Abstract
The shape control of metal nanoparticles, along with the size, is critical for most of their applications as they control their optical properties. Anisotropic metal nanoparticles show superior performance in a number of applications compared to spherical ones. Shape control is usually achieved by a two-step process, where the first involves the formation of spherical nanoparticles and the second is about the actual shape transformation. In this paper, we report on a fast and facile synthesis of silver nanoplates in a single step, involving laser ablation of a silver target in a liquid medium while this is exposed to light irradiation and hydrogen peroxide flow. We obtained anisotropic particles with a mixture of shapes, of 70–80 nm in size and 10–20 nm in thickness, which showed a plasmon sensitivity greater than 200 nm/RIU.
1. Introduction
Metal nanoparticles (NPs) have generated a great deal of interest in a range of fields including sensing, photonics, biology, and catalysis, owing to the unique interaction of their conduction electrons with electric fields [1,2,3,4]. Such an interaction is commonly known as Surface plasmon resonance (SPR) and is strongly related to the size and shape of the NPs, as well as the surrounding medium, which makes them particularly suitable for sensing applications [5]. While spherical NPs are easily obtained by chemical or physical methods, significant effort has been devoted to the synthesis of anisotropic metal NPs, with gold nanorods and silver nanoplates (NPTs) being among the most popular [6,7], due to their ability to better confine light below the diffraction limit, which enhances their sensitivity. Silver NPTs in particular show the highest sensitivity to the refractive index of the surrounding medium among metal NPs [7,8,9,10,11].
Ag NPT synthesis often occurs in two steps, since the first step is required to produce spherical NPs, while the second step transforms them into flat triangular nanoplates. This is necessary in order to separate the nucleation of new particles from their growth and shape transformation. The most widely used process so far is the so-called seed-mediated growth, where spherical particles are first produced by chemical reduction of Ag ions by NaBH4, then transformed chemically by hydrazine and citrate [7,12,13]. Other methods were also proposed in place of the first or second step of the seed-mediated growth or both. For example, chemically produced NPs can be transformed into NPTs through the use of light irradiation or H2O2 or a combination of both [14,15,16,17,18,19,20,21,22,23]. More recently, Ag NPTs were produced from laser-ablated Ag NPs using light irradiation and hydrogen peroxide (H2O2), fully avoiding the chemistry involved in the seed-mediated growth [10,11,23,24,25]. A few examples of a single-step synthesis of anisotropic Ag NPs have been reported [26,27,28,29,30]. However, most of the processes reported are very time consuming (from 12 h to 90 h). In this paper, we report on the synthesis of Ag NPTs in a single step, combining the laser ablation process with light irradiation and H2O2, a process that is completed within less than one hour. To our knowledge, this is the first time Ag NPTs have been obtained by a single laser ablation step. We also investigated the plasmon sensitivity of such nanoparticles under a varying refractive index.
2. Materials and Methods
Ag NPT synthesis took place according to the scheme shown in Figure 1. A quartz optically transparent reaction vessel was filled with a 1 mM solution of trisodium citrate (TSC). This gave a pH in the region of 8.5–9. A silver target was immersed in the vessel. The beam of a Nd:YAG pulsed laser (Quanta System Spa, Varese, Italy) at 1064 nm (fluence 0.6–1 J/cm2, 5 ns pulse duration, 10 Hz repetition rate) was focused by a lens onto the silver target and produces Ag NPs by laser ablation. During the laser ablation process, a white light LED (Ekoo IP66, 100 W; see the emission spectrum in Figure S1) illuminated the reaction vessel, and H2O2 flowed into the solution as regulated by a peristaltic pump at a flow of 69 μL/min. The overall setup is schematized in Figure 1. Small aliquots of the solution were taken at regular intervals to monitor the evolution of the reaction by UV-Vis spectroscopy (Agilent Cary 60 spectrometer, Santa Clara, CA, USA).
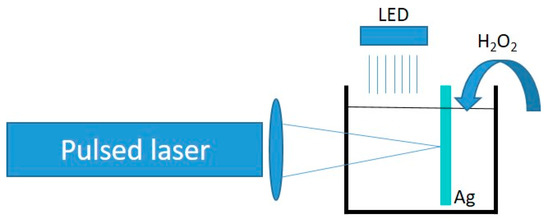
Figure 1.
Schematic of the reaction setup, including a pulsed laser, a focusing lens, a reaction vessel containing a silver target, an illuminating LED, and a flow of H2O2.
The morphology of the obtained Ag NPTs was characterized by scanning electron microscopy (SEM) using a Zeiss SUPRA 55-VP (Carl Zeiss Microscoy, Oberkochen, Germany) system and atomic force microscopy (AFM) using a Witec Alpha 300 RS system (WITec, Ulm, Germany). For the SEM and AFM analysis, the Ag NPTs were deposited onto a silane-functionalized Si substrate immediately after synthesis. SEM image analysis was performed using the software ImageJ (Author: Wayne Rasband, National Institute of Mental Health, Bethesda, MD, USA). Simulations were performed using the commercial COMSOL Multiphysics package (from COMSOL Inc., Stockholm, Sweden) in the frequency domain. The simulated spectra were obtained using radiation perpendicular to the flat/larger side of the NPT, with polarization parallel to the same side.
For plasmon sensitivity measurements, a solution obtained by the described process (15 mL) was initially centrifuged for 20 min at 10 K rpm. After the supernatant was removed, the deposit was redissolved in 1 mL of water and homogenized by very short ultrasonication (1 min). Then, 100 μL of the solution was added to 3 mL of water for a refractive index of 1.333 and to 3 mL of sucrose solution (22%, 40%, and 50% for a 1.367, 1.399, and 1.420 refractive index, respectively [31]), and the absorption spectrum was then measured.
3. Results and Discussion
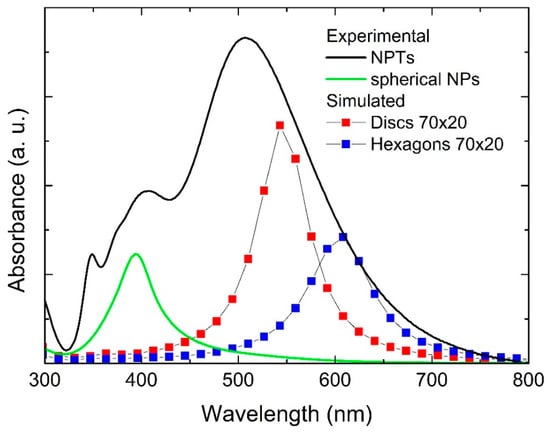
Figure 2 shows the UV-Vis absorption spectra of a solution that was exposed to our process for 30 min at 69 μL/min H2O2 flow, as well as a solution that was produced by laser ablation only. The latter showed a single sharp feature at 395 nm arising from the surface plasmon resonance of spherical Ag NPs. On the other hand, the spectrum from the solution exposed to irradiation and H2O2 flow during the laser ablation process appeared significantly different, as the main feature was red-shifted from that of spherical NPs, and additional features arose, including a low intensity one at 335 nm. Indeed, this is strong evidence that the Ag NPs in solution in this case were far from spherical and suggests that the nanoplates were formed during the process. The spectrum did not show significant variations by changing the H2O2 flow rate between 23 μL/min and 69 μL/min, while increasing the flow rate above 100 μL/min caused complete oxidation of the formed material, and the corresponding absorption spectrum would appear flat. Similarly, decreasing the TSC concentration to 0.1 mM did not change the absorption spectrum significantly, while increasing it up to 10 mM caused complete oxidation of the formed material (flat absorption spectrum). The main feature around 500 nm can be attributed to the in-plane dipole mode, while the peak at 335 nm can be attributed to the out-of-plane quadrupole mode, which is typical of Ag anisotropic structures [14,32]. The other feature around 400 nm may be attributed to larger spherical NPs and/or to the in-plane quadrupole and out-of-plane dipole modes for NPTs or both. We also performed control experiments using either light irradiation or H2O2 flow only. When only irradiation was used, without introducing any H2O2, the process yielded only spherical NPs, with an absorption spectrum close to that shown in Figure 2. When only H2O2 was introduced in the solution, without light irradiation, the transformation happened only to some extent (see Figure S2). We also performed a further control experiment, using no citrate in the solution. In this case, we observed an absorption spectrum with a single broad and asymmetrical feature (Figure S3), suggesting that only a broad distribution of spherical particles was formed. Such results indicate that citrate, light irradiation, and H2O2 are all essential ingredients that cooperate in a successful process, as already shown for a similar two-step process [11].
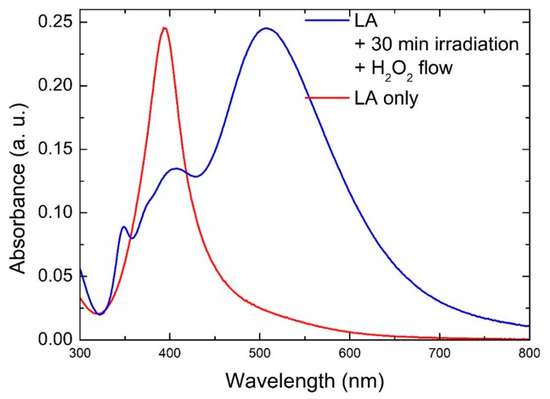
Figure 2.
UV-Vis absorption spectra of Ag NPTs obtained by laser ablation under irradiation and H2O2 flow (blue line) and Ag NPs obtained by laser ablation only (red line).
Figure 3a,b shows representative SEM images of Ag NPTs produced by our process. We can observe Ag NPTs showing a mixture of shapes, including triangles, hexagons, circles, and other irregular ones. The size distribution shows that most NPTs were 70–90 nm in size (Figure 3c).
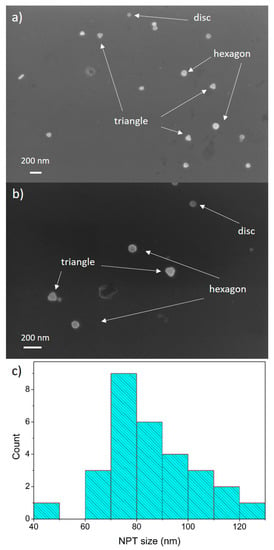
Figure 3.
(a,b) SEM images of Ag NPTs; (c) size distribution of Ag NPTs obtained from SEM image analysis.
Figure 4 shows an AFM image and the profiles obtained from the same samples analyzed by SEM. Figure 4a also shows NPTs with mixed shapes, and Figure 4b shows the thickness ranging between 10 nm and 25 nm.
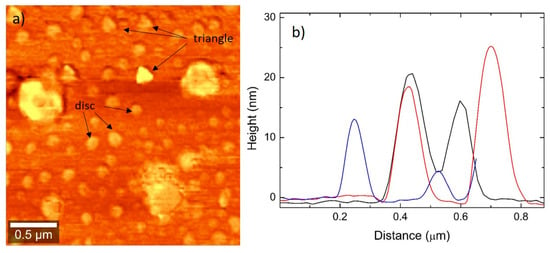
Figure 4.
(a) AFM image showing Ag NPTs of mixed shapes (mainly triangles and discs); (b) AFM profiles showing most profiles in the range of 10–20 nm in thickness.
The growth mechanism in this process can be elucidated within the framework of the reactions taking place among citrate, Ag, and H2O2 in the presence of light. It is known that, in the presence of light, citrate is able to reduce Ag+ to Ag0 [33]:
Citrate3− + λ → Acetone-1,3-dicarboxylate2− + CO2 + H+ + 2e−
Ag+ + e− → Ag0
At the same time, H2O2 can both oxidize and reduce silver [17,18]:
2Ag + H2O2 → 2Ag+ + 2OH−
H2O2 + 2Ag+ + 2OH− → 2Ag + 2H2O + O2
Besides reducing Ag in the presence of light, citrate also has the function of being a capping agent for Ag NPs, as it easily binds to the (111) facets of silver [34,35]. This allows both NP stabilization in solution and their preferential growth along the Ag (100) direction, which is less preferred by citrate. Hence, Ag NPs initially produced by the laser ablation process were first oxidized by H2O2, being partially dissolved in the liquid medium, and then reduced back by citrate, supported by H2O2, adding preferentially to the uncapped (100) Ag facet. It is interesting to note that the absorption spectrum shown in Figure 2 did not dramatically change its shape after about 10 min of this process, while the intensity increased and then stabilized over time (Figure S4). This suggests that an equilibrium was reached within the solution between the production of NPs by laser ablation and their conversion into NPT and that after reaching this equilibrium, the process only increased the NPT concentration in the solution.
Simulations of the extinction spectra were performed to support and complement the experimental results. Figure 5 shows simulated extinction spectra for NPTs of a round and hexagonal shape, with a size of 70 nm and 82.5 nm and a thickness of 10 and 20 nm, representative of the structures observed by SEM and AFM. Figure 6 shows the experimental absorption spectrum, already shown in Figure 2, with the simulated ones. It can be observed that the simulated spectra were mainly in agreement with the longer wavelength part of the experimental spectrum. This partial disagreement might be due to the fact that the simulated spectra were obtained for isolated NPTs, thus not taking into account the interaction among the particles. However, this aspect is still unclear and will be subject to further investigation.
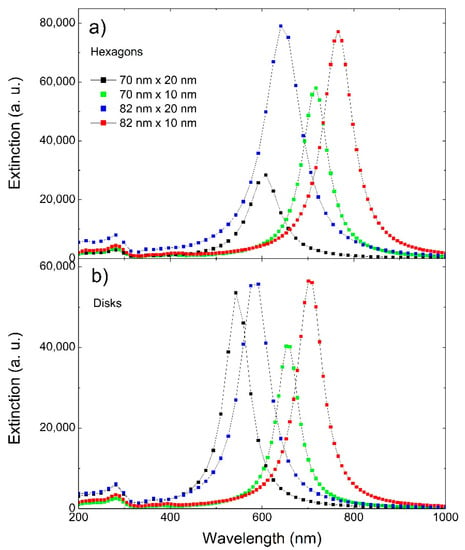
Figure 5.
Simulated extinction spectra for (a) hexagons and (b) disks of 70 nm and 82 nm in size and 10 nm and 20 nm in thickness.
The obtained NPTs were finally tested for plasmon sensitivity (S), i.e., the variation of the plasmon resonance peak position due to a change in the refractive index (S = Δλ/Δn), which is typically reported in terms of nm/RIU (refractive index unit). To change the refractive index, we used sucrose solutions at set concentrations, since the relation between sucrose concentration in water and the refractive index is well established [31]. Figure 7 shows the plasmon resonance peak position of a Ag NPT solution produced by our process by varying the refractive index, along with the data simulated for disks of 70 nm in size and 20 nm in thickness. Fitting the data yielded a plasmon sensitivity S of 216 nm/RIU, close to that obtained by the simulations. This value is comparable to the others, typically below 250 RIU, reported in the literature for Ag NPTs with a similar plasmon resonance [7,8,9,10,11], with the advantage of obtaining the nanostructures in a fast and simple one-step process, despite the poor SPR tunability. If we compare the experimental data with those obtained from the simulation of discs and hexagons (70 nm × 20 nm), we observe that the simulated data showed a higher plasmon sensitivity. This was probably due to a higher SPR wavelength in the simulated spectra, which was expected to exhibit higher plasmon sensitivity, in agreement with our previous work [36].
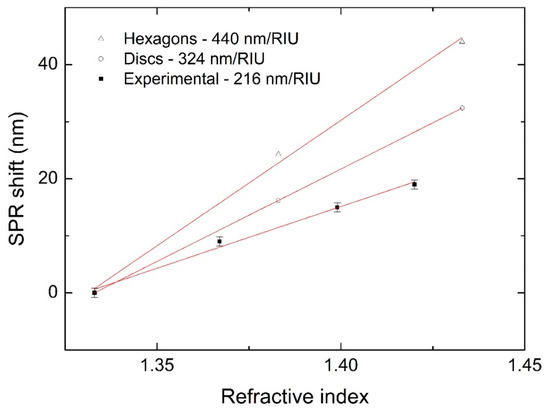
Figure 7.
Experimental (full squares) and simulated (empty shapes) SPR shift at different refractive indices.
4. Conclusions
We demonstrated the fast and facile synthesis of Ag NPTs in a single step by combining laser ablation in liquids with light irradiation in the presence of H2O2. We obtained flat NPTs of mixed shapes with sizes between 60 and 80 nm and thicknesses of 10–20 nm. The SPR resonance of these nanostructures fell around 500 nm. While at this stage, the process seems to be efficient in a narrow range of parameters, with poor SPR tuning capability, we foresee that exploring a wider range of conditions, such as laser ablation parameters or the chemical composition of the solution, might provide some SPR tuning capability to some extent. Changing the refractive index of the surrounding medium yielded a plasmon sensitivity of 216 nm/RIU.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11198949/s1, Figure S1: Emission spectrum of the LED used for irradiation during the laser ablation process. Figure S2: Absorption spectrum from a solution obtained when irradiation was taken out of the described process, Figure S3: Absorption spectrum from a solution when citrate was taken out of the described process, Figure S4: Absorption spectra taken at different times during the described process.
Author Contributions
Conceptualization, V.S. and G.C.; methodology, V.S. and M.P.; validation, M.E.F. and G.C.; formal analysis, V.S. and L.S.; investigation, V.S., M.C., M.B. and L.S.; data curation, V.S.; writing—original draft preparation, V.S.; writing—review and editing, G.C.; supervision, G.C.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Ministero della Università e della Ricerca-MIUR-(Project ARS01_00519-BEST4U).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this paper are available upon request from the corresponding author.
Acknowledgments
The authors gratefully acknowledge the PON project Bionanotech Research and Innovation Tower (BRIT) financed by the Italian Ministry for Education, University and Research (MIUR). G.F. Indelli (BRIT) is acknowledged for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Capek, I. Noble Metal Nanoparticles: Preparation, Composite Nanostructures, Biodecoration and Collective Properties; Springer: Tokyo, Japan, 2017. [Google Scholar]
- Fedlheim, D.L.; Foss, C.A. Metal Nanoparticles: Synthesis, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2001; p. 352. [Google Scholar]
- Tao, F. Metal Nanoparticles for Catalysis: Advances and Applications; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Condorelli, M.; Scardaci, V.; Pulvirenti, M.; D’Urso, L.; Neri, F.; Compagnini, G.; Fazio, E. Surface Plasmon Resonance Dependent Third-Order Optical Nonlinearities of Silver Nanoplates. Photonics 2021, 8, 299. [Google Scholar] [CrossRef]
- Trugler, A. Optical Properties of Metallic Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Niu, W.; Zhang, L.; Xu, G. Seed-mediated growth of noble metal nanocrystals: Crystal growth and shape control. Nanoscale 2013, 5, 3172–3181. [Google Scholar] [CrossRef]
- Compagnini, G.; Condorelli, M.; Fragala, M.; Scardaci, V.; Tinnirello, I.; Puglisi, O.; Neri, F.; Fazio, E. Growth Kinetics and Sensing Features of Colloidal Silver Nanoplates. J. Nanomater. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Potara, M.; Gabudean, A.; Astilean, S. Solution-phase, dual LSPR-SERS plasmonic sensors of high sensitivity and stability based on chitosan-coated anisotropic silver nanoparticles. J. Mater. Chem. 2011, 21, 3625–3633. [Google Scholar] [CrossRef]
- Guo, L.; Jackman, J.; Yang, H.; Chen, P.; Cho, N.; Kim, D. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef] [Green Version]
- Condorelli, M.; Scardaci, V.; D’Urso, L.; Puglisi, O.; Fazio, E.; Compagnini, G. Plasmon sensing and enhancement of laser prepared silver colloidal nanoplates. Appl. Surf. Sci. 2019, 475, 633–638. [Google Scholar] [CrossRef]
- Scardaci, V.; Pulvirenti, M.; Condorelli, M.; Compagnini, G. Monochromatic light driven synthesis and growth of flat silver nanoparticles and their plasmon sensitivity. J. Mater. Chem. C 2020, 8, 9734–9741. [Google Scholar] [CrossRef]
- Aherne, D.; Ledwith, D.M.; Gara, M.; Kelly, J.M. Optical Properties and Growth Aspects of Silver Nanoprisms Produced by a Highly Reproducible and Rapid Synthesis at Room Temperature. Adv. Funct. Mater. 2008, 18, 2005–2016. [Google Scholar] [CrossRef]
- Hu, G.; Jin, W.; Zhang, W.; Wu, K.; He, J.; Zhang, Y.; Chen, Q.; Zhang, W. Surfactant-assisted shape separation from silver nanoparticles prepared by a seed-mediated method. Colloids Surf. A-Physicochem. Eng. Asp. 2018, 540, 136–142. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.; Mirkin, C.A.; Kelly, K.L.; Schatz, G.C.; Zheng, J.G. Photoinduced Conversion of Silver Nanospheres to Nanoprisms. Science 2001, 294, 1901. [Google Scholar] [CrossRef] [Green Version]
- Mulvihill, M.; Ling, X.; Henzie, J.; Yang, P. Anisotropic Etching of Silver Nanoparticles for Plasmonic Structures Capable of Single-Particle SERS. J. Am. Chem. Soc. 2010, 132, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.N.; Nguyen, T.D.; Cao, M.T.; Pham, V.V. Fast and simple synthesis of triangular silver nanoparticles under the assistance of light. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 594, 124659. [Google Scholar] [CrossRef]
- Parnklang, T.; Lertvachirapaiboon, C.; Pienpinijtham, P.; Wongravee, K.; Thammacharoen, C.; Ekgasit, S. H2O2-triggered shape transformation of silver nanospheres to nanoprisms with controllable longitudinal LSPR wavelengths. Rsc Adv. 2013, 3, 12886–12894. [Google Scholar] [CrossRef]
- Parnklang, T.; Lamlua, B.; Gatemala, H.; Thammacharoen, C.; Kuimalee, S.; Lohwongwatana, B.; Ekgasit, S. Shape transformation of silver nanospheres to silver nanoplates induced by redox reaction of hydrogen peroxide. Mater. Chem. Phys. 2015, 153, 127–134. [Google Scholar] [CrossRef]
- Redmond, P.; Wu, X.; Brus, L. Photovoltage and photocatalyzed growth in citrate-stabilized colloidal silver nanocrystals. J. Phys. Chem. C 2007, 111, 8942–8947. [Google Scholar] [CrossRef] [Green Version]
- Rocha, T.; Winnischofer, H.; Westphal, E.; Zanchet, D. Formation kinetics of silver triangular nanoplates. J. Phys. Chem. C 2007, 111, 2885–2891. [Google Scholar] [CrossRef]
- Sherry, L.; Jin, R.; Mirkin, C.; Schatz, G.; Van Duyne, R. Localized surface plasmon resonance spectroscopy of single silver triangular nanoprisms. Nano Lett. 2006, 6, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Redmond, P.; Liu, H.; Chen, Y.; Steigerwald, M.; Brus, L. Photovoltage mechanism for room light conversion of citrate stabilized silver nanocrystal seeds to large nanoprisms. J. Am. Chem. Soc. 2008, 130, 9500–9506. [Google Scholar] [CrossRef]
- Scardaci, V. Anisotropic Silver Nanomaterials by Photochemical Reactions: Synthesis and Applications. Nanomaterials 2021, 11, 2226. [Google Scholar] [CrossRef]
- Tsuji, T.; Tsuji, M.; Hashimoto, S. Utilization of laser ablation in aqueous solution for observation of photoinduced shape conversion of silver nanoparticles in citrate solutions. J. Photochem. Photobiol. A-Chem. 2011, 221, 224–231. [Google Scholar] [CrossRef]
- Verma, S.; Rao, B.; Srivastava, A.; Srivastava, D.; Kaul, R.; Singh, B. A facile synthesis of broad plasmon wavelength tunable silver nanoparticles in citrate aqueous solutions by laser ablation and light irradiation. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 527, 23–33. [Google Scholar] [CrossRef]
- Tian, X.; Chen, K.; Cao, G. Seedless, surfactantless photoreduction synthesis of silver nanoplates. Mater. Lett. 2006, 60, 828–830. [Google Scholar] [CrossRef]
- Yang, L.-C.; Lai, Y.-S.; Tsai, C.-M.; Kong, Y.-T.; Lee, C.-I.; Huang, C.-L. One-Pot Synthesis of Monodispersed Silver Nanodecahedra with Optimal SERS Activities Using Seedless Photo-Assisted Citrate Reduction Method. J. Phys. Chem. C 2012, 116, 24292–24300. [Google Scholar] [CrossRef]
- Tang, B.; Sun, L.; Li, J.; Zhang, M.; Wang, X. Sunlight-driven synthesis of anisotropic silver nanoparticles. Chem. Eng. J. 2015, 260, 99–106. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, C.; Hao, R.; Zhang, D.; Fu, Y.; Moeendarbari, S.; Pickering, C.S.; Hao, Y.; Liu, Y. Morphological transformations of silver nanoparticles in seedless photochemical synthesis. Mater. Res. Express 2016, 3, 055014. [Google Scholar] [CrossRef]
- Ardianrama, A.D.; Wijaya, Y.N.; Hur, S.H.; Woo, H.-C.; Kim, M.H. Reshaping of triangular silver nanoplates by a non-halide etchant and its application in melamine sensing. J. Colloid Interface Sci. 2019, 552, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Yunus, W.M.b.M.; Rahman, A.b.A. Refractive index of solutions at high concentrations. Appl. Opt. 1988, 27, 3341–3343. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: Weinheim, Germany, 1998. [Google Scholar]
- Elechiguerra, J.; Reyes-Gasga, J.; Yacaman, M. The role of twinning in shape evolution of anisotropic noble metal nanostructures. J. Mater. Chem. 2006, 16, 3906–3919. [Google Scholar] [CrossRef]
- Lee, G.; Bignell, L.; Romeo, T.; Razal, J.; Shepherd, R.; Chen, J.; Minett, A.; Innis, P.; Wallace, G. The citrate-mediated shape evolution of transforming photomorphic silver nanoparticles. Chem. Commun. 2010, 46, 7807–7809. [Google Scholar] [CrossRef]
- Kilin, D.S.; Prezhdo, O.V.; Xia, Y. Shape-controlled synthesis of silver nanoparticles: Ab initio study of preferential surface coordination with citric acid. Chem. Phys. Lett. 2008, 458, 113–116. [Google Scholar] [CrossRef]
- Condorelli, M.; Litti, L.; Pulvirenti, M.; Scardaci, V.; Meneghetti, M.; Compagnini, G. Silver nanoplates paved PMMA cuvettes as a cheap and re-usable plasmonic sensing device. Appl. Surf. Sci. 2021, 150701. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).