Molecular Profile of Skin Cancer
Abstract
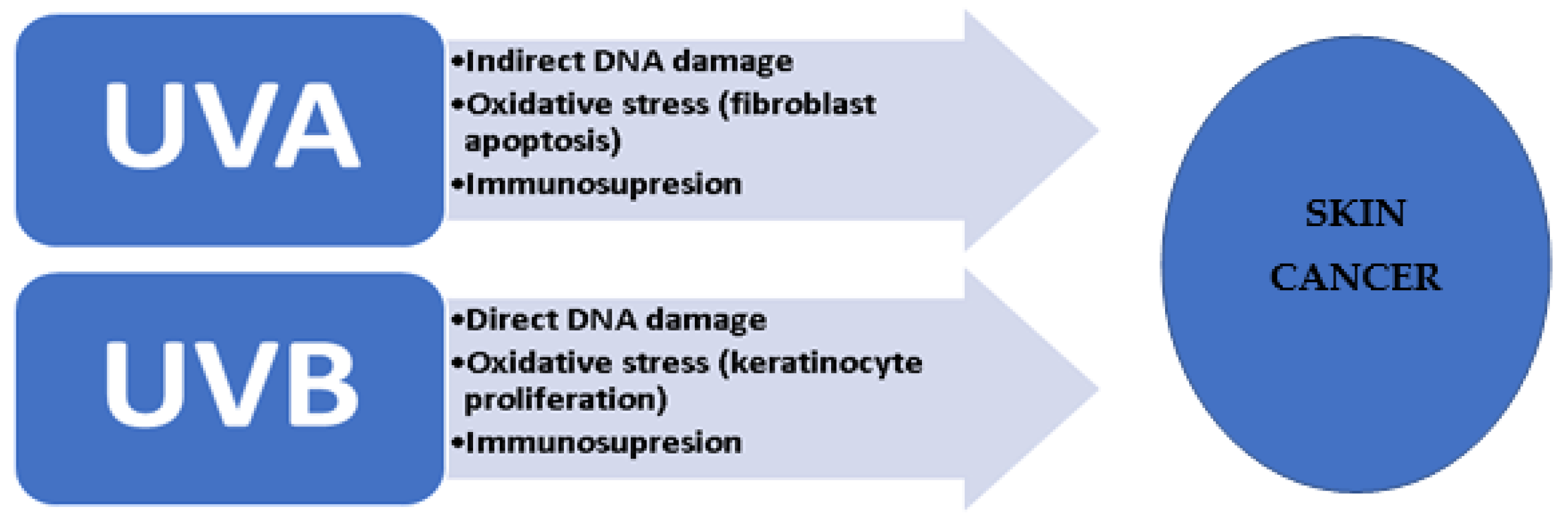
:1. Introduction
2. UV Signature in Skin Cancer
3. Gene Mutations in Melanoma
4. Gene Mutations in NMSC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Stransky, N.; Egloff, A.M.; Tward, A.D.; Kostic, A.; Cibulskis, K.; Sivachenko, A.; Kryukov, G.; Lawrence, M.S.; Sougnez, C.; McKenna, A.; et al. The Mutational Landscape of Head and Neck Squamous Cell Carcinoma. Science 2011, 333, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.J.; Vousden, K.H. P53 mutations in cancer. Nature 2013, 15, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Freed-Pastor, W.A.; Prives, C. Mutant p53: One name, manyproteins. Genes Dev. 2012, 26, 1268–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, C.L.; Ananthaswamy, H.N. P53 and the pathogenesis of skin cancer. Toxicol. Appl. Pharmacol. 2007, 224, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conforti, C.; Beninanti, E.; Dianzani, C. Are actinic keratoses really squamous cell cancer? How do we know if they would become malignant? Clin. Dermatol. 2017, 36, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yuan, R.; Gu, H.; Liu, T.; Tu, Y.; Yang, Z.; He, L. The effect of ultraviolet radiation on the transforming growth factor beta 1/Smads pathway and p53 in actinic keratosis and normal skin. Arch. Dermatol. Res. 2013, 305, 777–786. [Google Scholar] [CrossRef]
- Hussein, M.R.; Haemel, A.K.; Wood, G.S. Apoptosis and melanoma: Molecular mechanisms. J. Pathol. 2003, 199, 275–288. [Google Scholar] [CrossRef]
- Zhang, H.; Rosdahl, I. Deletion in p16INK4a and loss of p16 expression in human skin primary and metastatic melanoma cells. Int. J. Oncol. 2004, 24, 331–335. [Google Scholar] [CrossRef]
- van Kempen, L.C.L.; Redpath, M.; Elchebly, M.; Klein, K.O.; Papadakis, A.I.; Wilmott, J.S.; Scolyer, R.A.; Edqvist, P.-H.; Pontén, F.; Schadendorf, D.; et al. The protein phosphatase 2A regulatory subunit PR70 is a gonosomal melanoma tumor suppressor gene. Sci. Transl. Med. 2016, 8, 369ra177. [Google Scholar] [CrossRef] [PubMed]
- Arafeh, R.; Qutob, N.; Emmanuel, R.; Keren-Paz, A.; Madore, J.; Elkahloun, A.; Wilmott, J.; Gartner, J.J.; Di Pizio, A.; Winograd-Katz, S.; et al. Recurrent inactivating RASA2 mutations in melanoma. Nat. Genet. 2015, 47, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Albino, A.P.; Le Strange, R.; Oliff, A.I.; Furth, M.E.; Old, L.J. Transforming ras genes from human melanoma: A manifestation of tumour heterogeneity? Nature 1984, 308, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Lee, J.T.; Wang, W.; Zhang, J.; Cho, H.; Mamo, S.; Bremer, R.; Gillette, S.; Kong, J.; Haass, N.K.; et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl. Acad. Sci. USA 2008, 105, 3041–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosso, S.; Zanetti, R.; Martinez, C.; Tormo, M.J.; Schraub, S.; Sancho-Garnier, H.; Franceschi, S.; Gafà, L.; Perea, E.; Navarro, C.; et al. The multicentre south European study ‘Helios’. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br. J. Cancer 1996, 73, 1447–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotti, T.; Bruscino, N.; Hercogová, J.; De Giorgi, V. Controversial issues on melanoma. Dermatol. Ther. 2012, 25, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Poon, T.S.; Barnetson, R.S.C.; Halliday, G.M. Sunlight-Induced Immunosuppression in Humans Is Initially Because of UVB, Then UVA, Followed by Interactive Effects. J. Investig. Dermatol. 2005, 125, 840–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheucă Solovăstru, L.; Vâță, D.; Stătescu, L.; Constantin, M.M.; Andrese, E. Skin cancer between myth and reality, yet ethically constrained. Rev. Rom. Bioet. 2014, 2, 47–52. [Google Scholar]
- Tassone, P.; Old, M.; Teknos, T.N.; Pan, Q. p53-based therapeutics for head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 733–737. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, A.; Al-Hraishawi, H.; Simhadri, S.; Hirshfield, K.M.; Chen, S.; Pine, S.; Jeyamohan, C.; Sokol, L.; Ali, S.; Teo, M.L.; et al. BRAF Fusion as a Novel Mechanism of Acquired Resistance to Vemurafenib in BRAFV600E Mutant Melanoma. Clin. Cancer Res. 2017, 23, 5631–5638. [Google Scholar] [CrossRef] [Green Version]
- Roberts, A.E.; Allanson, J.E.; Tartaglia, M.; Gelb, B.D. Noonan syndrome. Lancet 2013, 381, 333–342. [Google Scholar] [CrossRef] [Green Version]
- Curtin, J.; Fridlyand, J.; Kageshita, T.; Patel, H.N.; Busam, K.J.; Kutzner, H.; Cho, K.-H.; Aiba, S.; Bröcker, E.-B.; LeBoit, P.E.; et al. Distinct Sets of Genetic Alterations in Melanoma. N. Engl. J. Med. 2005, 353, 2135–2147. [Google Scholar] [CrossRef]
- Shinozaki, M.; Fujimoto, A.; Morton, D.L.; Hoon, D. Incidence of BRAF Oncogene Mutation and Clinical Relevance for Primary Cutaneous Melanomas. Clin. Cancer Res. 2004, 10, 1753–1757. [Google Scholar] [CrossRef] [Green Version]
- Tschernitz, S.; Flossbach, L.; Bonengel, M.; Roth, S.; Rosenwald, A.; Geissinger, E. AlternativeBRAFmutations inBRAFV600E-negative hairy cell leukaemias. Br. J. Haematol. 2014, 165, 529–533. [Google Scholar] [CrossRef]
- Tiacci, E.; Trifonov, V.; Schiavoni, G.; Holmes, A.; Kern, W.; Martelli, M.P.; Pucciarini, A.; Bigerna, B.; Pacini, R.; Wells, V.A.; et al. BRAFMutations in Hairy-Cell Leukemia. N. Engl. J. Med. 2011, 364, 2305–2315. [Google Scholar] [CrossRef] [Green Version]
- Schindler, G.; Capper, D.; Meyer, J.; Janzarik, W.; Omran, H.; Herold-Mende, C.; Schmieder, K.; Wesseling, P.; Mawrin, C.; Hasselblatt, M.; et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011, 121, 397–405. [Google Scholar] [CrossRef]
- Madhunapantula, S.; Robertson, G.P. Is B-Raf a Good Therapeutic Target for Melanoma and Other Malignancies? Cancer Res. 2008, 68, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Sharma, A.; Peng, H.-H.; Robertson, G.; Dong, C. Targeting Mutant (V600E)B-Rafin Melanoma Interrupts Immunoediting of Leukocyte Functions and Melanoma Extravasation. Cancer Res. 2007, 67, 5814–5820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaev, S.I.; Rimoldi, D.; Iseli, C.; Valsesia, A.; Robyr, D.; Gehrig, C.; Harshman, K.; Guipponi, M.; Bukach, O.; Zoete, V.; et al. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat. Genet. 2011, 44, 133–139. [Google Scholar] [CrossRef]
- Mandarà, M.; Nortilli, R.; Sava, T.; Cetto, G.L. Chemotherapy for metastatic melanoma. Expert Rev. Anticancer Ther. 2006, 6, 121–130. [Google Scholar] [CrossRef]
- Ranhotra, H.S. The estrogen-related receptors in metabolism and cancer: Newer insights. J. Recept. Signal. Transduct. 2018, 38, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Gumaste, P.; Penn, L.; Cymerman, R.; Kirchhoff, T.; Polsky, D.; McLellan, B. Skin cancer risk in BRCA1/2 mutation carriers. Br. J. Dermatol. 2014, 172, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Berger, M.; Richtig, G.; Kashofer, K.; Aigelsreiter, A.; Richtig, E. The window of opportunities for targeted therapy in BRAFwt/NRASwt/KITwt melanoma: Biology and clinical implications of fusion proteins and other mutations. Ital. J. Dermatol. Venereol. 2018, 153, S0392–S0488. [Google Scholar] [CrossRef]
- Mologni, L.; Costanza, M.; Sharma, G.G.; Viltadi, M.; Massimino, L.; Citterio, S.; Purgante, S.; Raman, H.; Pirola, A.; Zucchetti, M.; et al. Concomitant BCORL1 and BRAF Mutations in Vemurafenib-Resistant Melanoma Cells. Neoplasia 2018, 20, 467–477. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Edmunds, S.C.; Kelsell, D.; Hungerford, J.L.; Cree, I.A. Mutational analysis of selected genes in the TGFbeta, Wnt, pRb, and p53 pathways in primary uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2845–2851. [Google Scholar]
- Van Raamsdonk, C.; Griewank, K.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations inGNA11in Uveal Melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef] [Green Version]
- Chae, Y.K.; Anker, J.; Carneiro, B.A.; Chandra, S.; Kaplan, J.; Kalyan, A.; Santa-Maria, C.A.; Platanias, L.C.; Giles, F.J. Genomic landscape of DNA repair genes in cancer. Oncotarget 2016, 7, 23312–23321. [Google Scholar] [CrossRef] [Green Version]
- De Martino, E.; Brunetti, D.; Canzonieri, V.; Conforti, C.; Eisendle, K.; Mazzoleni, G.; Nobile, C.; Rao, F.; Zschocke, J.; Jukic, E.; et al. The Association of Residential Altitude on the Molecular Profile and Survival of Melanoma: Results of an Interreg Study. Cancers 2020, 12, 2796. [Google Scholar] [CrossRef]
- de Feraudy, S.; Ridd, K.; Richards, L.M.; Kwok, P.-Y.; Revet, I.; Oh, D.; Feeney, L.; Cleaver, J.E. The DNA Damage-Binding Protein XPC Is a Frequent Target for Inactivation in Squamous Cell Carcinomas. Am. J. Pathol. 2010, 177, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, E.; Fargnoli, M.C.; Gandini, S.; Maisonneuve, P.; Cornelius, L.A.; Kayser, M.; Nijsten, T.; Han, J.; Kumar, R.; Gruis, N.A.; et al. MC1R gene variants and non-melanoma skin cancer: A pooled-analysis from the M-SKIP project. Br. J. Cancer 2015, 113, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regl, G.; Neill, G.W.; Eichberger, T.; Kasper, M.; Ikram, M.S.; Koller, J.; Hintner, H.; Quinn, A.G.; Frischauf, A.-M.; Aberger, F. Human GLI2 and GLI1 are part of a positive feedback mechanism in Basal Cell Carcinoma. Oncogene 2002, 21, 5529–5539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S. Liposome encapsulation of doxorubicin and celecoxib in combination inhibits progression of human skin cancer cells. Int. J. Nanomed. 2018, 13, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Conforti, C.; Paolini, F.; Venuti, A.; Dianzani, C.; Zalaudek, I. The detection rate of human papillomavirus in well-differentiated squamous cell carcinoma and keratoacanthoma: Is there new evidence for a viral pathogenesis of keratoacanthoma? Br. J. Dermatol. 2019, 181, 1309–1311. [Google Scholar] [CrossRef]
- Yao, C.D.; Haensel, D.; Gaddam, S.; Patel, T.; Atwood, S.X.; Sarin, K.Y.; Whitson, R.J.; McKellar, S.; Shankar, G.; Aasi, S.; et al. AP-1 and TGFß cooperativity drives non-canonical Hedgehog signaling in resistant basal cell carcinoma. Nat. Commun. 2020, 11, 5079. [Google Scholar] [CrossRef]
- O’Connor, C.; Perl, A.; Leonard, D.; Sangodkar, J.; Narla, G. Therapeutic targeting of PP2A. Int. J. Biochem. Cell Biol. 2018, 96, 182–193. [Google Scholar] [CrossRef]
- Ponti, G.; Manfredini, M.; Greco, S.; Pellacani, G.; Depenni, R.; Tomasi, A.; Maccaferri, M.; Cascinu, S. BRAF, NRAS and C-KIT Advanced Melanoma: Clinico-pathological Features, Targeted-Therapy Strategies and Survival. Anticancer Res. 2017, 37, 7043–7048. [Google Scholar] [CrossRef]
- Loureiro, J.; Abrantes, M.; Oliveira, P.; Saraiva, L. P53 in skin cancer: From a master player to a privileged target for prevention and therapy. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1874, 188438. [Google Scholar] [CrossRef]
- Noubissi, F.K.; Kim, T.; Kawahara, T.N.; Aughenbaugh, W.D.; Berg, E.; Longley, B.; Athar, M.; Spiegelman, V.S. Role of CRD-BP in the Growth of Human Basal Cell Carcinoma Cells. J. Investig. Dermatol. 2014, 134, 1718–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanovic, A.; Mus-Veteau, I. Targeting the Multidrug Transporter Ptch1 Potentiates Chemotherapy Efficiency. Cells 2018, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Dupuy, A.; Valton, J.; LeDuc, S.; Armier, J.; Galetto, R.; Gouble, A.; Lebuhotel, C.; Stary, A.; Pâques, F.; Duchateau, P.; et al. Targeted Gene Therapy of Xeroderma Pigmentosum Cells Using Meganuclease and TALEN™. PLoS ONE 2013, 8, e78678. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Maturo, M.G.; Di Nardo, L.; Ciciarelli, V.; García-Rodrigo, C.G.; Fargnoli, M.C. Understanding the Molecular Genetics of Basal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 2485. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Nelson, M.; Basu, M.; Srinivasan, P.; Lazarski, C.; Zhang, P.; Zheng, P.; Sandler, A.D. MYC oncogene is associated with suppression of tumor immunity and targeting Myc induces tumor cell immunogenicity for therapeutic whole cell vaccination. J. Immunother. Cancer 2021, 9, e001388. [Google Scholar] [CrossRef]
- Welss, T.; Papoutsaki, M.; Reifenberger, J.; Chimenti, S.; Ruzicka, T.; Abts, H.F. Molecular basis of basal cell carcinoma: Analysis of differential gene expression by differential display PCR and expression array. Int. J. Cancer 2002, 104, 66–72. [Google Scholar] [CrossRef]
- Heller, E.R.; Gor, A.; Wang, D.; Hu, Q.; Lucchese, A.; Kanduc, D.; Katdare, M.; Liu, S.; Sinha, A.A. Molecular signatures of basal cell carcinoma susceptibility and pathogenesis: A genomic approach. Int. J. Oncol. 2012, 42, 583–596. [Google Scholar] [CrossRef]
- Jäger, K.; Walter, M. Therapeutic Targeting of Telomerase. Genes 2016, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Kiniwa, Y.; Nakamura, K.; Mikoshiba, A.; Ashida, A.; Akiyama, Y.; Morimoto, A.; Okuyama, R. Usefulness of monitoring circulating tumor cells as a therapeutic biomarker in melanoma with BRAF mutation. BMC Cancer 2021, 21, 287. [Google Scholar] [CrossRef]
- Marsavela, G.; Johansson, P.A.; Pereira, M.R.; McEvoy, A.C.; Reid, A.L.; Robinson, C.; Warburton, L.; Khattak, M.A.; Meniawy, T.M.; Amanuel, B.; et al. The Prognostic Impact of Circulating Tumour DNA in Melanoma Patients Treated with Systemic Therapies—Beyond BRAF Mutant Detection. Cancers 2020, 12, 3793. [Google Scholar] [CrossRef] [PubMed]
- Marczynski, G.T.; Laus, A.C.; Dos Reis, M.B.; Reis, R.M.; Vazquez, V.D.L. Circulating tumor DNA (ctDNA) detection is associated with shorter progression-free survival in advanced melanoma patients. Sci. Rep. 2020, 10, 18682. [Google Scholar] [CrossRef] [PubMed]
- Zocco, D.; Bernardi, S.; Novelli, M.; Astrua, C.; Fava, P.; Zarovni, N.; Carpi, F.M.; Bianciardi, L.; Malavenda, O.; Quaglino, P.; et al. Isolation of extracellular vesicles improves the detection of mutant DNA from plasma of metastatic melanoma patients. Sci. Rep. 2020, 10, 15745. [Google Scholar] [CrossRef]
- Colombino, M.; Rozzo, C.; Paliogiannis, P.; Casula, M.; Manca, A.; Doneddu, V.; Fedeli, M.; Sini, M.; Palomba, G.; Pisano, M.; et al. Comparison of BRAF Mutation Screening Strategies in a Large Real-Life Series of Advanced Melanoma Patients. J. Clin. Med. 2020, 9, 2430. [Google Scholar] [CrossRef] [PubMed]
- Herbreteau, G.; Vallée, A.; Knol, A.-C.; Théoleyre, S.; Quéreux, G.; Frénard, C.; Varey, E.; Hofman, P.; Khammari, A.; Dréno, B.; et al. Circulating Tumour DNA Is an Independent Prognostic Biomarker for Survival in Metastatic BRAF or NRAS-Mutated Melanoma Patients. Cancers 2020, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
| Gene | Type | Incidence (%) | Type of Melanoma | Comments | Therapeutic Modalities |
|---|---|---|---|---|---|
| P53 (Benjamin et al., 2007; Xu et al., 2013) | tumor suppressor gene | 92–100 or 7–27 | Cutaneous | - associated with advanced-stage disease directly - correlated with the exposure to UV-type B | PRIMA-1 |
| TP 53 (Oliver et al., 2010) | tumor suppressor gene | 50 | Cutaneous | - somatic mutations | PRIMA-1MET |
| P16 (Zhang et al., 2004; Borg et al., 2000) | tumor suppressor gene | 10 | Familial malignant melanoma | - loss of p16 protein expression was common event in melanoma | ABT-737 ABT-263 (oral administration) 3MR (novel suicide gene therapy) |
| Protein phosphatase 2A regulatory subunit PR70 (O’Connor et al., 2018) | tumor suppressor gene | 1 | Gonosomal melanoma | - PPP2R3B expression was lower in males than in females - independently correlated with poor clinical outcome. | SMAPs, Phenothyazines |
| RASA2 (Arafeh et al., 2015) | tumor suppressor gene | 5 | Cutaneous melanoma | - encodes a GTPase-activating protein (GAP) | MEK inhibitors |
| RAS (Albino et al., 1984) | proto-oncogene | 15 | Cutaneous melanoma | - activates the mitogen-activated protein kinases (MAPKs) and other signaling pathways involved in cell survival, proliferation and apoptosis | Salirasib |
| BRAF V600K (Kulkarni et al., 2017) | proto-oncogene | 10 | Melanoma in situ Lentigo maligna | - tumors appear over 50, in males, and the tumors often occur in the head and neck area (prone to sun damage) | Sorafenib Farnesyl-transferase inhibitors MEK inhibitors PLX4032 Vemurafenib and Dabrafenib |
| BRAFV600E (Kulkarni et al., 2017) | proto-oncogene | 40 | Cutaneous melanoma | - has been reported to be more frequent in benign than in dysplastic nevi or melanoma | |
| BCORL1 (Mologni et al., 2018) | tumor suppressor | 10 | Cutaneous melanoma | - correlated with resistance of the disease to previous effective drugs - represses E-cadherin expression via interaction with CtBP | immunosuppressive therapy Azacitidine Lenalidomide |
| CTNNB1 (Cerami et al., 2012) | tumor-suppressor gene | 23 | Malignant melanoma | - is a central component of the Wnt (wingless) signal-transduction pathway | TTK inhibitors |
| GNA11 and GNAQ (Van Raamsdonk et al., 2010) | proto-oncogene | 50–85 | Uveal melanoma Non-epithelial melanocytic lesions cutaneous melanoma | - the reduction in melanoblast numbers - encode G-protein alpha subunit q and alpha subunit 11, respectively, and are paralogs | Selumetinib Sotrastaurin (AEB071) |
| C-KIT (Ponti et al., 2017) | proto-oncogene | 11 | Melanomas located in acral regions and mucosae | - resistance to anti-BRAF or anti-MEK targeted therapy | Imatinib Milotinib |
| Author, Year | BRAF Detection Method | Results and Conclusion |
|---|---|---|
| Kiniwa et al., 2021 |
|
|
| Marsavela et al., 2020 |
|
|
| Marczynski et al., 2020 |
|
|
| Zocco et al., 2020 |
|
|
| Colombino et al., 2020 |
|
|
| Herbreteau et al., 2020 |
|
|
| Gene/Gene Product | Type | Incidence (%) | Type of Skin Cancer | Comments | Therapeutic Modality |
|---|---|---|---|---|---|
| P53 (Loureiro et al., 2020) | tumor suppressor gene | 66, 50 | Actinic keratosis SCC | - identified even in premalignant lesios - encodes p53 proteine, a well-known tumor suppressor - causes the cell cycle to stop in the presence of DNA damage | Analogous to melanoma therapy |
| P16 (Zhang et al., 2004) | tumor suppressor gene | 41 non-metastatic and 30 metastatic tumours squamous cell carcinoma | SCC | - frequently inactivated in human cancers, consists of two overlapping genes that encode two unrelated proteins, p16INK4a and p14ARF, functioning as cell cycle inhibitors | Analogous to melanoma therapy |
| PTCH1 (Noubisi et al., 2014; Hasanovic et al., 2018) | tumor suppressor gene | mutations PTCH in 90 sporadic BCC | BCC | - overexpressed in BCC - induces GL 1 promotor-driven luciferase activation in keratinocytes | PTCH1 drug efflux antagonist |
| RAS (de Feraudy et al., 2010) | proto-oncogene | 33 | SCC Keratoacanthoma | - the molecular mechanism is consistent with the paradoxical activation of MAPK signaling and leads to accelerated growth of these lesions | Anti-EGFR agents |
| XPC (Dupuy et al., 2013) | DNA repair gene | 10–90 (more prevalent in Africa) | Xeroderma pigmentosum | - early during skin carcinogenesis | Meganucleases, zinc-finger nucleases or TALE nucleases |
| MC1R (Tagliabue et al., 2015) | DNA repair gene | 24–67 (66–67 for European origin) | BCC SCC | - important role in normal pigmentation | BMS-470539 |
| GLI1 and GLI2 (Pellegrini et al., 2017) | transcription factor | 17 | BCC Melanoma | - frequently overexpressed - increased expression following mutations at any level of the HH signaling pathway (PTCH1, SMO, SUFU) - GLI transcription factors regulate angiogenesis - GLI 1 activity is positively influenced by KRAS, TGF, AKT and negatively by p53,PKA, PKC | TAK-441 |
| TP53 (Pellegriniet al., 2017) | tumor suppressor gene | 50 | BCC, CSC | - TP 53 inactivation is detected in 50% of human cancers, including all skin cancers - inactivation of TP 53 gene is the second most common event associated with BCC pathogenesis | APR-246 COTI-2 |
| SMO (Yao et al., 2020) | proto-oncogene | 10–20 | BCC | - coupling to G protein Gαi in the regulation of Hedgehog | SMO inhibitors: LDE225, LEQ506, BMS833923 |
| MYCN (Wu et al., 2021) | proto-oncogene | 30 | CSC, Melanoma | - member of the MYC family of transcriptional activators, downstream effector of the HH pathway - identified in 30% of BCC - influences cell growth, proliferation, differentiation and apoptosis | DFMO (2-(difluoromethyl)ornithine), an ODC inhibitor (ornithine decarboxylase) |
| CRD-BP (Noubisis et al., 2014) | multifunctional RNA binding protein, anti-apoptotic, | 10–15 | BCC, CSC Melanoma | - correlates with the activation of both WnT and Hh signaling pathways - induces abnormal cell proloferations and suppression of apoptosis - controls the activity of other genes involved in proliferation, invasion and inhibition of apoptosis (TrCP1,c-myc) | Dacarbazine VBN, TMZ |
| MCP-1 CCL2 (Wells et al., 2003) | chemokine with potent monocyte chemotactic activity | 30–40 | Melanoma | - member of C-C family of chemokines - involved in the chemotaxis of monocytes, T lymphocytes and skin dendritic cells - expressed and secreted by keratinocytes - MCP-1 expression may be induced by TNF or INF treatment | MCP-1-blocking antibodies CCR-2B antagonists |
| PPP6C (Pellegriniet al., 2017) | tumor suppressor | 15 | CBC | - mutations were detected in 15% of BCC - regulates cell cycle progression in humans cells by controlling cyclin D1, inactivating RB1 - participates in the activation LATS1 | DMBA/TPA (12-Otetradecanoylphorbol 13-acetate) |
| Jak3 (Wells et al., 2003) | cytoplasmic non-receptor tyrosine kinases. | 18–21 | CSC of the head and neck Melanoma | - differential hybridization showed induction of tyrosine kinase 3 (Jak3) in BCC compared to normal skin - associated with keratinocyte differentiation | JAK inhibitors (Tofacitinib) |
| E2F5 (Heller et al., 2013) | tumor suppressor. transcription factor | 10 | CBC | - recent evidence shows that E2F5 contributes to tumorigenesis - has a stable role by inhibiting MYC | Paclitaxel |
| DAPK1 (Heller et al., 2013) | tumor suppressor | 60 | Head and neck cancers | - a tumor suppressor with increased expression in BCC - inhibits ERK - affects the Ras-MAPK and TGF-β pathways | Decitibane, gliotoxin and paclitaxel |
| TERT (Jager et al., 2016; Pellegrini et al., 2017) | ribonucleoprotein polymerase | 39, 22 | Basal cell carcinomas, cutaneous melanomas squamous cell carcinoma (tongue and skin) | - TERT promotor mutations are found at a high frequency in many cancers (melanoma, non-melanoma skin cancer, bladder cancer, glioma) - associated with UV exposure | oncolytic virotherapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porumb-Andrese, E.; Scutariu, M.M.; Luchian, I.; Schreiner, T.G.; Mârţu, I.; Porumb, V.; Popa, C.G.; Sandu, D.; Ursu, R.G. Molecular Profile of Skin Cancer. Appl. Sci. 2021, 11, 9142. https://doi.org/10.3390/app11199142
Porumb-Andrese E, Scutariu MM, Luchian I, Schreiner TG, Mârţu I, Porumb V, Popa CG, Sandu D, Ursu RG. Molecular Profile of Skin Cancer. Applied Sciences. 2021; 11(19):9142. https://doi.org/10.3390/app11199142
Chicago/Turabian StylePorumb-Andrese, Elena, Mihaela Monica Scutariu, Ionut Luchian, Thomas Gabriel Schreiner, Ioana Mârţu, Vlad Porumb, Cosmin Gabriel Popa, Darius Sandu, and Ramona Gabriela Ursu. 2021. "Molecular Profile of Skin Cancer" Applied Sciences 11, no. 19: 9142. https://doi.org/10.3390/app11199142
APA StylePorumb-Andrese, E., Scutariu, M. M., Luchian, I., Schreiner, T. G., Mârţu, I., Porumb, V., Popa, C. G., Sandu, D., & Ursu, R. G. (2021). Molecular Profile of Skin Cancer. Applied Sciences, 11(19), 9142. https://doi.org/10.3390/app11199142








