parsek, M.R.; Whiteley, M. Going local: Technologies for exploring bacterial microenvironments. Nat. Rev. Genet. 2013, 11, 337–348. [Google Scholar] [CrossRef]
- Zhou, W.; Le, J.; Chen, Y.; Cai, Y.; Hong, Z.; Chai, Y. Recent advances in microfluidic devices for bacteria and fungus research. TrAC Trends Anal. Chem. 2019, 112, 175–195. [Google Scholar] [CrossRef]
- Weibel, D.B.; DiLuzio, W.R.; Whitesides, G.M. Microfabrication meets microbiology. Nat. Rev. Microbiol. 2007, 5, 209–218. [Google Scholar] [CrossRef]
- Seymour, J.R.; Ahmed, T.; Stocker, R. A microfluidic chemotaxis assay to study microbial behavior in diffusing nutrient patches. Limnol. Oceanogr. Methods 2008, 6, 477–488. [Google Scholar] [CrossRef]
- Kim, H.J.; Boedicker, J.Q.; Choi, J.W.; Ismagilov, R.F. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc. Natl. Acad. Sci. USA 2008, 105, 18188–18193. [Google Scholar] [CrossRef]
- Boedicker, J.Q.; Vincent, M.E.; Ismagilov, R.F. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angew. Chem. Int. Ed. 2009, 48, 5908–5911. [Google Scholar] [CrossRef]
- Mukhopadhyay, R. When PDMS isn’t the best. What are its weaknesses, and which other polymers can researchers add to their toolboxes? Anal. Chem. 2007, 79, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T. Review general fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Connell, J.L.; Wessel, A.K.; Parsek, M.R.; Ellington, A.D.; Whiteley, M.; Shear, J.B. Probing prokaryotic social behaviors with bacterial “lobster traps”. mBio 2010, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baca, H.K.; Ashley, C.; Carnes, E.; Lopez, D.; Flemming, J.; Dunphy, D.; Singh, S.; Chen, Z.; Liu, N.; Fan, H.; et al. Cell-directed assembly of lipid-silica nanostructures providing extended cell viability. Science 2006, 313, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Carnes, E.C.; Lopez, D.M.; Donegan, N.; Cheung, A.; Gresham, H.; Timmins, G.; Brinker, C.J. Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat. Chem. Biol. 2009, 6, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Orner, E.; Chau, J.F.; Anderson, E.M.; Kadilak, A.L.; Rubinstein, R.; Bouchillon, G.M.; Goodwin, R.; Gage, D.; Shor, L.M. Synergistic effects of soil microstructure and bacterial EPS on drying rate in emulated soil micromodels. Soil Biol. Biochem. 2015, 83, 116–124. [Google Scholar] [CrossRef]
- Rubinstein, R.; Kadilak, A.L.; Cousens, V.C.; Gage, D.; Shor, L.M. Protist-facilitated particle transport using emulated soil micromodels. Environ. Sci. Technol. 2015, 49, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Wondraczek, L.; Pohnert, G.; Schacher, F.H.; Köhler, A.; Gottschaldt, M.; Schubert, U.S.; Küsel, K.; Brakhage, A.A. Artificial microbial arenas: Materials for observing and manipulating microbial consortia. Adv. Mater. 2019, 31, e1900284. [Google Scholar] [CrossRef]
- Gimeno, A.; Stanley, C.E.; Ngamenie, Z.; Hsung, M.-H.; Walder, F.; Schmieder, S.S.; Bindschedler, S.; Junier, P.; Keller, B.; Vogelgsang, S. A versatile microfluidic platform measures hyphal interactions between Fusarium graminearum and Clonostachys rosea in real-time. Commun. Biol. 2021, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.L.; Nicolau, D.V.; Filipponi, L.; Wang, L.; Lee, A.P. Fungi use efficient algorithms for the exploration of microfluidic networks. Small 2006, 2, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.Y.; Wang, S.; Dick, G.J.; Young, V.B.; Sherman, D.H.; Burns, M.A.; Lin, X.N. Co-cultivation of microbial sub-communities in microfluidic droplets facilitates high-resolution genomic dissection of microbial ‘dark matter’. Integr. Biol. 2020, 12, 263–274. [Google Scholar] [CrossRef]
- Kaehr, B.; Shear, J.B. Multiphoton fabrication of chemically responsive protein hydrogels for microactuation. Proc. Natl. Acad. Sci. USA 2008, 105, 8850–8854. [Google Scholar] [CrossRef]
- Zhang, Z.; Boccazzi, P.; Choi, H.-G.; Perozziello, G.; Sinskey, A.J.; Jensen, K. Microchemostat—Microbial continuous culture in a polymer-based, instrumented microbioreactor. Lab Chip 2006, 6, 906–913. [Google Scholar] [CrossRef]
- Pamp, S.J.; Sternberg, C.; Tolker-Nielsen, T. Insight into the microbial multicellular lifestyle via flow-cell technology and confocal microscopy. Cytom. Part A 2008, 75, 90–103. [Google Scholar] [CrossRef]
- Massalha, H.; Korenblum, E.; Malitsky, S.; Shapiro, O.H.; Aharoni, A. Live imaging of root–bacteria interactions in a microfluidics setup. Proc. Natl. Acad. Sci. USA 2017, 114, 4549–4554. [Google Scholar] [CrossRef]
- Schmieder, S.S.; Stanley, C.; Rzepiela, A.; van Swaay, D.; Sabotič, J.; Nørrelykke, S.; Demello, A.J.; Aebi, M.; Künzler, M. Bidirectional propagation of signals and nutrients in fungal networks via specialized hyphae. Curr. Biol. 2019, 29, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Tayyrov, A.; Stanley, C.E.; Azevedo, S.; Künzler, M. Combining microfluidics and RNA-sequencing to assess the inducible defensome of a mushroom against nematodes. BMC Genom. 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Uehling, J.K.; Entler, M.R.; Meredith, H.R.; Millet, L.J.; Timm, C.M.; Aufrecht, J.A.; Bonito, G.M.; Engle, N.L.; Labbé, J.L.; Doktycz, M.J.; et al. Microfluidics and metabolomics reveal symbiotic bacterial–fungal interactions between Mortierella elongata and Burkholderia include metabolite exchange. Front. Microbiol. 2019, 10, 2163. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.; Stöckli, M.; van Swaay, D.; Sabotič, J.; Kallio, P.T.; Künzler, M.; Demello, A.J.; Aebi, M. Probing bacterial–fungal interactions at the single cell level. Integr. Biol. 2014, 6, 935–945. [Google Scholar] [CrossRef]
- Eun, Y.J.; Utada, A.S.; Copeland, M.F.; Takeuchi, S.; Weibel, D.B. Encapsulating bacteria in agarose microparticles using microfluidics for high-throughput cell analysis and isolation. ACS Chem. Biol. 2010, 6, 260–266. [Google Scholar] [CrossRef]
- Churski, K.; Kaminski, T.S.; Jakiela, S.; Kamysz, W.; Barańska-Rybak, W.; Weibel, D.B.; Garstecki, P. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip 2012, 12, 1629–1637. [Google Scholar] [CrossRef]
- Abraham, W.-R. Applications and impacts of stable isotope probing for analysis of microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 4817–4828. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Groth, I.; Schmitt, I.; Richter, W.; Roth, M.; Hertweck, C. Burkholderia rhizoxinica sp. nov. and Burkholderia endofungorum sp. nov., bacterial endosymbionts of the plant-pathogenic fungus Rhizopus microsporus. Int. J. Syst. Evol. Microbiol. 2007, 57, 2583–2590. [Google Scholar] [CrossRef]
- Söderström, B.; Erland, S. Isolation of fluorescein diacetate stained hyphae from soil by micromanipulation. Trans. Br. Mycol. Soc. 1986, 86, 465–468. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, A.J.; Clark, I.M.; Taylor, R.G.; Valsami-Jones, E.; Hirsch, P.R. A novel method for sampling bacteria on plant root and soil surfaces at the microhabitat scale. J. Microbiol. Methods 2008, 75, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ashida, N.; Ishii, S.; Hayano, S.; Tago, K.; Tsuji, T.; Yoshimura, Y.; Otsuka, S.; Senoo, K. Isolation of functional single cells from environments using a micromanipulator: Application to study denitrifying bacteria. Appl. Microbiol. Biotechnol. 2009, 85, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Tago, K.; Uei, Y.; Ishii, S.; Isobe, K.; Otsuka, S.; Senoo, K. Advantages of functional single-cell isolation method over standard agar plate dilution method as a tool for studying denitrifying bacteria in rice paddy soil. AMB Express 2012, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, J.; König, H. New techniques for isolation of single prokaryotic cells. FEMS Microbiol. Rev. 2000, 24, 567–572. [Google Scholar] [CrossRef]
- Whitley, K.D.; Comstock, M.J.; Chemla, Y.R. High-resolution “fleezers”: Dual-trap optical tweezers combined with single-molecule fluorescence detection. In Optical Tweezers. Methods in Molecular Biology; Gennerich, A., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1486, pp. 183–256. [Google Scholar] [CrossRef]
- Ringeisen, B.R.; Rincon, K.; Fitzgerald, L.A.; Fulmer, P.A.; Wu, P.K. Printing soil: A single-step, high-throughput method to isolate micro-organisms and near-neighbour microbial consortia from a complex environmental sample. Methods Ecol. Evol. 2014, 6, 209–217. [Google Scholar] [CrossRef]
- Sato, Y.; Narisawa, K.; Tsuruta, K.; Umezu, M.; Nishizawa, T.; Tanaka, K.; Yamaguchi, K.; Komatsuzaki, M.; Ohta, H. Detection of betaproteobacteria inside the mycelium of the fungus Mortierella elongata. Microbes Environ. 2010, 25, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Ghodsalavi, B.; Svenningsen, N.B.; Hao, X.; Olsson, S.; Nicolaisen, M.H.; Abu Al-Soud, W.; Sørensen, S.J.; Nybroe, O. A novel baiting microcosm approach used to identify the bacterial community associated with Penicillium bilaii hyphae in soil. PLoS ONE 2017, 12, e0187116. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.; Altemüller, H.-J. Bacteria in thin soil sections stained with the fluorescent brightener calcofluor white M2R. Soil Biol. Biochem. 1990, 22, 89–96. [Google Scholar] [CrossRef]
- Hoffman, M.T.; Gunatilaka, M.K.; Wijeratne, K.; Gunatilaka, L.; Arnold, A.E. Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLoS ONE 2013, 8, e73132. [Google Scholar] [CrossRef] [PubMed]
- Bertaux, J.; Schmid, M.; Prevost-Boure, N.C.; Churin, J.L.; Hartmann, A.; Garbaye, J.; Frey-Klett, P. In situ identification of intracellular bacteria related to Paenibacillus spp. in the mycelium of the ectomycorrhizal fungus Laccaria bicolor S238N. Appl. Environ. Microbiol. 2003, 69, 4243–4248. [Google Scholar] [CrossRef]
- Hoffman, M.T.; Arnold, A.E. Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes. Appl. Environ. Microbiol. 2010, 76, 4063–4075. [Google Scholar] [CrossRef]
- Naumann, M.; Schüssler, A.; Bonfante, P. The obligate endobacteria of arbuscular mycorrhizal fungi are ancient heritable components related to the mollicutes. ISME J. 2010, 4, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.-Y.; Chen, A. Fluorescence in situ hybridization. J. Investig. Dermatol. 2013, 133, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Eickhorst, T.; Mußmann, M. Gold-FISH: A new approach for the in situ detection of single microbial cells combining fluorescence and scanning electron microscopy. Syst. Appl. Microbiol. 2012, 35, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Eickhorst, T.; Tippkötter, R. Evaluation of tyramide solutions for an improved detection and enumeration of single microbial cells in soil by CARD-FISH. J. Microbiol. Methods 2012, 91, 399–405. [Google Scholar] [CrossRef]
- Schmidt, H.; Eickhorst, T. Detection and quantification of native microbial populations on soil-grown rice roots by catalyzed reporter deposition-fluorescencein situhybridization. FEMS Microbiol. Ecol. 2014, 87, 390–402. [Google Scholar] [CrossRef]
- Behrens, S.; Lösekann, T.; Pett-Ridge, J.; Weber, P.K.; Ng, W.-O.; Stevenson, B.; Hutcheon, I.D.; Relman, D.A.; Spormann, A.M. Linking microbial phylogeny to metabolic activity at the single-cell level by using enhanced element labeling-catalyzed reporter deposition fluorescence in situ hybridization (EL-FISH) and NanoSIMS. Appl. Environ. Microbiol. 2008, 74, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Nunan, N.; Ritz, K.; Crabb, D.; Harris, K.; Wu, K.; Crawford, J.W.; Young, I.M. Quantification of the in situ distribution of soil bacteria by large-scale imaging of thin sections of undisturbed soil. FEMS Microbiol. Ecol. 2001, 37, 67–77. [Google Scholar] [CrossRef]
- Hapca, S.; Baveye, P.; Wilson, C.; Lark, R.; Otten, W. Three-dimensional mapping of soil chemical characteristics at micrometric scale by combining 2D SEM-EDX data and 3D X-ray CT images. PLoS ONE 2015, 10, e0137205. [Google Scholar] [CrossRef] [PubMed]
- Nunan, N.; Wu, K.; Young, I.M.; Crawford, J.W.; Ritz, K. Spatial distribution of bacterial communities and their relationships with the micro-architecture of soil. FEMS Microbiol. Ecol. 2003, 44, 203–215. [Google Scholar] [CrossRef]
- Oburger, E.; Schmidt, H. New methods to unravel rhizosphere processes. Trends Plant Sci. 2016, 21, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.S.; Robinson, I.; Yusuf, M. 3D X-ray nanotomography of cells grown on electrospun scaffolds. Macromol. Biosci. 2016, 17, 1–8. [Google Scholar] [CrossRef]
- Bouckaert, L.; van Loo, D.; Ameloot, N.; Buchan, D.; van Hoorebeke, L.; Sleutel, S. Compatibility of X-ray micro-computed tomography with soil biological experiments. Soil Biol. Biochem. 2013, 56, 10–12. [Google Scholar] [CrossRef][Green Version]
- Sturrock, C.; Woodhall, J.; Brown, M.; Walker, C.; Mooney, S.; Ray, R.V. Effects of damping-off caused by Rhizoctonia solani anastomosis group 2-1 on roots of wheat and oil seed rape quantified using X-ray computed tomography and real-time PCR. Front. Plant Sci. 2015, 6, 461. [Google Scholar] [CrossRef]
- Hingley-Wilson, S.M.; Ma, N.; Hu, Y.; Casey, R.; Bramming, A.; Curry, R.J.; Tang, H.L.; Wu, H.; Butler, R.E.; Jacobs, W.R.; et al. Loss of phenotypic inheritance associated with ydcI mutation leads to increased frequency of small, slow persisters in Escherichia coli. Proc. Natl. Acad. Sci. USA 2020, 117, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Juyal, A.; Otten, W.; Baveye, P.C.; Eickhorst, T. Influence of soil structure on the spread of Pseudomonas fluorescens in soil at microscale. Eur. J. Soil Sci. 2021, 72, 141–153. [Google Scholar] [CrossRef]
- Zappala, S.; Helliwell, J.R.; Tracy, S.; Mairhofer, S.; Sturrock, C.; Pridmore, T.; Bennett, M.; Mooney, S. Effects of X-ray dose on rhizosphere studies using X-ray computed tomography. PLoS ONE 2013, 8, e67250. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Vetterlein, D.; Köhne, J.M.; Eickhorst, T. Negligible effect of X-ray μ-CT scanning on archaea and bacteria in an agricultural soil. Soil Biol. Biochem. 2015, 84, 21–27. [Google Scholar] [CrossRef]
- Arendt, K.R.; Hockett, K.; Araldi-Brondolo, S.J.; Baltrus, D.A.; Arnold, A.E. Isolation of Endohyphal bacteria from foliar ascomycota and in vitro establishment of their symbiotic associations. Appl. Environ. Microbiol. 2016, 82, 2943–2949. [Google Scholar] [CrossRef]
- Benoit, I.; van den Esker, M.H.; Patyshakuliyeva, A.; Mattern, D.J.; Blei, F.; Zhou, M.; Dijksterhuis, J.; Brakhage, A.A.; Kuipers, O.P.; de Vries, R.P.; et al. Bacillus subtilis attachment to Aspergillus nigerhyphae results in mutually altered metabolism. Environ. Microbiol. 2014, 17, 2099–2113. [Google Scholar] [CrossRef]
- Schuster, M.; Kilaru, S.; Guo, M.; Sommerauer, M.; Lin, C.; Steinberg, G. Red fluorescent proteins for imaging Zymoseptoria tritici during invasion of wheat. Fungal Genet. Biol. 2015, 79, 132–140. [Google Scholar] [CrossRef][Green Version]
- Lichius, A. Live-cell imaging in Trichoderma. In New and Future Developments in Microbial Biotechnology and Bioengineering: Recent Developments in Trichoderma Research; Gupta, V.K., Zeilinger, S., Singh, H.B., Druzhinina, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–108. [Google Scholar]
- Bard, A.J.; Mirkin, M.V. (Eds.) Scanning Electrochemical Microscopy; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Liu, X.; Ramsey, M.M.; Chen, X.; Koley, D.; Whiteley, M.; Bard, A.J. Real-time mapping of a hydrogen peroxide concentration profile across a polymicrobial bacterial biofilm using scanning electrochemical microscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 2668–2673. [Google Scholar] [CrossRef]
- Buchberger, A.R.; Delaney, K.; Johnson, J.; Jillian, J. Mass spectrometry imaging: A review of emerging advancements and future insights. Anal. Chem. 2018, 90, 240–265. [Google Scholar] [CrossRef]
- Watrous, J.D.; Dorrestein, P.C. Imaging mass spectrometry in microbiology. Nat. Rev. Genet. 2011, 9, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, E.; Yang, Y.-L.; Watrous, J.; Gerwick, W.H.; Dorrestein, P.C. Imaging mass spectrometry of natural products. Nat. Prod. Rep. 2009, 26, 1521–1534. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Xu, Y.; Straight, P.; Dorrestein, P.C. Translating metabolic exchange with imaging mass spectrometry. Nat. Chem. Biol. 2009, 5, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Watrous, J.; Hendricks, N.; Meehan, M.; Dorrestein, P.C. capturing bacterial metabolic exchange using thin film desorption electrospray ionization-imaging mass spectrometry. Anal. Chem. 2010, 82, 1598–1600. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Esquenazi, E.; Coates, C.; Simmons, L.; Gonzalez, D.; Gerwick, W.H.; Dorrestein, P.C. Visualizing the spatial distribution of secondary metabolites produced by marine cyanobacteria and sponges via MALDI-TOF imaging. Mol. BioSyst. 2008, 4, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-T.; Yang, Y.-L.; Xu, Y.; Lamsa, A.; Haste, N.M.; Yang, J.Y.; Ng, J.; Gonzalez, D.; Ellermeier, C.; Straight, P.; et al. Imaging mass spectrometry of intraspecies metabolic exchange revealed the cannibalistic factors of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2010, 107, 16286–16290. [Google Scholar] [CrossRef] [PubMed]
- Holzlechner, M.; Reitschmidt, S.; Gruber, S.; Zeilinger, S.; Marchetti-Deschmann, M. Visualizing fungal metabolites during mycoparasitic interaction by MALDI mass spectrometry imaging. Proteomics 2016, 16, 1742–1746. [Google Scholar] [CrossRef]
- Moree, W.J.; Yang, J.Y.; Zhao, X.; Liu, W.-T.; Aparicio, M.; Atencio, L.; Ballesteros, J.; Sanchez, J.; Gavilan, R.; Gutiérrez, M.; et al. Imaging mass spectrometry of a coral microbe interaction with fungi. J. Chem. Ecol. 2013, 39, 1045–1054. [Google Scholar] [CrossRef]
- Boya, P.C.A.; Fernández-Marín, H.; Mejia, L.C.; Spadafora, C.; Dorrestein, P.C.; Gutiérrez, M. Imaging mass spectrometry and MS/MS molecular networking reveals chemical interactions among cuticular bacteria and pathogenic fungi associated with fungus-growing ants. Sci. Rep. 2017, 7, 5604. [Google Scholar] [CrossRef]
- Lane, A.L.; Nyadong, L.; Galhena, A.S.; Shearer, T.L.; Stout, E.P.; Parry, R.M.; Kwasnik, M.; Wang, M.D.; Hay, M.E.; Fernandez, F.M.; et al. Desorption electrospray ionization mass spectrometry reveals surface-mediated antifungal chemical defense of a tropical seaweed. Proc. Natl. Acad. Sci. USA 2009, 106, 7314–7319. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Weber, P.K. NanoSIP: NanoSIMS applications for microbial biology. In Microbial Systems Biology. Methods in Molecular Biology (Methods and Protocols); Navid, A., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 881, pp. 375–408. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; Fletcher, J.; Goodacre, R.; Lockyer, N.P.; Micklefield, J.; Vickerman, J.C. Subsurface biomolecular imaging of Streptomyces coelicolor using secondary ion mass spectrometry. Anal. Chem. 2008, 80, 1942–1951. [Google Scholar] [CrossRef]
- Murrell, J.C.; Whiteley, A.S. Stable Isotope Probing and Related Technologies; American Society for Microbiology Press: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Dumont, M.G.; Murrell, J.C. Stable isotope probing—Linking microbial identity to function. Nat. Rev. Genet. 2005, 3, 499–504. [Google Scholar] [CrossRef]
- Rime, T.; Hartmann, M.; Frey, B. Potential sources of microbial colonizers in an initial soil ecosystem after retreat of an alpine glacier. ISME J. 2016, 10, 1625–1641. [Google Scholar] [CrossRef]
- Pinto-Tomás, A.A.; Anderson, M.A.; Suen, G.; Stevenson, D.M.; Chu, F.S.T.; Cleland, W.W.; Weimer, P.J.; Currie, C.R. Symbiotic nitrogen fixation in the fungus gardens of leaf-cutter ants. Science 2009, 326, 1120–1123. [Google Scholar] [CrossRef]
- Lee, N.; Nielsen, P.H.; Andreasen, K.H.; Juretschko, S.; Nielsen, J.L.; Schleifer, K.-H.; Wagner, M. Combination of fluorescent in situ hybridization and microautoradiography—A new tool for structure-function analyses in microbial ecology. Appl. Environ. Microbiol. 1999, 65, 1289–1297. [Google Scholar] [CrossRef]
- Ouverney, C.C.; Fuhrman, J.A. Combined microautoradiography–16S rRNA probe technique for determination of radioisotope uptake by specific microbial cell types in situ. Appl. Environ. Microbiol. 1999, 65, 1746–1752. [Google Scholar] [CrossRef]
- Adamczyk, J.; Hesselsoe, M.; Iversen, N.; Horn, M.; Lehner, A.; Nielsen, P.H.; Schloter, M.; Roslev, P.; Wagner, M. The isotope array, a new tool that employs substrate-mediated labeling of rRNA for determination of microbial community structure and function. Appl. Environ. Microbiol. 2003, 69, 6875–6887. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, G.; von Netzer, F.; Engel, M.; Lueders, T. Electron acceptor-dependent identification of key anaerobic toluene degraders at a tar-oil-contaminated aquifer by Pyro-SIP. FEMS Microbiol. Ecol. 2011, 78, 165–175. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Kuske, C.R. Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. Appl. Environ. Microbiol. 2012, 78, 2316–2327. [Google Scholar] [CrossRef] [PubMed]
- López-Mondéjar, R.; Brabcova, V.; Štursová, M.; Davidová, A.; Jansa, J.; Cajthaml, T.; Baldrian, P. Decomposer food web in a deciduous forest shows high share of generalist microorganisms and importance of microbial biomass recycling. ISME J. 2018, 12, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Sheik, A.R.; Brussaard, C.P.D.; Lavik, G.; Lam, P.; Musat, N.; Krupke, A.; Littmann, S.; Strous, M.; Kuypers, M.M.M. Responses of the coastal bacterial community to viral infection of the algae Phaeocystis globosa. ISME J. 2014, 8, 212–225. [Google Scholar] [CrossRef]
- Musat, N.; Musat, F.; Weber, P.K.; Pett-Ridge, J. Tracking microbial interactions with NanoSIMS. Curr. Opin. Biotechnol. 2016, 41, 114–121. [Google Scholar] [CrossRef]
- Dekas, A.E.; Poretsky, R.S.; Orphan, V.J. Deep-sea archaea fix and share nitrogen in methane-consuming microbial consortia. Science 2009, 326, 422–426. [Google Scholar] [CrossRef]
- Orphan, V.J.; House, C.H.; Hinrichs, K.-U.; McKeegan, K.D.; DeLong, E.F. Methane-consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 2001, 293, 484–487. [Google Scholar] [CrossRef]
- Tai, V.; Carpenter, K.J.; Weber, P.K.; Nalepa, C.A.; Perlman, S.J.; Keeling, P.J. Genome evolution and nitrogen fixation in bacterial ectosymbionts of a protist inhabiting wood-feeding cockroaches. Appl. Environ. Microbiol. 2016, 82, 4682–4695. [Google Scholar] [CrossRef] [PubMed]
- Woebken, D.; Burow, L.C.; Prufert-Bebout, L.; Bebout, B.M.; Hoehler, T.M.; Pett-Ridge, J.; Spormann, A.M.; Weber, P.K.; Singer, S.W. Identification of a novel cyanobacterial group as active diazotrophs in a coastal microbial mat using NanoSIMS analysis. ISME J. 2012, 6, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Fike, D.A.; Gammon, C.L.; Ziebis, W.; Orphan, V.J. Micron-scale mapping of sulfur cycling across the oxycline of a cyanobacterial mat: A paired nanoSIMS and CARD-FISH approach. ISME J. 2008, 2, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Musat, N.; Adam, B.; Kuypers, M.; Amann, R. HISH–SIMS analysis of bacterial uptake of algal-derived carbon in the Río de la Plata estuary. Syst. Appl. Microbiol. 2012, 35, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.K.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as complementary techniques for analysis of volatiles: A tutorial review. Anal. Chim. Acta 2018, 1035, 1–13. [Google Scholar] [CrossRef] [PubMed]


| BIB-SEM | Broad ion-beam | GFP | Green fluorescent protein |
| BioLP | Biological laser printing | HISH | Halogen in situ hybridization |
| CARD-FISH | Catalysed reporter deposition | IMS | Imaging mass spectrometry |
| CLSM | Confocal laser scanning microscope | MALDI-IMS | Matrix-assisted laser desorption-ionization |
| DESI-IMS | Desorption electrospray ionization | OCT | Optical coherence tomography |
| DFA | Direct fluorescent antibody | PTR-MS | Proton transfer reaction |
| EL-FISH | Elemental labelling | SECM | Scanning electrochemical microscope |
| FACS | Fluorescence-activated cell sorter | SEM | Scanning electron microscope |
| FADS | Fluorescence-activated droplet sorter | (nano)SIMS | Secondary ion mass spectrometry |
| FIB-SEM | Focused ion-beam | SIP | Stable isotope probing |
| FISH | Fluorescence in situ hybridization | SR-CT | Synchroton computer tomography |
| FPT | Fluorescence probe techniques | TEM | Transmission electron microscope |
| GC-MS | Gas chromatography | X-ray CT | X-ray computer tomography |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandolini, E.; Probst, M.; Peintner, U. Methods for Studying Bacterial–Fungal Interactions in the Microenvironments of Soil. Appl. Sci. 2021, 11, 9182. https://doi.org/10.3390/app11199182
Mandolini E, Probst M, Peintner U. Methods for Studying Bacterial–Fungal Interactions in the Microenvironments of Soil. Applied Sciences. 2021; 11(19):9182. https://doi.org/10.3390/app11199182
Chicago/Turabian StyleMandolini, Edoardo, Maraike Probst, and Ursula Peintner. 2021. "Methods for Studying Bacterial–Fungal Interactions in the Microenvironments of Soil" Applied Sciences 11, no. 19: 9182. https://doi.org/10.3390/app11199182
APA StyleMandolini, E., Probst, M., & Peintner, U. (2021). Methods for Studying Bacterial–Fungal Interactions in the Microenvironments of Soil. Applied Sciences, 11(19), 9182. https://doi.org/10.3390/app11199182







