Increased Expression of TGF-β1 by 4-hexylresorcinol Is Mediated by Endoplasmic Reticulum and Mitochondrial Stress in Human Umbilical Endothelial Vein Cells
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. HUVEC Culture
2.2. Western Blot and Sp1 Transcription Factor Assay
2.3. ATP, Mitochondrial Membrane Potential (MMP), and Oxygen Consumption Assay
2.4. Confocal Microscopic Exam
2.5. Immunoprecipitation High-Performance Liquid Chromatography (IP-HPLC)
2.6. Statistical Analysis
3. Results
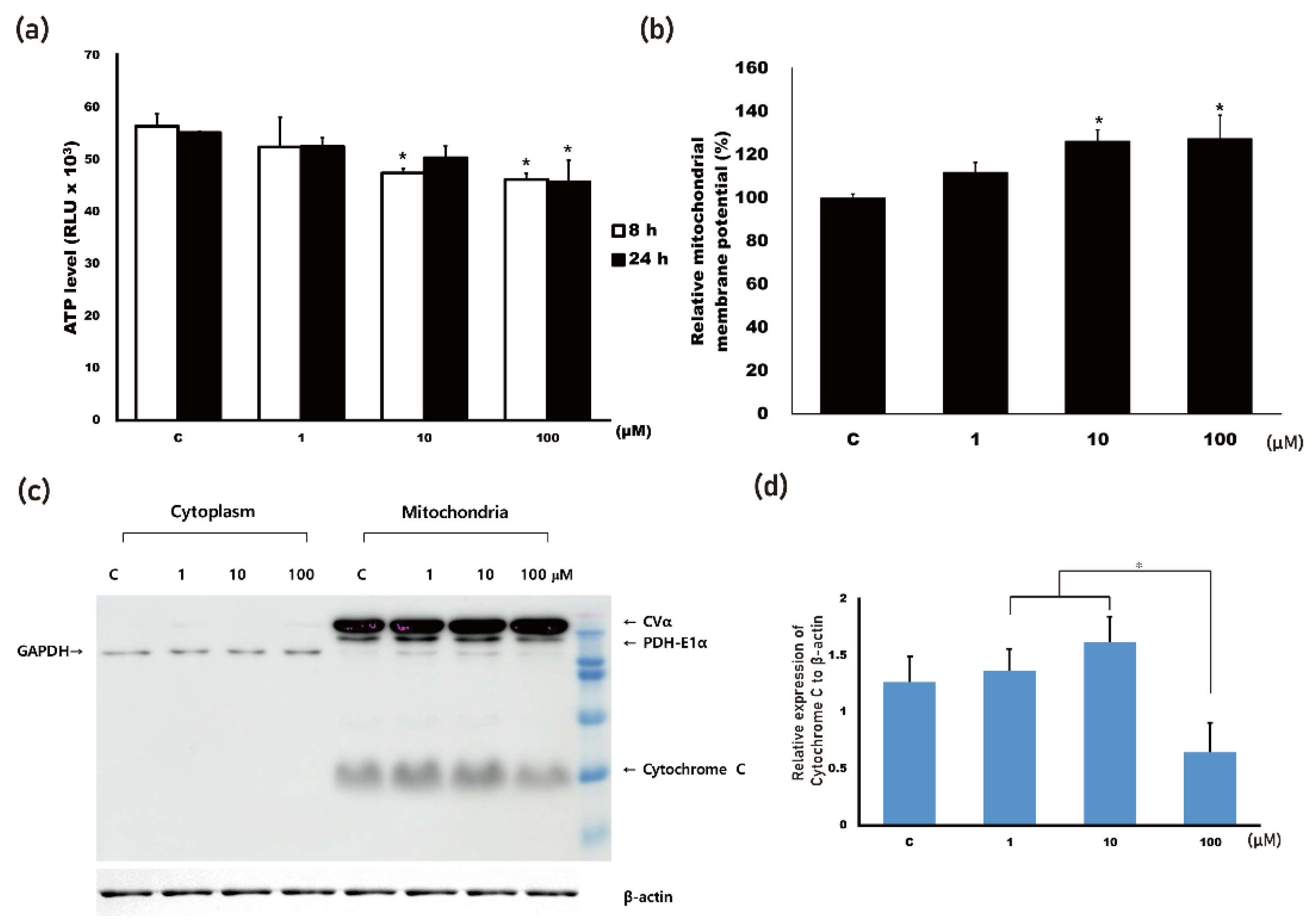
3.1. 4HR Increased Mitochondrial Stress
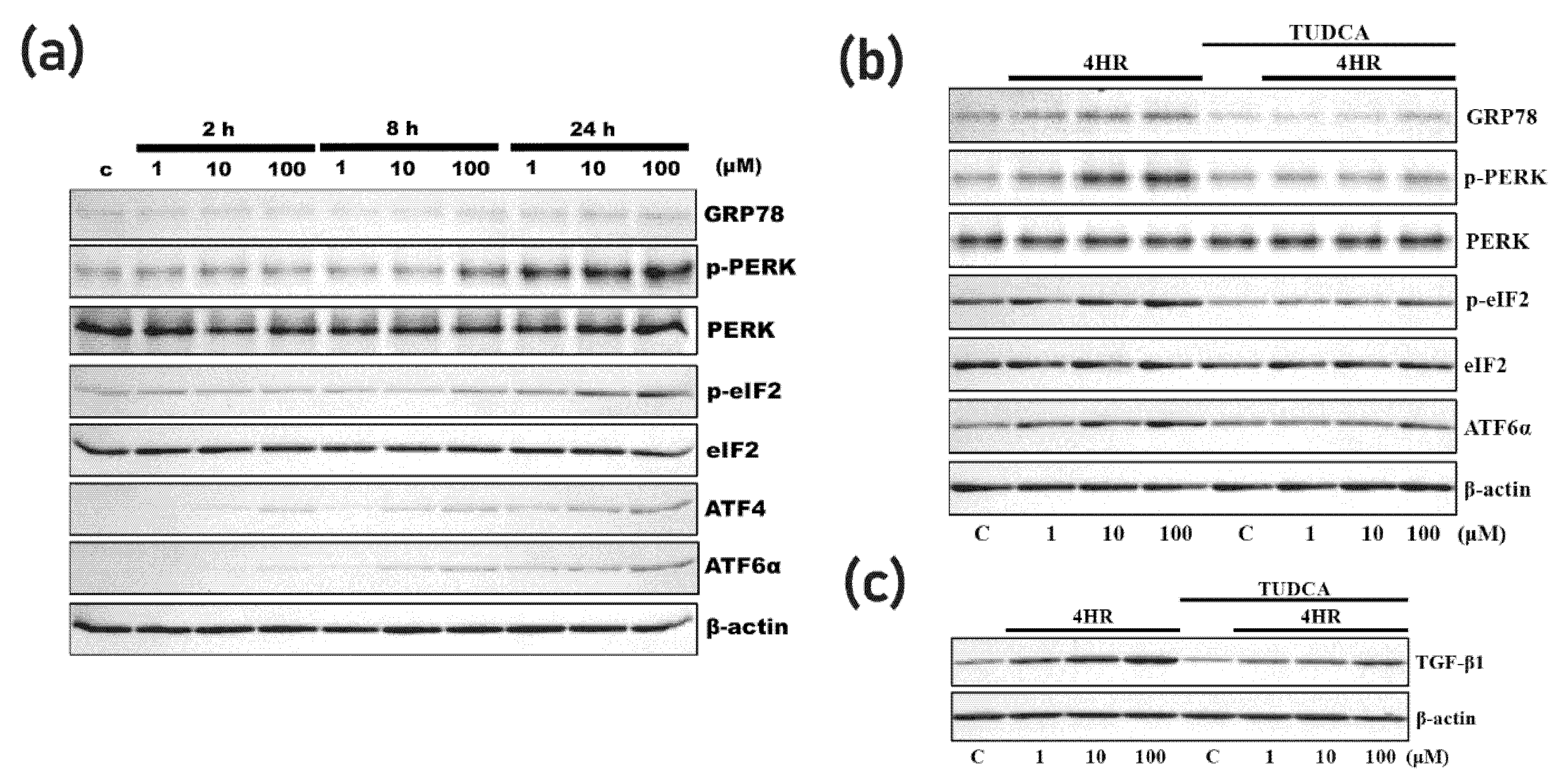
3.2. 4HR Increased ER Stress
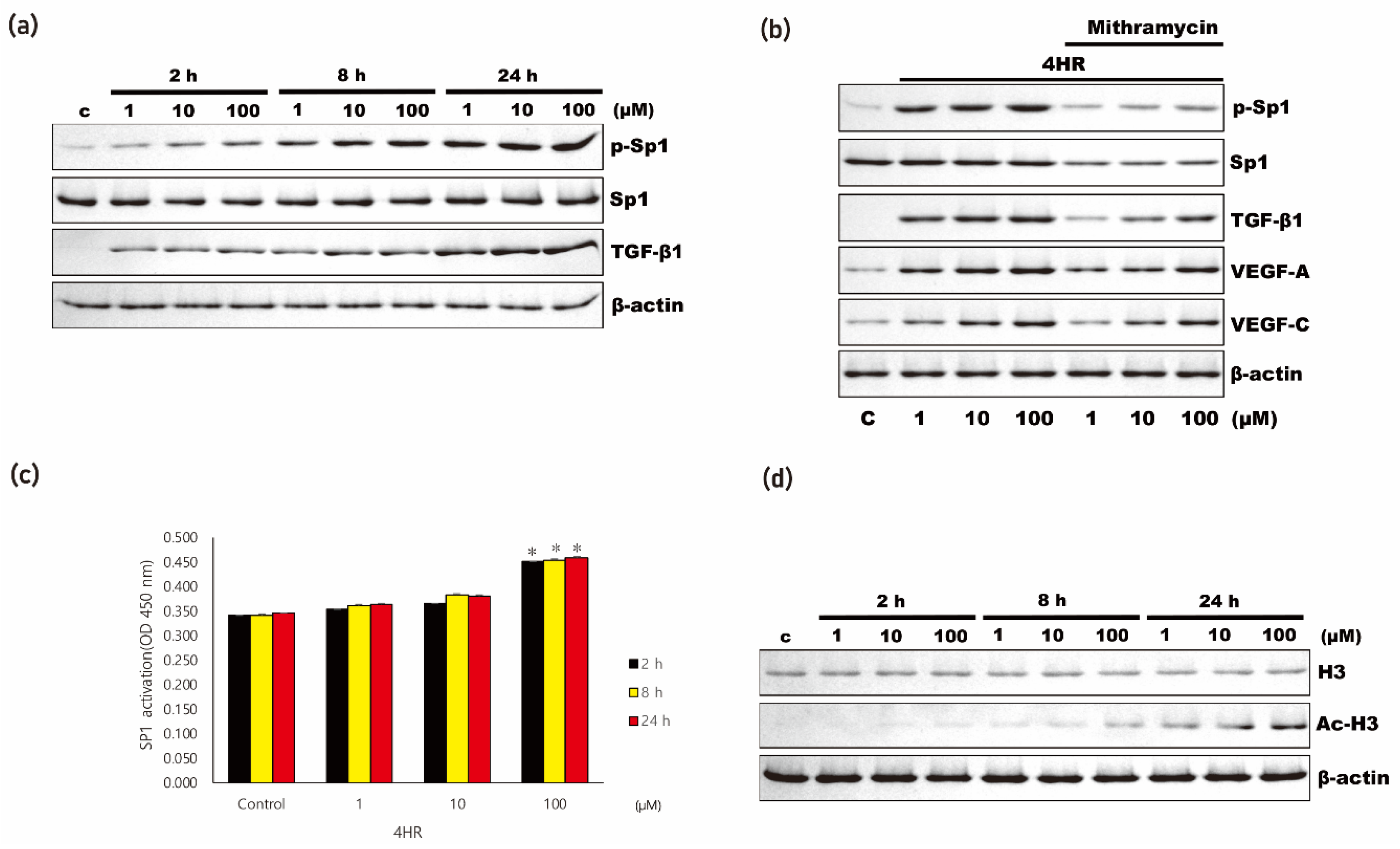
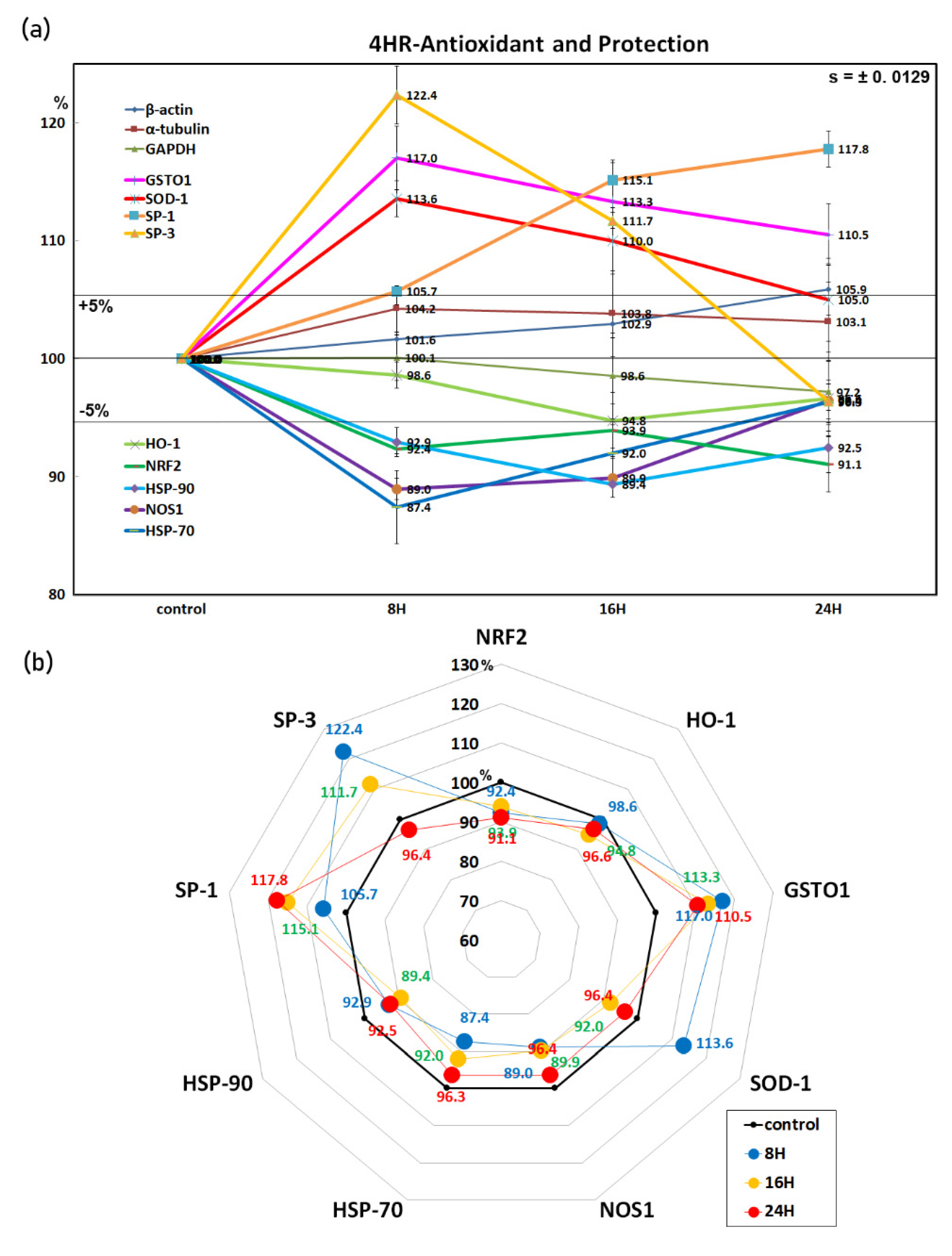
3.3. 4HR Increased Antioxidant Proteins and Sp1/Sp3 Ratio
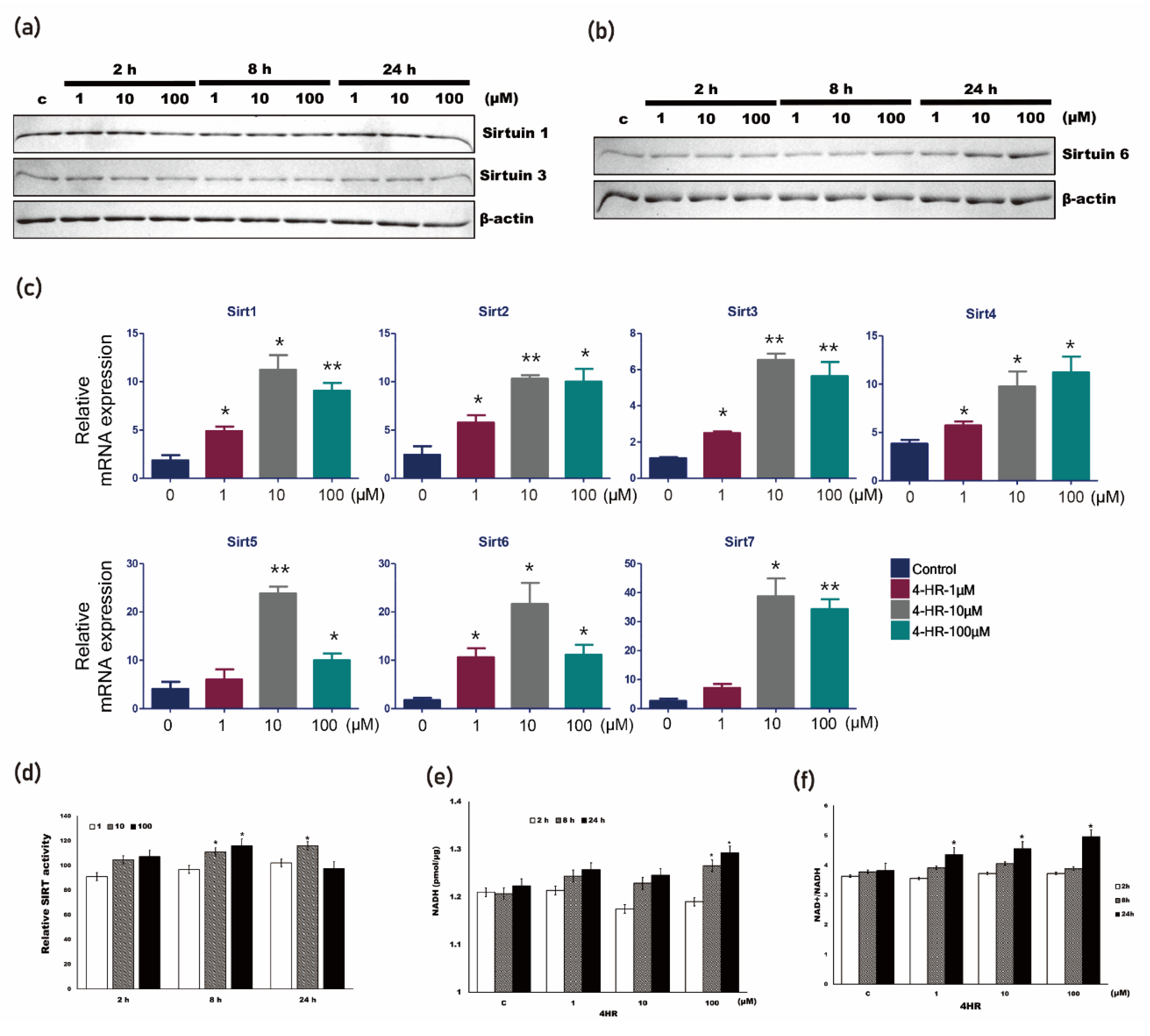
3.4. 4HR Increased SIRT Activity
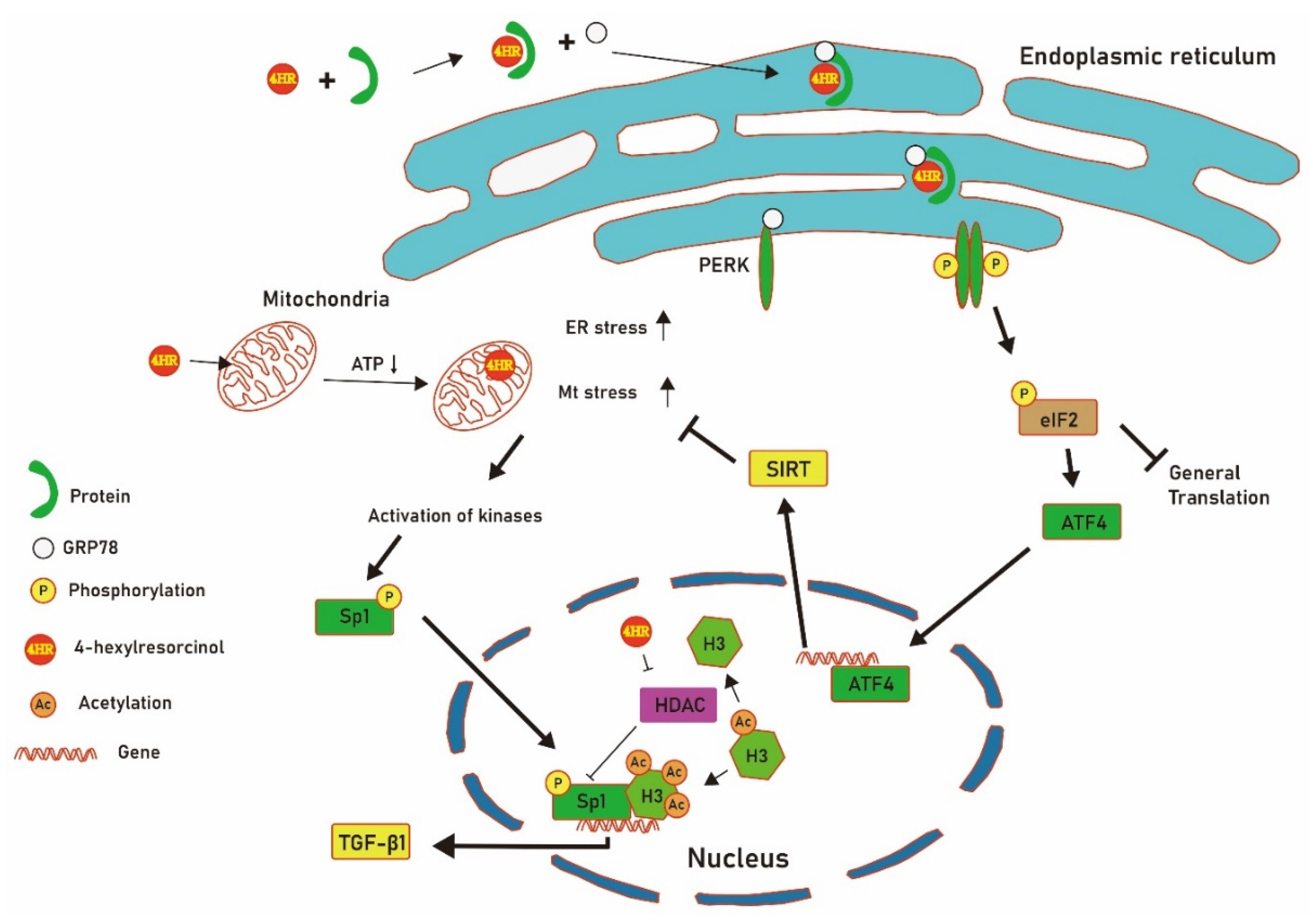
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kozubek, A.; Tyman, J.H.P. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–26. [Google Scholar] [CrossRef]
- Muliukin, A.L.; Demkina, E.V.; Kozlova, A.N.; Soina, V.S.; El’-Registan, G.I. Synthesis of anabiosis autoinducers in non-spore-forming bacteria as a mechanism regulating their activity in soil and subsoil sedimentary rocks. Mikrobiologiia 2001, 70, 620–628. [Google Scholar] [PubMed]
- Nikolaev, Y.A.; Tutel’yan, A.V.; Loiko, N.G.; Buck, J.; Sidorenko, S.V.; Lazareva, I.; Gostev, V.; Manzen’yuk, O.Y.; Shemyakin, I.G.; Abramovich, R.A.; et al. The use of 4-Hexylresorcinol as antibiotic adjuvant. PLoS ONE 2020, 15, e0239147. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, E.; Hassanzadeh, A.; Marofi, F.; Alivand, M.R.; Solali, S. Flavonoid-based cancer therapy: An updated review. Anticancer Agents Med. Chem. 2020, 20, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Tamaddoni, A.; Mohammadi, E.; Sedaghat, F.; Qujeq, D.; As’Habi, A. The anticancer effects of curcumin via targeting the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway. Pharmacol. Res. 2020, 156, 104798. [Google Scholar] [CrossRef]
- Havalová, H.; Ondrovičová, G.; Keresztesová, B.; Bauer, J.A.; Pevala, V.; Kutejová, E.; Kunová, N. Mitochondrial HSP70 chaperone system-The influence of post-translational modifications and involvement in human diseases. Int. J. Mol. Sci. 2021, 22, 8077. [Google Scholar] [CrossRef]
- Healy, S.J.M.; Verfaillie, T.; Jäger, R.; Agostinis, P.; Samali, A. Biology of the Endoplasmic Reticulum. In Endoplasmic Reticulum Stress in Health and Disease; Agostinis, P., Samali, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–22. ISBN 978-94-007-4350-2. [Google Scholar]
- Jo, Y.Y.; Kim, D.W.; Choi, J.Y.; Kim, S.G. 4-Hexylresorcinol and silk sericin increase the expression of vascular endothelial growth factor via different pathways. Sci. Rep. 2019, 9, 3448. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.; Jo, Y.Y.; Garagiola, U.; Choi, J.Y.; Kang, Y.J.; Oh, J.H.; Kim, S.G. Increased level of vascular endothelial growth factors by 4-hexylresorcinol is mediated by transforming growth factor-β1 and accelerates capillary regeneration in the burns in diabetic animals. Int. J. Mol. Sci. 2020, 21, 3473. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Choudhury, S.; Chaudhary, G.; Singh, V.; Prabhu, S.N.; Pandey, S.; Garg, S.K. Chitosan and gelatin biopolymer supplemented with mesenchymal stem cells (Velgraft®) enhanced wound healing in goats (Capra hircus): Involvement of VEGF, TGF and CD31. J. Tissue Viability 2021, 30, 59–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zou, T.; Qi, Y.; Yi, B.; Dissanayaka, W.L.; Zhang, C. DPSCs treated by TGF-β1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling. Stem Cell Res. Ther. 2021, 12, 281. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Chen, S.; Qiu, J.; Li, Q.; Wang, S.; Zhou, W.; Chen, D.; Yang, G.; Guo, L. Transcription factor EB-mediated autophagy promotes dermal fibroblast differentiation and collagen production by regulating endoplasmic reticulum stress and autophagy-dependent secretion. Int. J. Mol. Med. 2021, 47, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Haller, D. Structure-function analysis of the tertiary bile acid TUDCA for the resolution of endoplasmic reticulum stress in intestinal epithelial cells. Biochem. Biophys. Res. Commun. 2011, 409, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Vassilopoulos, A.; Fritz, K.S.; Petersen, D.R.; Gius, D. The human sirtuin family: Evolutionary divergences and functions. Hum. Genom. 2011, 5, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aka, J.A.; Kim, G.W.; Yang, X.J. K-Acetylation and Its Enzymes: Overview and New Developments. In Histone Deactylases: The Biology and Clinical Implication; Yao, T.P., Seto, E., Eds.; Springer: New York, NY, USA, 2011; pp. 1–12. ISBN 978-3-642-21630-5. [Google Scholar]
- Kim, M.K.; Yoon, C.S.; Kim, S.G.; Park, Y.W.; Lee, S.K. Effects of 4-hexylresorcinol on protein expressions in RAW 264.7 cells as determined by immunoprecipitation high performance liquid chromatography. Sci. Rep. 2019, 9, 3379. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, S.G.; Lee, S.K. 4-Hexylresorcinol-induced angiogenesis potential in human endothelial cells. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 23. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Lin, C.W. Effects of resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced oxidative DNA damage in human lymphocytes. Free Radic. Res. 2003, 37, 509–514. [Google Scholar] [CrossRef]
- Dauer, P.; Gupta, V.K.; McGinn, O.; Nomura, A.; Sharma, N.S.; Arora, N.; Giri, B.; Dudeja, V.; Saluja, A.K.; Banerjee, S. Inhibition of Sp1 prevents ER homeostasis and causes cell death by lysosomal membrane permeabilization in pancreatic cancer. Sci. Rep. 2017, 7, 1564. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Béguet-Crespel, F.; Marinelli, L.; Jamet, A.; Ledue, F.; Blottière, H.M.; Lapaque, N. Butyrate produced by gut commensal bacteria activates TGF-beta1 expression through the transcription factor SP1 in human intestinal epithelial cells. Sci. Rep. 2018, 8, 9742. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, A.S.; Jeong, J.H.; Choi, J.Y.; Kweon, H. 4-hexylresorcinol stimulates the differentiation of SCC-9 cells through the suppression of E2F2, E2F3 and Sp3 expression and the promotion of Sp1 expression. Oncol. Rep. 2012, 28, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kweon, H.Y.; Kim, D.W.; Choi, J.Y.; Kim, S.G. 4-Hexylresorcinol inhibits class I histone deacetylases in human umbilical cord endothelial cells. Appl. Sci. 2021, 11, 3486. [Google Scholar] [CrossRef]
- Deb, D.K.; Chen, Y.; Sun, J.; Wang, Y.; Li, Y.C. ATP-citrate lyase is essential for high glucose-induced histone hyperacetylation and fibrogenic gene upregulation in mesangial cells. Am. J. Physiol. Renal Physiol. 2017, 313, F423–F429. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Kim, D.W.; Kim, S.G.; Lee, S.K. 4-hexylresorcinol-induced protein expression changes in human umbilical cord vein endothelial cells as determined by immunoprecipitation high-performance liquid chromatography. PLoS ONE 2020, 15, e0243975. [Google Scholar] [CrossRef]
- Yoon, Y.M.; Lee, J.H.; Yun, S.P.; Han, Y.S.; Yun, C.W.; Lee, H.J.; Noh, H.; Lee, S.J.; Han, H.J.; Lee, S.H. Tauroursodeoxycholic acid reduces ER stress by regulating of Akt-dependent cellular prion protein. Sci. Rep. 2016, 6, 39838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Yang, Z.; Shen, R.; Zhong, W.; Zheng, H.; Chen, Z.; Tang, J.; Zhu, J. Resveratrol Improves Mitochondrial Biogenesis Function and Activates PGC-1α Pathway in a Preclinical Model of Early Brain Injury Following Subarachnoid Hemorrhage. Front. Mol. Biosci. 2021, 8, 620683. [Google Scholar] [CrossRef] [PubMed]
- Chu, S. Transcriptional regulation by post-transcriptional modification—Role of phosphorylation in Sp1 transcriptional activity. Gene 2012, 508, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Sun, S.; Cai, J.; Cao, J. Sp1 promotes ovarian cancer cell migration through repressing miR-335 expression. Biochem. Biophys. Res. Commun. 2020, 524, 211–216. [Google Scholar] [CrossRef]
- Amuthan, G.; Biswas, G.; Ananadatheerthavarada, H.K.; Vijayasarathy, C.; Shephard, H.M.; Avadhani, N.G. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene 2002, 21, 7839–7849. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.H.; Kim, S.G.; Kim, M.K.; Kim, D.W.; Lee, J.H.; Seok, H.; Choi, J.Y. Topical delivery of 4-hexylresorcinol promotes wound healing via tumor necrosis factor-α suppression. Burns 2016, 42, 1534–1541. [Google Scholar] [CrossRef]
- Kweon, H.Y.; Kim, S.G.; Choi, J.Y. Inhibition of foreign body giant cell formation by 4-hexylresorcinol through suppression of diacylglycerol kinase delta gene expression. Biomaterials 2014, 35, 8576–8584. [Google Scholar] [CrossRef]
- Kim, S.G. Immunomodulation for maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 5. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, T.; Matsui, T.; Sato, M.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell 2007, 13, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Dave, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Douglas, P.M.; Summers, D.W.; Cyr, D.M. Molecular chaperones antagonize proteotoxicity by differentially modulating protein aggregation pathways. Prion 2009, 3, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.D.; Harding, H.P.; Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004, 167, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Flory, M.R.; Lee, H.; Bonneau, R.; Mallick, P.; Serikawa, K.; Morris, D.R.; Aebersold, R. Quantitative proteomic analysis of the budding yeast cell cycle using acid-cleavable isotope-coded affinity tag reagents. Proteomics 2006, 6, 6146–6157. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. Discrepancy between mRNA and protein abundance: Insight from information retrieval process in computers. Comp. Biol. Chem. 2008, 32, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Karbasforooshan, H.; Roohbakhsh, A.; Karimi, G. SIRT1 and microRNAs: The role in breast, lung and prostate cancers. Exp. Cell Res. 2018, 367, 1–6. [Google Scholar] [CrossRef]
- Xiao, C.; Kim, H.S.; Lahusen, T.; Wang, R.H.; Xu, X.; Gavrilova, O.; Jou, W.; Gius, D.; Deng, C.X. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J. Biol. Chem. 2010, 285, 36776–36784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [Green Version]
- Easlon, E.; Tsang, F.; Dilova, I.; Wang, C.; Lu, S.P.; Skinner, C.; Lin, S.J. The dihydrolipoamide acetyltransferase is a novel metabolic longevity factor and is required for calorie restriction-mediated life span extension. J. Biol. Chem. 2007, 282, 6161–6171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.A.; Madsen, A.S.; Olsen, C.A.; Hirschey, M.D. Metabolic control by sirtuins and other enzymes that sense NAD+, NADH, or their ratio. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 991–998. [Google Scholar] [CrossRef]
- Williamson, D.H.; Lund, P.; Krebs, H.A. The redox state of free nicotinamide-adenine dinucleotide in cytoplasm and mitochondria of rat liver. Biochem. J. 1967, 103, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, T.; Baur, J.A.; Perez, E.; Matsui, T.; Carmona, J.J.; Lamming, D.W.; Souza-Pinto, N.C.; Bohr, V.A.; Rosenzweig, A.; et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 2007, 130, 1095–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Gao, Y.; Zhang, Q.; Wei, S.; Chen, Z.; Dai, X.; Zeng, Z.; Zhao, K.S. SIRT1/3 activation by resveratrol attenuates acute kidney injury in a septic rat model. Oxid. Med. Cell. Longev. 2016, 2016, 7296092. [Google Scholar] [CrossRef]
- Basseri, S.; Austin, R.C. Endoplasmic Reticulum Stress and the Unfolded Protein Response in Lipid Metabolism and Obesity. In Endoplasmic Reticulum Stress in Health and Disease; Agostinis, P., Samali, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 231–256. ISBN 978-94-007-4350-2. [Google Scholar]
- Liao, M.; Zhang, Y.; Dufau, M.L. Protein kinase Calpha-induced derepression of the human luteinizing hormone receptor gene transcription through ERK mediated release of HDAC1/Sin3A repressor complex from Sp1 sites. Mol. Endocrinol. 2008, 22, 1449–1463. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.T.; Baker, P.J.; Coburn, R.A.; Fischman, S.L.; Genco, R.J. In vitro antiplaque effects of antiseptic phenols. J. Periodontol. 1977, 48, 156–162. [Google Scholar] [CrossRef]
- Kushneruk, M.A.; Tugarova, A.V.; Il’chukova, A.V.; Slavkina, E.A.; Starichkova, N.I.; Bogatyrev, V.A.; Antoniuk, L.P. Factors inducing transition from growth to dormancy in rhizobacteria Azospirillum brasilense. Mikrobiologiia 2013, 82, 563–570. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Kim, D.-W.; Lee, S.K.; Choi, J.-Y.; Che, X.; Kim, S.-G.; Garagiola, U. Increased Expression of TGF-β1 by 4-hexylresorcinol Is Mediated by Endoplasmic Reticulum and Mitochondrial Stress in Human Umbilical Endothelial Vein Cells. Appl. Sci. 2021, 11, 9128. https://doi.org/10.3390/app11199128
Kim J-Y, Kim D-W, Lee SK, Choi J-Y, Che X, Kim S-G, Garagiola U. Increased Expression of TGF-β1 by 4-hexylresorcinol Is Mediated by Endoplasmic Reticulum and Mitochondrial Stress in Human Umbilical Endothelial Vein Cells. Applied Sciences. 2021; 11(19):9128. https://doi.org/10.3390/app11199128
Chicago/Turabian StyleKim, Jwa-Young, Dae-Won Kim, Suk Keun Lee, Je-Yong Choi, Xiangguo Che, Seong-Gon Kim, and Umberto Garagiola. 2021. "Increased Expression of TGF-β1 by 4-hexylresorcinol Is Mediated by Endoplasmic Reticulum and Mitochondrial Stress in Human Umbilical Endothelial Vein Cells" Applied Sciences 11, no. 19: 9128. https://doi.org/10.3390/app11199128
APA StyleKim, J.-Y., Kim, D.-W., Lee, S. K., Choi, J.-Y., Che, X., Kim, S.-G., & Garagiola, U. (2021). Increased Expression of TGF-β1 by 4-hexylresorcinol Is Mediated by Endoplasmic Reticulum and Mitochondrial Stress in Human Umbilical Endothelial Vein Cells. Applied Sciences, 11(19), 9128. https://doi.org/10.3390/app11199128








