Fresh State, Rheological and Microstructural Characteristics of Alkali-Activated Mortars Developed Using Novel Dry Mixing Technique under Ambient Conditions
Abstract
:Highlights
- Development of supplementary cementitious materials-based alkali-activated mortars
- Use of powder-based reagents/activators, novel dry mixing techniques and ambient curing
- Evaluation of fresh state, rheological and microstructural characteristics
- Influence of binary/ternary combinations of supplementary cementitious materials and combinations/dosages of reagents
- Relationships between slump flow, yield stress, plastic viscosity and shear rate of mortars
1. Introduction
2. Experimental Program
2.1. Materials
2.2. Mix Proportions
2.3. Mixing, Casting and Curing of Specimens
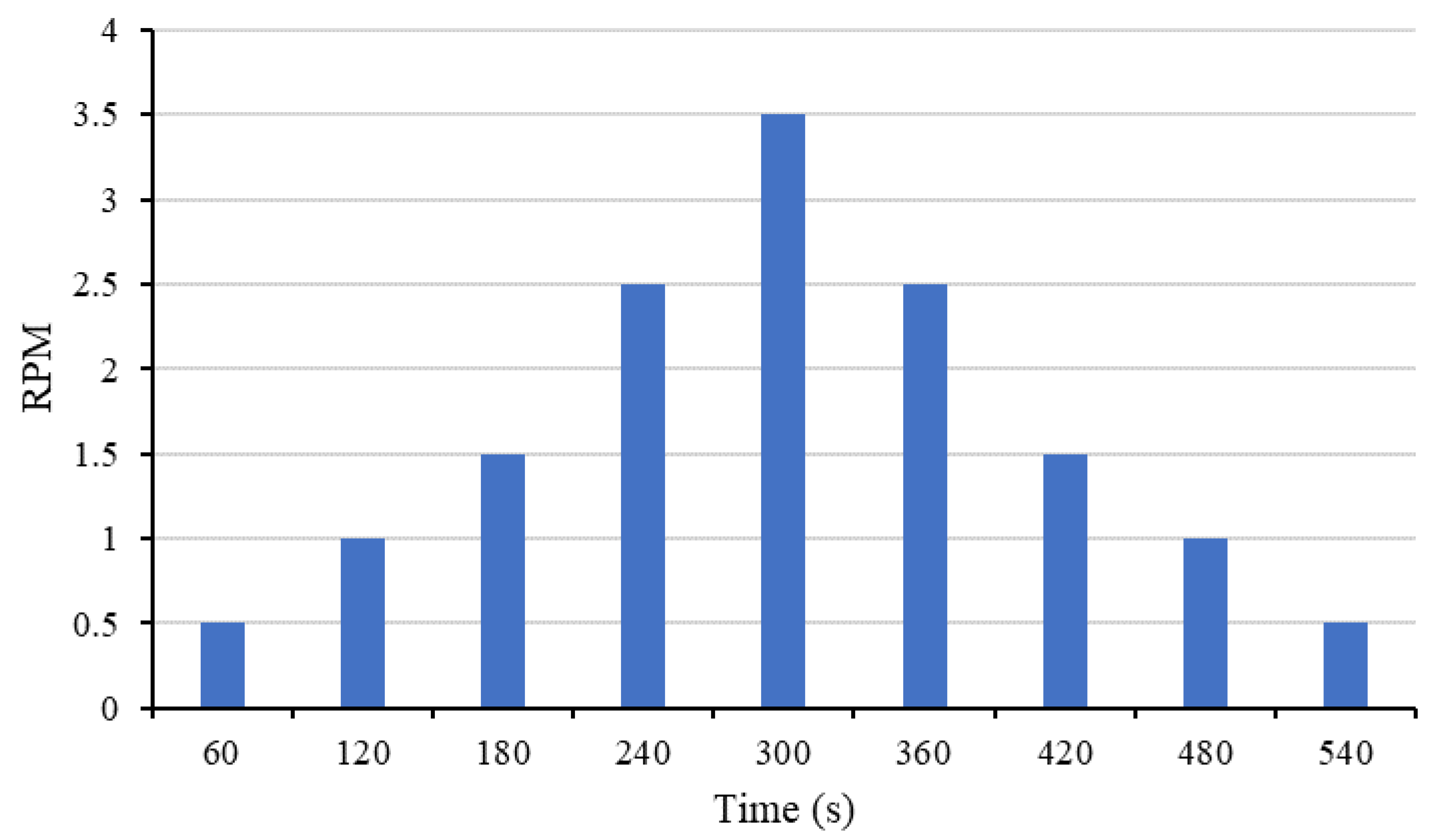
2.4. Test Methods
3. Results and Discussions
3.1. Fresh State and Rheological Characteristics
3.1.1. Slump Flow, Viscosity and Setting Times
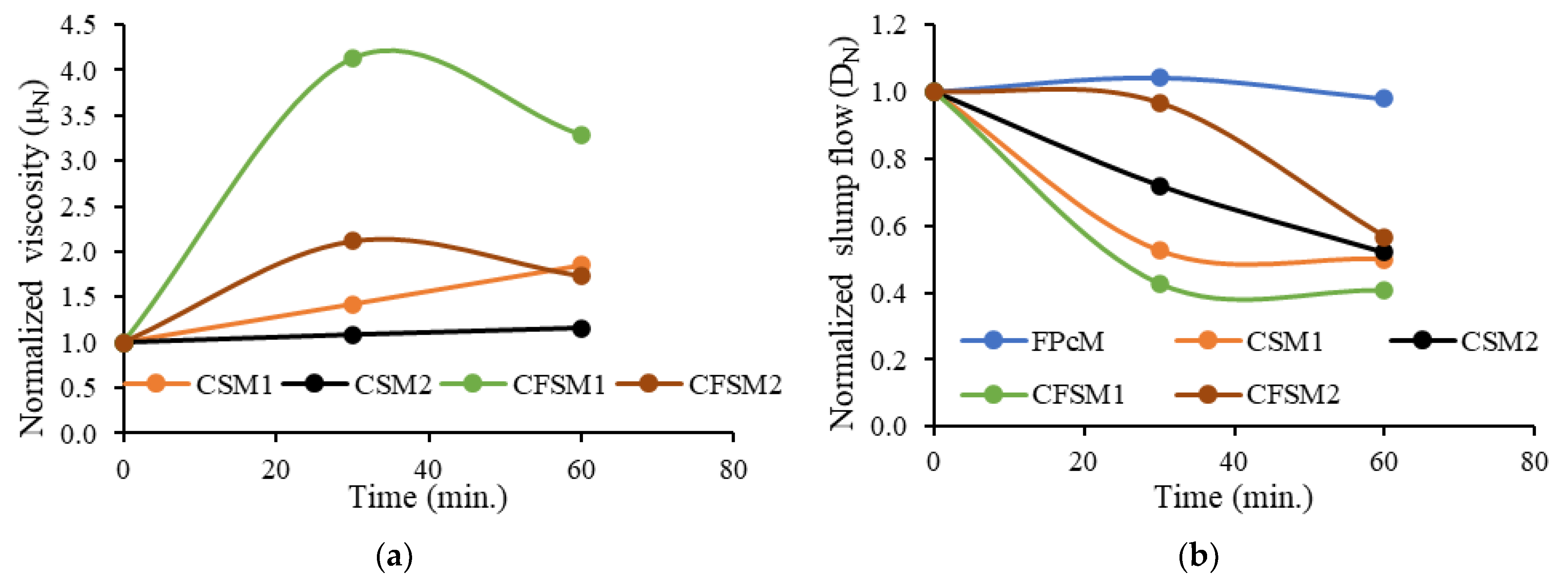
3.1.2. Hysteretic Viscosity Behavior of Mortars
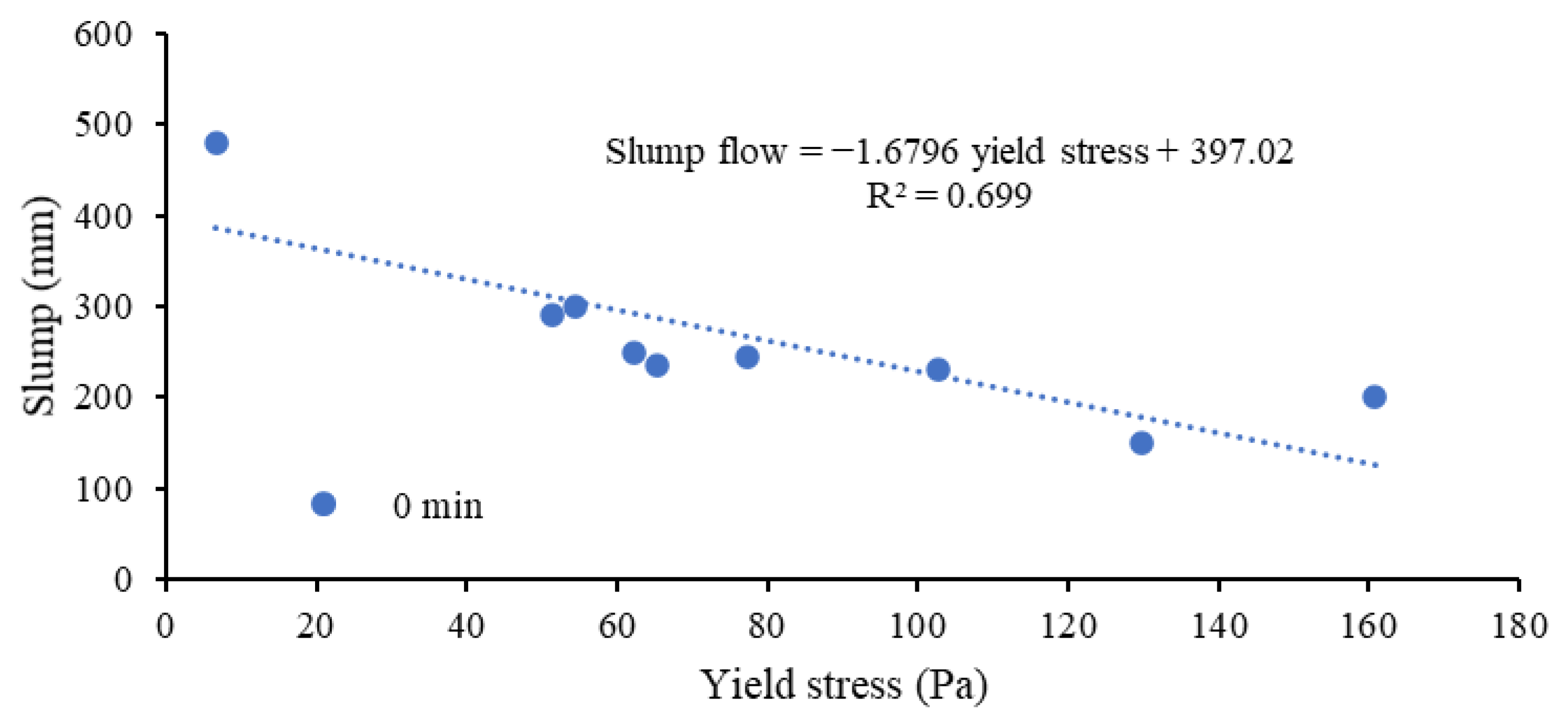
3.1.3. Yield Stress of Mortars
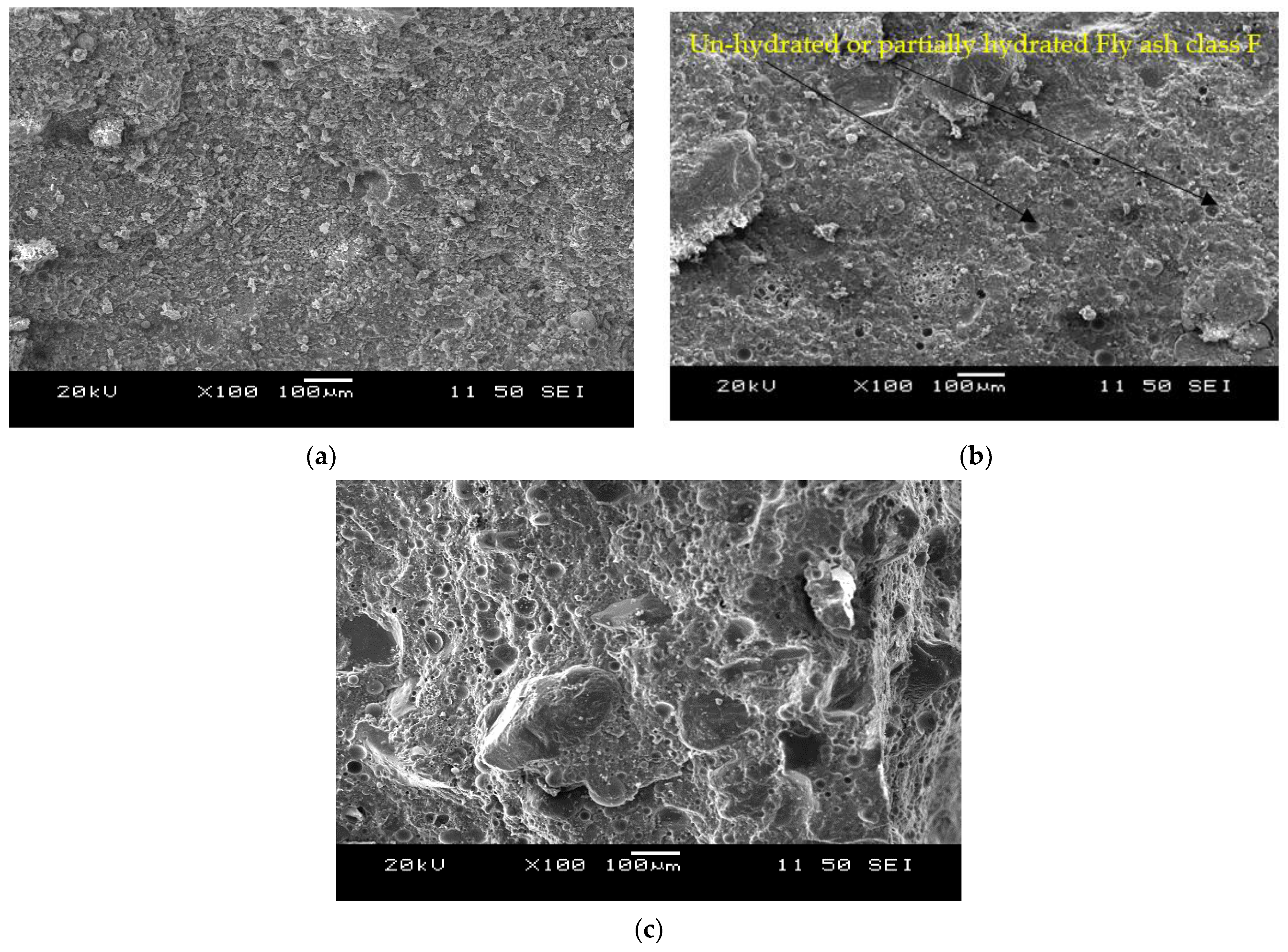
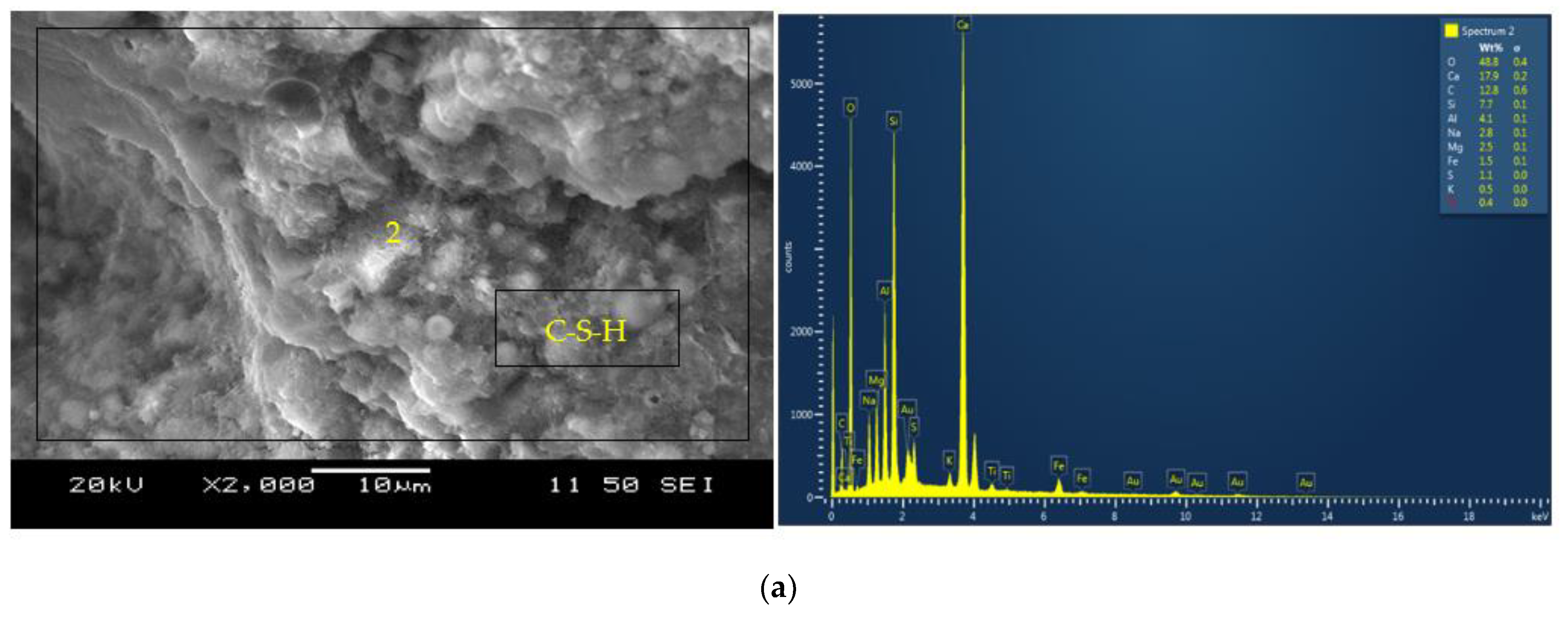
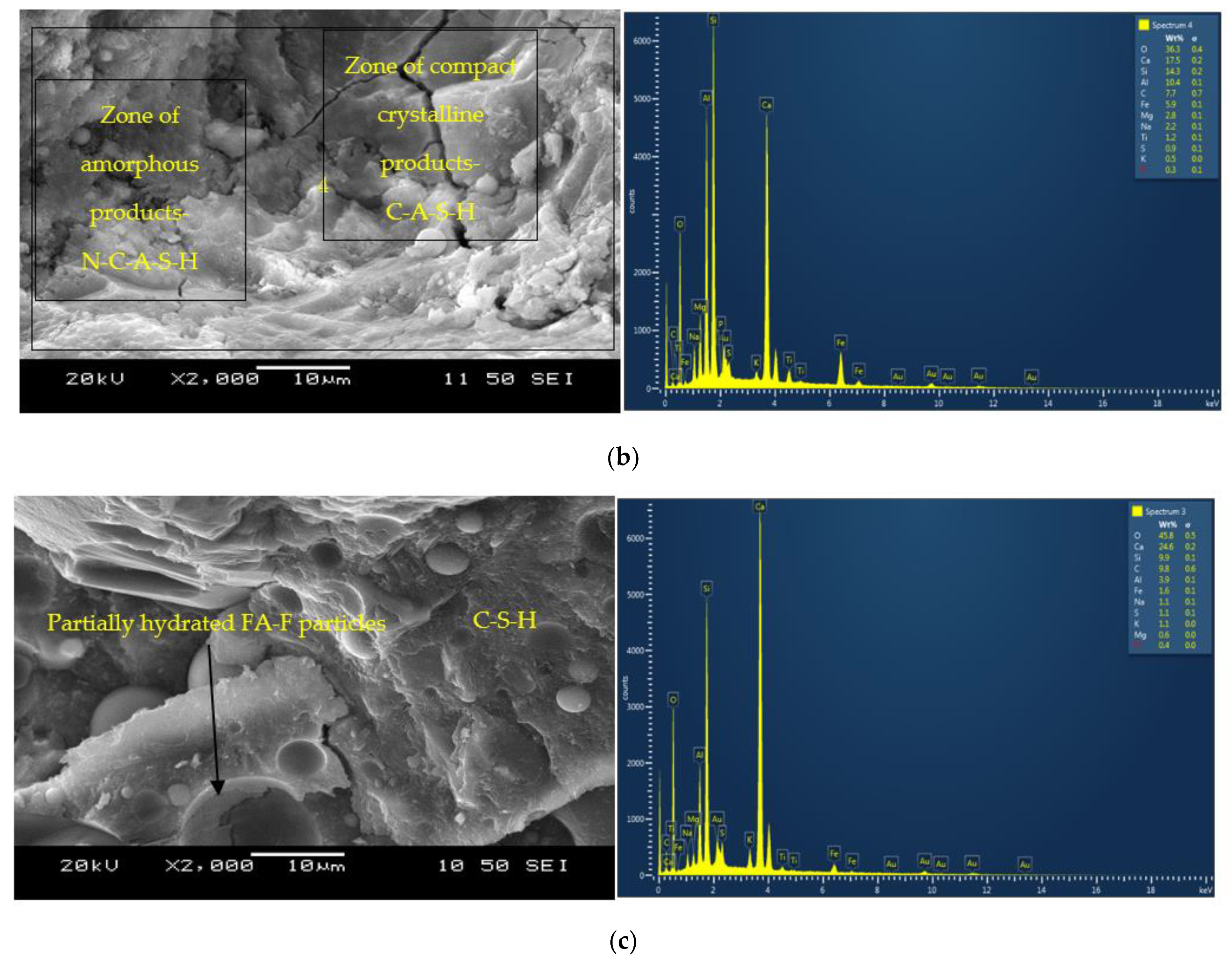
3.2. Microstructural Analysis
4. Conclusions
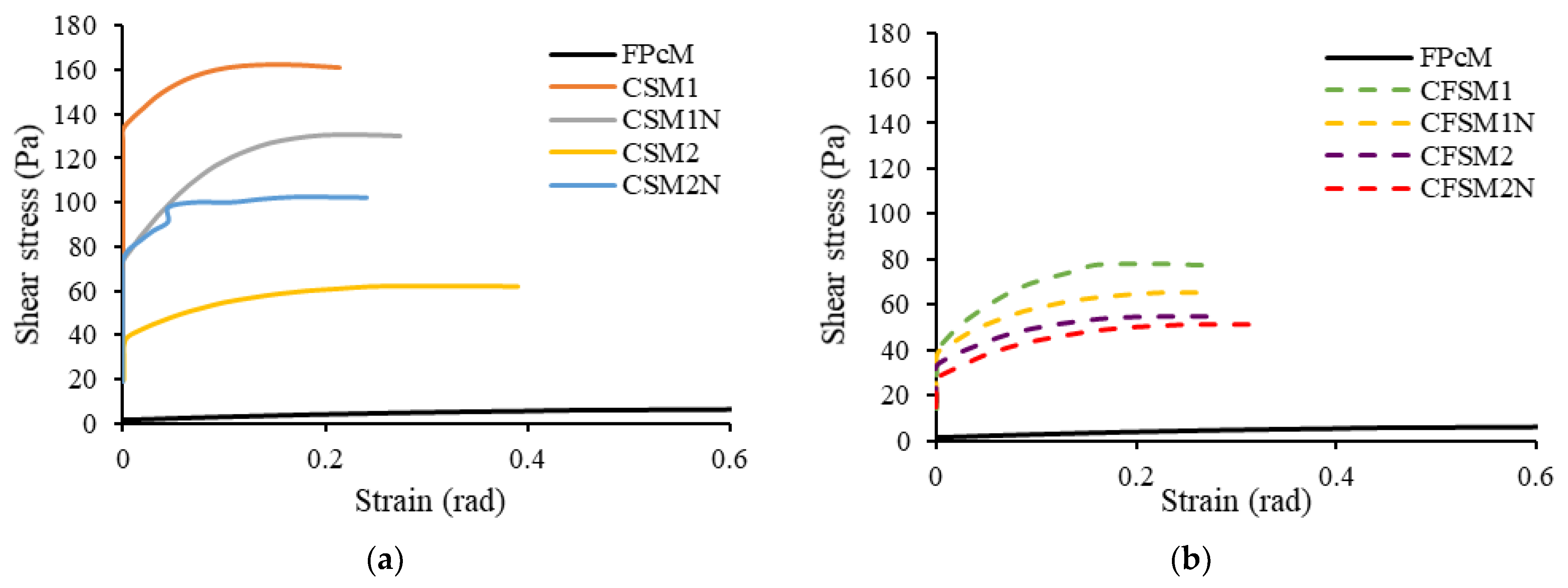
- The AAM mortars incorporating reagent one (Ca(OH)2:Na2SiO3 = 1:2.5) obtained higher yield stresses than those with reagent two (Ca(OH)2:Na2SO4 = 2.5:1) owing to the relatively more significant formation of colloidal complexes (Si-O-H-Na) and Si-Al binding phases. FA-F in ternary (CFS) mixes led to lower yield stress ranging from 51.2 to 77.4 Pa than their binary (CS) counterparts (62.2 to 160.8 Pa). The shear modulus and yield stress were observed to decrease with the increase in the slump flow spread of the mortar mixes.
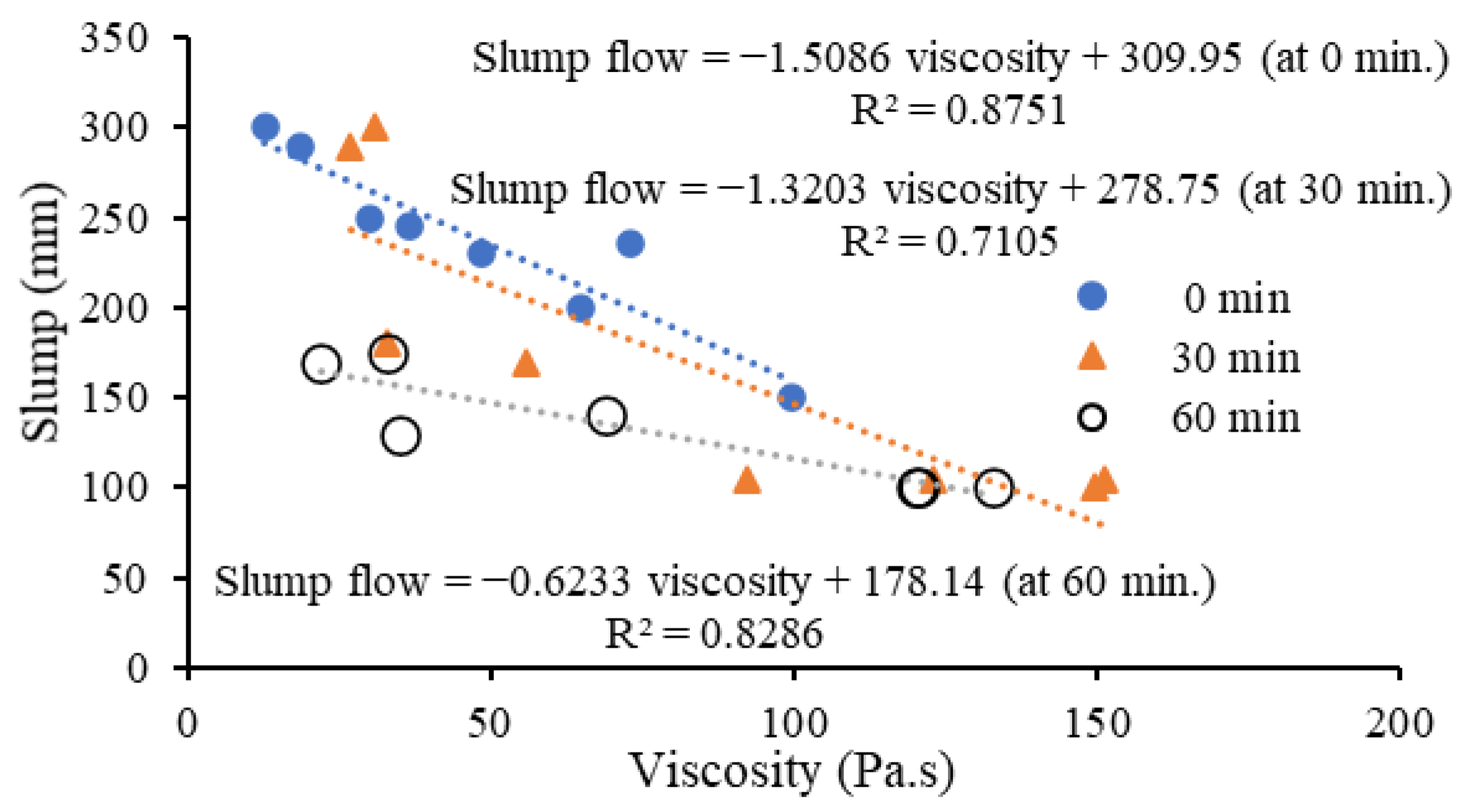
- The slump flow gradually reduced, and the plastic viscosity increased with time from 0 to 60 min after mixing. Lower slump flow/relative slump reductions were noted for mortars with reagent two (250 to 180 mm)/(5.2 to 2.2) than their counterparts with reagent one (245 to 105 mm)/(5.0 to 0.1) after 30 min of mixing. This lower decrease in slump flow spread of mixes with reagent two can be attributed to their higher liquid to solid ratios and the predominant formation of cementitious reaction products having shorter chains than the Si-Al linkages in alkali-activation products formed in mixes with reagent one.
- There was no change in the slump flow of cement-based control mortar (FPCM) with time (0 to 60 min after mixing). The high volume (55%) fly ash class F with characteristic round shape particles enhanced the workability, and the formation of shorter C-S-H linkages led to the lowest viscosity, lowest yield stress and highest compressive strength of the control mortar.
- All the AAM mixes showed decreased viscosity with an increase in shear rate up to 3.5 RPM depicting pseudoplastic behavior. The mixes with reagent two exhibited slightly lower or similar initial and final viscosities at 0.5 RPM. These binary and ternary mix compositions with reagent two (CSM2, CSM2N, CFSM2 and CFSM2N) also obtained lower viscosities/yield stresses and a higher slump flow spread than their counterparts with reagent one. This rheological behavior of reagent two mixes can be attributed to the dominant formation of cementitious gels (C-A-S-H and C-S-H) with shorter chains or linkages than the polymeric chains associated with geopolymeric products (N-A-S-H and N-C-A-S-H) formed in mixes with reagent one.
- The formation of both amorphous and crystalline phases in AAMs is evident from the SEM/EDS analysis. Portlandite formation in the mortars with reagent two is responsible for synthesizing additional binding phases resulting in higher compressive strengths than their counterparts with reagent one. The formation of C-A-S-H/C-S-H and C-A-S-H/N-C-A-S-H as the primary binding phases for binary and ternary mortars is characterized from the SEM/EDS analysis.
- The shorter linkages/chains present in the cementitious (C-A-S-H and C-S-H) binding phases and higher liquid to solid (L/S) ratios of mixes with reagent two resulted in a higher flow spread/relative slump, lower plastic viscosities and yield stresses compared to mixes with reagent one with a more significant formation of geopolymeric (N-C-A-S-H and N-A-S-H) products. However, the AAM mixes with reagent one exhibited lower setting times than their counterparts with reagent two due to the high alkalinity of sodium metasilicate, leading to a comparatively higher degree of alkali-activation with a quicker formation of reaction products.
- The findings of this research bolster the feasibility of producing commercially viable cement-free one-part ambient cured AAMs incorporating binary or ternary combinations of SCMs, powder form reagents in lesser quantities and fine aggregates with fresh and hardened properties comparable to their heat-cured two-part solution-based counterparts and control cement-based mortars.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Farasat, S.; Shah, A.; Chen, B.; Yousefi, S.; Haque, M.A.; Riaz, M. Improvement of early strength of fly ash-slag based one-part alkali activated mortar. Constr. Build. Mater. 2020, 246, 1–10. [Google Scholar]
- Hossain, K.M.A. Blended cement using volcanic ash and pumice. Cem. Concr. Res. 2003, 33, 1601–1605. [Google Scholar] [CrossRef]
- Hossain, K.M.A. Performance of Volcanic Ash Based Precast and In Situ Blended Cement Concretes in Marine Environment. J. Mater. Civ. Eng. 2005, 17, 694–702. [Google Scholar] [CrossRef]
- Shah, F.A.; Chen, B.; Oderji, S.Y.; Haque, M.A.; Ahmad, M.R. Comparative study on the effect of fiber type and content on the performance of one-part alkali-activated mortar. Constr. Build. Mater. 2020, 243, 1–9. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Illikainen, M. One-part alkali-activated blast furnace slag for sustainable construction at subzero temperatures. Constr. Build. Mater. 2021, 276, 1–11. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Mastali, M.; Falah, M.; Shaad, K.M.; Luukkonen, T.; Illikainen, M. Durability of the Reinforced One-Part Alkali-Activated Slag Mortars with Different Fibers. Waste Biomass Valorization 2021, 12, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Alrefaei, Y.; Wang, Y.-S.; Dai, J.-G. The effectiveness of different superplasticizers in ambient cured one-part alkali activated pastes. Cem. Concr. Compos. 2019, 97, 166–174. [Google Scholar] [CrossRef]
- Luukkonen, T.; Sreenivasan, H.; Abdollahnejad, Z.; Yliniemi, J.; Kantola, A.; Telkki, V.V.; Kinnunen, P.; Illikainen, M. Influence of sodium silicate powder silica modulus for mechanical and chemical properties of dry-mix alkali-activated slag mortar. Constr. Build. Mater. 2020, 233, 1–10. [Google Scholar] [CrossRef]
- Alonso, M.M.; Gismera, S.; Blanco, M.T.; Lanzon, M.; Puertas, F. Alkali-activated mortars: Workability and rheological behaviour. Constr. Build. Mater. 2017, 145, 576–587. [Google Scholar] [CrossRef]
- Kwan, A.K.H.; Li, Y. Effects of fly ash microsphere on rheology, adhesiveness and strength of mortar. Constr. Build. Mater. 2013, 42, 137–145. [Google Scholar] [CrossRef]
- Kwasny, J.; Sonebi, M.; Plasse, J.; Amziane, S. Influence of rheology on the quality of surface finish of cement-based mortars. Constr. Build. Mater. 2015, 89, 102–109. [Google Scholar] [CrossRef]
- Hafid, H.; Ovarlez, G.; Toussaint, F.; Jezequel, P.H.; Roussel, N. Effect of particle morphological parameters on sand grains packing properties and rheology of model mortars. Cem. Concr. Res. J. 2016, 80, 44–51. [Google Scholar] [CrossRef]
- Celikten, S.; Sarıdemir, M.; Deneme, I.O. Mechanical and microstructural properties of alkali-activated slag and slag + fly ash mortars exposed to high temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Palacios, M.; Banfill, P.F.G.; Puertas, F. Rheology and setting of alkali-activated slag pastes and mortars: Effect of organic admixture. ACI Mater. J. 2008, 105, 140–148. [Google Scholar]
- Yang, K.H.; Song, J.K.; Ashour, A.F.; Lee, E.T. Properties of cementless mortars activated by sodium silicate. Constr. Build. Mater. 2008, 22, 1981–1989. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Yang, Y.; Yao, Y. Use of slag to improve mechanical properties of engineered cementitious composites (ECCs) with high volumes of fly ash. Constr. Build. Mater. 2012, 36, 1076–1081. [Google Scholar] [CrossRef]
- Puertas, F.; Varga, C.; Alonso, M.M. Rheology of alkali-activated slag pastes. Effect of the nature and concentration of the activating solution. Cem. Concr. Compos. 2014, 53, 279–288. [Google Scholar] [CrossRef]
- Criado, M.; Palomo, A.; Fernández-Jiménez, A.; Banfill, P.F.G. Alkali activated fly ash: Effect of admixtures on paste rheology. Rheol. Acta 2009, 48, 447–455. [Google Scholar] [CrossRef]
- Banfill, P.F.G. Simultaneous rheological and kinetic measurements on cement pastes. Cem. Concr. Res. 1991, 21, 1148–1154. [Google Scholar] [CrossRef]
- Lachemi, M.; Hossain, K.M.A.; Lambros, V.; Nkinamubanzi, P.-C.; Bouzoubaâ, N. Performance of new viscosity modifying admixtures in enhancing the rheological properties of cement paste. Cem. Concr. Res. 2004, 34, 185–193. [Google Scholar] [CrossRef]
- Wallevik, J.E. Relationship between the Bingham parameters and slump. Cem. Concr. Res. 2006, 36, 1214–1221. [Google Scholar] [CrossRef]
- Sood, D.; Hossain, K.M.A. Optimizing Precursors and Reagents for the Development of Alkali-Activated Binders in Ambient Curing Conditions. J. Compos. Sci. 2021, 5, 59. [Google Scholar] [CrossRef]
- Sood, D.; Hossain, K.M.A.; Manzur, T.; Hasan, M.J. Developing Geopolymer Pastes Using Dry Mixing Technique. In Proceedings of the 7th International Conference on Engineering Mechanics and Materials, Laval, QC, Canada, 12–15 June 2019; pp. 1–8. [Google Scholar]
- Sherir, M.A.A.; Hossain, K.M.A.; Lachemi, M. Self-healing and expansion characteristics of cementitious composites with high volume fly ash and MgO-type expansive agent. Constr. Build. Mater. 2016, 127, 80–92. [Google Scholar] [CrossRef]
- Sherir, M.A.A.; Hossain, K.M.A.; Lachemi, M. Permeation and Transport Properties of Self-Healed Cementitious Composite Produced with MgO Expansive Agent. J. Mater. Civ. Eng. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Matrix design of strain hardening fiber reinforced engineered geopolymer composite. Compos. Part B Eng. 2016, 89, 253–265. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Effect of different superplasticizers and activator combinations on workability and strength of fly ash based geopolymer. Mater. Des. 2014, 57, 667–672. [Google Scholar] [CrossRef]
- ASTM International. ASTM C1437, Standard Test Method for Flow of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM International. ASTM C191-18a, Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM International. ASTM C109/C109 M. Standard test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- American Concrete Institute. ACI Committee 318. Building Code Requirements for Structural Concrete and Commentary; ACI 318-19; American Concrete Institute: Farmington Hills, MI, USA, 2019. [Google Scholar]
- Palacios, M.; Alonso, M.M.; Varga, C.; Puertas, F. Influence of the alkaline solution and temperature on the rheology and reactivity of alkali-activated fly ash pastes. Cem. Concr. Compos. 2019, 95, 277–284. [Google Scholar] [CrossRef]
- Panda, B.; Unluer, C.; Tan, M.J. Extrusion and rheology characterization of geopolymer nanocomposites used in 3D printing. Compos. Part B 2019, 176, 1–9. [Google Scholar] [CrossRef]
- Vance, K.; Dakhane, A.; Sant, G.; Neithalath, N. Observations on the rheological response of alkali activated fly ash suspensions: The role of activator type and concentration. Rheol Acta 2014, 53, 843–855. [Google Scholar] [CrossRef]
- Tognonvi, M.T.; Massiot, D.; Lecomte, A.; Rossignol, S.; Bonnet, J.-P. Identification of solvated species present in concentrated and dilute sodium silicate solutions by combined 29 Si NMR and SAXS studies. J. Colloid Interface Sci. 2010, 352, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Aboulayt, A.; Jaafri, R.; Samouh, H.; El Idrissi, A.C.; Roziere, E.; Moussa, R.; Loukili, A. Stability of a new geopolymer grout: Rheological and mechanical performances of metakaolin-fly ash binary mixtures. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T. Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 2018, 86, 9–18. [Google Scholar] [CrossRef]
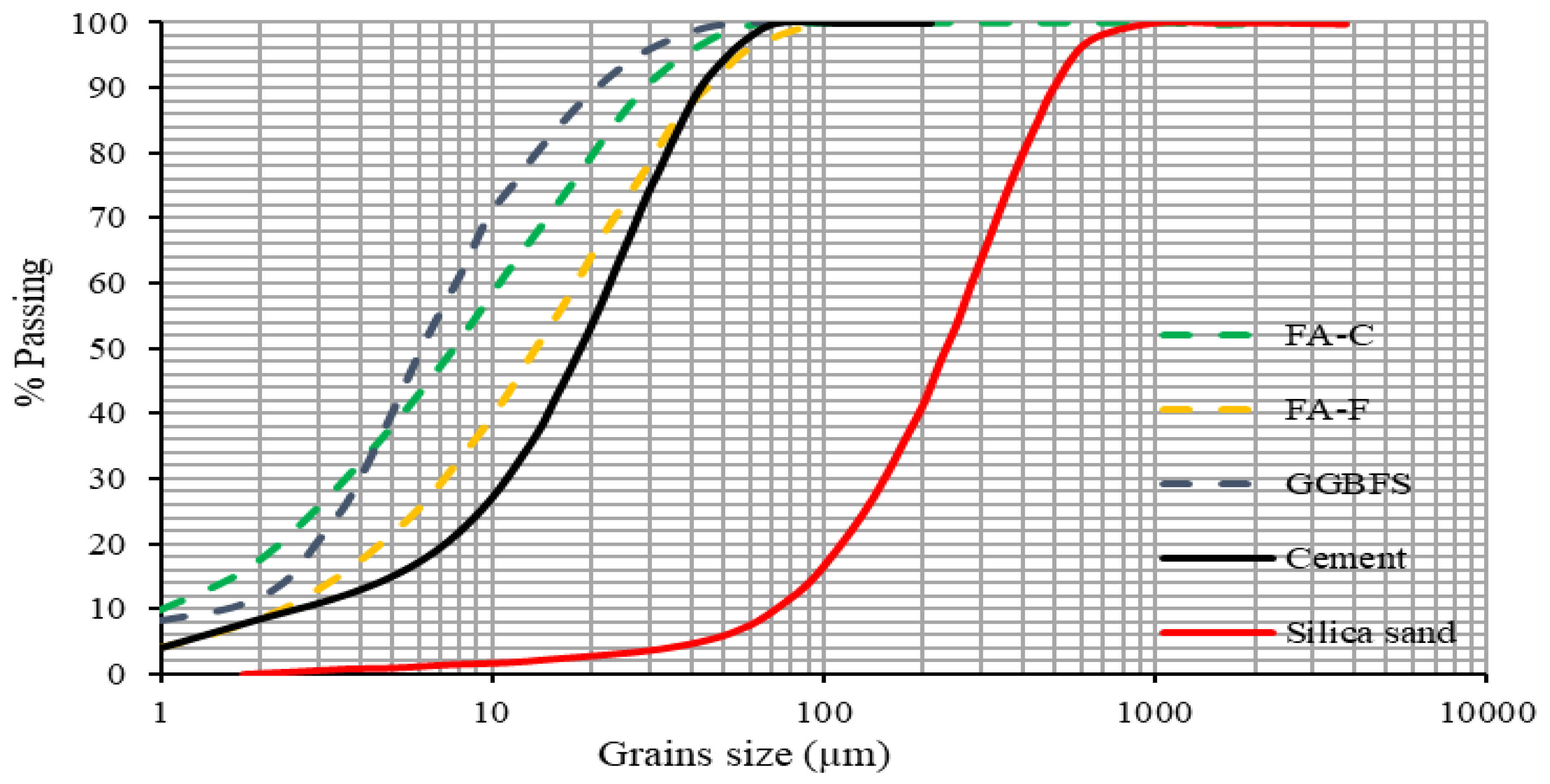


| Chemical Composition (%) | FA-C | FA-F | GGBFS | Cement |
|---|---|---|---|---|
| SiO2 | 36.53 | 55.66 | 35.97 | 19.35 |
| Al2O3 | 18.26 | 22.09 | 9.18 | 5.31 |
| Fe2O3 | 5.66 | 4.26 | 0.50 | 3.10 |
| CaO | 20.97 | 7.97 | 38.61 | 62 |
| MgO | 5.08 | 1.16 | 10.99 | 3 |
| K2O | 0.68 | 1.49 | 0.36 | - |
| Na2O | 4.04 | 4.10 | 0.28 | 0.23 |
| MnO | 0.03 | 0.03 | 0.25 | - |
| TiO2 | 1.26 | 0.61 | 0.39 | - |
| P2O5 | 0.96 | 0.43 | 0.01 | - |
| LOI. | 2.18 | 1.05 | 0.74 | 2.40 |
| Physical properties | FA-C | FA-F | GGBFS | Cement |
| Density (g/cm3) | 2.61 | 2.02 | 2.87 | 3.15 |
| Retained on 45 µ (%) | <5 | <10 | <5 | <10 |
| Blaine fineness (m2/kg) | 315 | 306 | 489.30 | 410 |
| Mix-Des. | Total SCMs (Binder *) | Cement | FA-C | FA-F | GGBFS | Reagent Type | Reagent/ Binder | Silica Sand | Water/ Binder | HR-WRA ** |
|---|---|---|---|---|---|---|---|---|---|---|
| Alkali activated mortars (AAMs)—CS: Binary and CFS: Ternary | ||||||||||
| CSM1 | 1 | 0 | 0.55 | 0 | 0.45 | 1 | 0.09 | 0.3 | 0.35 | 0.02 |
| CSM1N | 1 | 0 | 0.50 | 0 | 0.50 | 1 | 0.09 | 0.3 | 0.35 | 0.02 |
| CFSM1 | 1 | 0 | 0.25 | 0.35 | 0.40 | 1 | 0.09 | 0.3 | 0.35 | 0.02 |
| CFSM1N | 1 | 0 | 0.25 | 0.25 | 0.50 | 1 | 0.09 | 0.3 | 0.35 | 0.02 |
| CSM2 | 1 | 0 | 0.55 | 0 | 0.45 | 2 | 0.12 | 0.3 | 0.375 | 0.02 |
| CSM2N | 1 | 0 | 0.50 | 0 | 0.50 | 2 | 0.12 | 0.3 | 0.375 | 0.02 |
| CFSM2 | 1 | 0 | 0.25 | 0.35 | 0.40 | 2 | 0.12 | 0.3 | 0.375 | 0.02 |
| CFSM2N | 1 | 0 | 0.25 | 0.25 | 0.50 | 2 | 0.12 | 0.3 | 0.375 | 0.02 |
| Control mortar | ||||||||||
| FPCM | 1 | 0.45 | 0 | 0.55 | 0 | N.A. | NA. | 0.36 | 0.27 | 0.006 |
| Mix Designation | R. Type | R. Component Ratio | Chemical Ratios (SCMs + Reagents) | 28d-Compressive Strength (MPa) | 28d-Density (kg/m3) | |||
|---|---|---|---|---|---|---|---|---|
| SiO2/Al2O3 | Na2O/SiO2 | CaO/SiO2 | Na2O/Al2O3 | |||||
| CSM1 | 1 | 1:2.5 | 2.62 | 0.09 | 0.84 | 0.23 | 42.6 | 2088 |
| CSM1N | 1 | 1:2.5 | 2.71 | 0.08 | 0.87 | 0.23 | 35.0 | 2075 |
| CFSM1 | 1 | 1:2.5 | 2.75 | 0.08 | 0.59 | 0.22 | 40.4 | 2030 |
| CFSM1N | 1 | 1:2.5 | 2.86 | 0.07 | 0.69 | 0.21 | 34.0 | 2010 |
| CSM2 | 2 | 2.5:1 | 2.56 | 0.14 | 1.02 | 0.35 | 41.2 | 2042 |
| CSM2N | 2 | 2.5:1 | 2.64 | 0.13 | 1.02 | 0.35 | 35.8 | 2032 |
| CFSM2 | 2 | 2.5:1 | 2.69 | 0.12 | 0.73 | 0.32 | 42.0 | 1983 |
| CFSM2N | 2 | 2.5:1 | 2.80 | 0.12 | 0.84 | 0.33 | 38.1 | 2055 |
| FPCM | - | - | 2.70 | 0.06 | 0.82 | 0.16 | 43.5 | 1937 |
| Mix-ID | Slump Flow (mm) with Time (min) | Relative Slump (T) with Time (min) | Viscosity (Pa.s) with Time (min) | Yield Stress (Pa) | Shear Modulus (Pa/rad) | Setting Time (min.) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 0 | 30 | 60 | 0 | 30 | 60 | Initial | Final | |||
| FPcM | 480 | 500 | 490 | 22.0 | 24.0 | 23.0 | 1.8 | - | - | 6.6 | 9.5 | 474 | 522 |
| CSM1 | 200 | 105 | 100 | 3.0 | 0.1 | 0.0 | 64.7 | 92.2 | 120.1 | 160.8 | 300.1 | 216 | 248 |
| CSM1N | 150 | 105 | 100 | 1.2 | 0.1 | 0.0 | 99.8 | 123.1 | 133.0 | 129.8 | 373.4 | 225 | 250 |
| CSM2 | 250 | 180 | 130 | 5.2 | 2.2 | 0.7 | 30.2 | 32.7 | 34.8 | 62.2 | 129.4 | 311 | 369 |
| CSM2N | 230 | 170 | 140 | 4.3 | 1.9 | 1.0 | 48.3 | 56.0 | 69.1 | 102.6 | 383.9 | 277 | 345 |
| CFSM1 | 245 | 105 | 100 | 5.0 | 0.1 | 0.0 | 36.7 | 151.3 | 120.6 | 77.4 | 250.9 | 145 | 230 |
| CFSM1N | 235 | 100 | 100 | 4.5 | 0.0 | 0.0 | 73.2 | 149.8 | - | 65.4 | 163.9 | 122 | 215 |
| CFSM2 | 300 | 290 | 170 | 8.0 | 7.4 | 1.9 | 12.7 | 26.9 | 22.0 | 54.4 | 128.0 | 458 | 483 |
| CFSM2N | 290 | 300 | 175 | 7.4 | 8.0 | 2.1 | 18.6 | 30.7 | 33.0 | 51.2 | 123.0 | 438 | 472 |
| Speed | Shear Rate (RPM/60) | Mix-ID | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FPcM | CSM1 | CSM1N | CSM2 | CSM2N | CFSM1 | CFSM1N | CFSM2 | CFSM2N | ||
| (RPM) | (1/s) | Viscosity (Pa.s) | ||||||||
| 0.5 | 0.01 | 12.8 | 311.4 | 332.8 | 280.3 | 234.3 | 195.8 | 571.4 | 93.1 | 67.4 |
| 1 | 0.02 | 8.0 | 173.9 | 156.8 | 142.8 | 122.5 | 114.5 | 344.0 | 48.2 | 36.4 |
| 1.5 | 0.03 | 5.7 | 122.7 | 125.2 | 95.9 | 84.9 | 83.1 | 235.0 | 33.5 | 25.3 |
| 2.5 | 0.04 | 4.1 | 73.8 | 90.7 | 58.0 | 53.5 | 51.6 | 130.3 | 21.8 | 16.9 |
| 3.5 | 0.06 | 3.2 | 52.1 | 66.0 | 41.3 | 39.6 | 37.1 | 86.4 | 16.7 | 13.2 |
| 2.5 | 0.04 | 3.4 | 76.8 | 100.8 | 53.9 | 52.6 | 54.8 | 120.3 | 22.3 | 18.0 |
| 1.5 | 0.03 | 5.0 | 139.5 | 184.8 | 83.8 | 84.2 | 98.8 | 211.1 | 35.0 | 28.5 |
| 1 | 0.02 | 7.0 | 229.5 | 305.0 | 120.4 | 123.6 | 161.6 | 329.0 | 51.4 | 42.8 |
| 0.5 | 0.01 | 12.8 | 513.6 | 676.2 | 227.9 | 240.8 | 368.1 | 701.9 | 99.5 | 85.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sood, D.; Hossain, K.M.A. Fresh State, Rheological and Microstructural Characteristics of Alkali-Activated Mortars Developed Using Novel Dry Mixing Technique under Ambient Conditions. Appl. Sci. 2021, 11, 8920. https://doi.org/10.3390/app11198920
Sood D, Hossain KMA. Fresh State, Rheological and Microstructural Characteristics of Alkali-Activated Mortars Developed Using Novel Dry Mixing Technique under Ambient Conditions. Applied Sciences. 2021; 11(19):8920. https://doi.org/10.3390/app11198920
Chicago/Turabian StyleSood, Dhruv, and Khandaker M. A. Hossain. 2021. "Fresh State, Rheological and Microstructural Characteristics of Alkali-Activated Mortars Developed Using Novel Dry Mixing Technique under Ambient Conditions" Applied Sciences 11, no. 19: 8920. https://doi.org/10.3390/app11198920
APA StyleSood, D., & Hossain, K. M. A. (2021). Fresh State, Rheological and Microstructural Characteristics of Alkali-Activated Mortars Developed Using Novel Dry Mixing Technique under Ambient Conditions. Applied Sciences, 11(19), 8920. https://doi.org/10.3390/app11198920







