Prediction of Efficacy of Taeumjowi-Tang for Treatment of Metabolic Risk Factors Based on Machine Learning
Abstract
1. Introduction
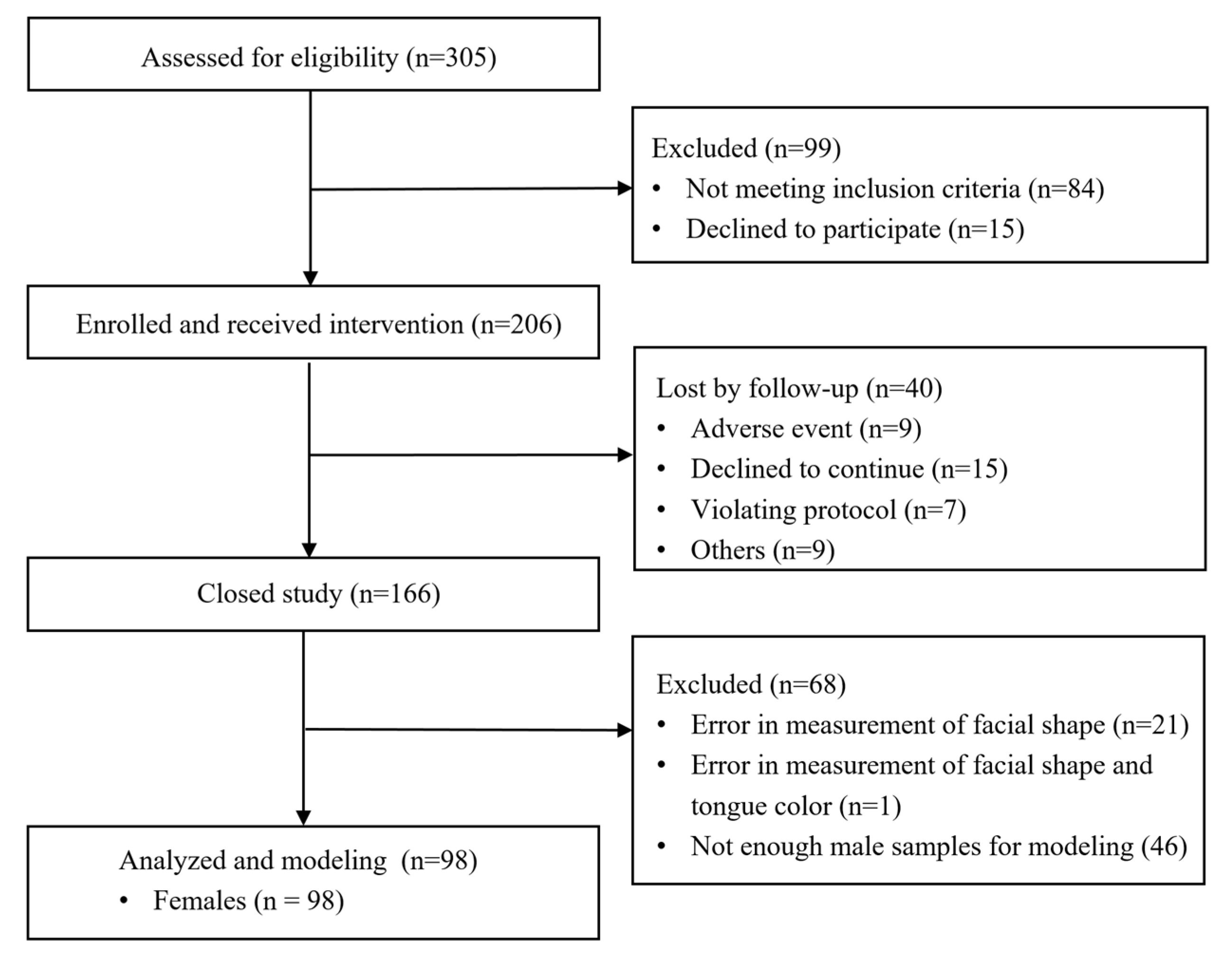
2. Materials and Methods
2.1. Subjects
2.2. Definition
2.3. Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahima, R.S.; Lazar, M.A. The Health Risk of Obesity—Better Metrics Imperative. Science 2013, 341, 856–858. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nat. Cell Biol. 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Huxley, R.R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.L.N.; Chan, J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2009, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nat. Cell Biol. 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.Y. Identification of Type 2 Diabetes Risk Factors Using Phenotypes Consisting of Anthropometry and Triglycerides based on Machine Learning. IEEE J. Biomed. Health Inform. 2016, 20, 39–46. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Lee, E.-J.; Kwak, C.-K.; Sohn, E.-H.; Koh, B.-H.; Song, I.-B.; Lee, K.-S. Clinical Trial of Herbal Formula on Weight Loss in Obese Korean Children. Am. J. Chin. Med. 2005, 33, 713–722. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, Y.J.; Cho, S.-J.; Kwon, E.-Y.; Ryu, R.; Choi, M.-S. Metabolic Effect of an Oriental Herbal Medicine on Obesity and Its Comorbidities with Transcriptional Responses in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2017, 18, 747. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Park, T.-J.; Chaudhari, H.N.; Choi, J.H.; Choi, J.-Y.; Kim, Y.J.; Choi, M.-S.; Yun, J.W. Hepatic proteome and its network response to supplementation of an anti-obesity herbal mixture in diet-induced obese mice. Biotechnol. Bioprocess. Eng. 2015, 20, 775–793. [Google Scholar] [CrossRef]
- Park, J.; Youn, D.-H.; Kang, J.; Ahn, K.S.; Kwak, H.J.; Um, J.-Y. Taeumjowi-tang, a Traditional Korean Sasang Remedy, Improves Obesity-Atopic Dermatitis Comorbidity by Regulating Hypoxia-Inducible Factor 1 Alpha. Front. Pharmacol. 2019, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.Y.; Seo, G.S.; Song, J.H.; Baek, C.H. The Retrospective Analysis on Obese and Overweight Female Patients with Korean Medical Treatment and Its Effectiveness for Clinical Setting of Seasonal Treatment. J. Korean Med. Obes. Res. 2017, 17, 10–19. [Google Scholar] [CrossRef]
- Kim, B.-M.; Jo, H.-G. Progress of Short-term Herbal Medicine Administration for Hypertriglyceridemia: A Case Report. J. Intern. Korean Med. 2019, 40, 517–524. [Google Scholar] [CrossRef]
- Park, K.-M.; Song, Y.-K.; Lim, H.-H.; Lee, J.-A.; Ko, H.-Y.; Park, J.-H.; Kim, H.-J.; Park, S.-J.; Park, J.-S.; Ko, S.-G. Review on the research relative to Taeeumjowui-Tang (Taiyintiaowei-tang). J. Korean Med. Obes. Res. 2009, 9, 23–36. [Google Scholar]
- Seo, N.-J.; Nam, D.-W.; Lee, E.-O.; Shim, B.-S.; Ahn, K.-S.; Kim, S.-H. Clinical study of Gamitaeeumjowi-tang for obese patients. J. Physiol. Pathol. Korean Med. 2008, 22, 446–452. [Google Scholar]
- Li, J.-E.; Song, Y.-K.; Lim, H.-H. Clinical Trial of Taeeumjowui-Tang (Taiyintiaowei-tang) on Obese Patients-Randomized, Double Blind, Placebo-Controlled Study. J. Korean Med. Rehabil. 2010, 20, 97–213. [Google Scholar]
- Park, S.; Park, J.-S.; Cheon, C.; Yang, Y.J.; An, C.; Jang, B.-H.; Song, Y.-K.; Go, H.; Lee, J.A.; Shin, Y.; et al. A pilot study to evaluate the effect of Taeumjowi-tang on obesity in Korean adults: Study protocol for a randomised, double-blind, placebo-controlled, multicentre trial. Trials 2012, 13, 33. [Google Scholar] [CrossRef]
- Do, J.-H.; Ku, B.; Jang, J.-S.; Kim, H.; Kim, J.Y. Analysis of Sasang constitutional types using facial features with compensation for photographic distance. Integr. Med. Res. 2012, 1, 26–35. [Google Scholar] [CrossRef]
- Nam, J.; Jang, J.-S.; Kim, H.; Kim, J.Y.; Do, J.-H. Modification of the Integrated Sasang Constitutional Diagnostic Model. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Vaughan, C.J.; Gotto, A.M., Jr. Update on statins: 2003. Circulation 2004, 110, 886–892. [Google Scholar] [CrossRef]
- Collaboration, P.S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Tsagkrasoulis, D.; Hysi, P.; Spector, T.; Montana, G. Heritability maps of human face morphology through large-scale auto-mated three-dimensional phenotyping. Sci. Rep. 2017, 7, 45885. [Google Scholar] [CrossRef]
- Zabatani, A.; Surazhsky, V.; Sperling, E.; Moshe, S.B.; Menashe, O.; Silver, D.H.; Karni, T.; Bronstein, A.M.; Bronstein, M.M.; Kimmel, R. Intel® RealSense™ SR300 Coded light depth Camera. IEEE Trans. Pattern Anal. Mach. Intell. 2020, 42, 2333–2345. [Google Scholar] [CrossRef]
- Siena, F.L.; Byrom, B.; Watts, P.; Breedon, P. Utilising the Intel RealSense Camera for Measuring Health Outcomes in Clinical Research. J. Med. Syst. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Cha, S.; Lim, J.E.; Park, A.Y.; Do, J.-H.; Lee, S.W.; Shin, C.; Cho, N.H.; Kang, J.-O.; Nam, J.M.; Kim, J.-S.; et al. Identification of five novel genetic loci related to facial morphology by genome-wide association studies. BMC Genom. 2018, 19, 481. [Google Scholar] [CrossRef]
- Jung, C.J.; Jeon, Y.J.; Kim, J.Y.; Kim, K.H. Review on the current trends in tongue diagnosis systems. Integr. Med. Res. 2012, 1, 13–20. [Google Scholar] [CrossRef]
- Kim, J.; Jung, C.J.; Nam, D.-H.; Kim, K.H. Different trends of teeth marks according to qi blood yin yang deficiency pattern in patients with chronic fatigue. Eur. J. Integr. Med. 2017, 12, 122–128. [Google Scholar] [CrossRef]
- Jang, J.-S.; Ku, B.; Kim, Y.-S.; Nam, J.; Kim, K.H.; Kim, J.Y. A practical approach to Sasang constitutional diagnosis using vocal features. BMC Complement. Altern. Med. 2013, 13, 307. [Google Scholar] [CrossRef]
- Lee, B.J.; Ku, B.; Nam, J.; Pham, D.D.; Kim, J.Y. Prediction of Fasting Plasma Glucose Status Using Anthropometric Measures for Diagnosing Type 2 Diabetes. IEEE J. Biomed. Health Inform. 2013, 18, 555–561. [Google Scholar] [CrossRef]
- Lee, B.J.; Ku, B. A comparison of trunk circumference and width indices for hypertension and type 2 diabetes in a large-scale screening: A retrospective cross-sectional study. Sci. Rep. 2018, 8, 13284. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.Y. Identification of Hemoglobin Levels Based on Anthropometric Indices in Elderly Koreans. PLoS ONE 2016, 11, e0165622. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.Y. Identification of the best anthropometric predictors of serum high-and low-density lipoproteins using ma-chine learning. IEEE J. Biomed. Health Inform. 2014, 19, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Kim, J.Y. Indicators of hypertriglyceridemia from anthropometric measures based on data mining. Comput. Biol. Med. 2015, 57, 201–211. [Google Scholar] [CrossRef][Green Version]
- Cho, J.-H.; Song, B.-J.; Jang, Y.-J. Effect of Taeyeumjowee-Tang and Electroacupuncture Combined-therapy on. J. Korean Med. Obes. Res. 2001, 1, 77–83. [Google Scholar]
- Park, S.; Nahmkoong, W.; Cheon, C.; Park, J.-S.; Jang, B.-H.; Shin, Y.; Kim, K.-S.; Go, H.; Song, Y.-K.; Ko, S.-G. Efficacy and Safety of Taeeumjowi-tang in Obese Korean Adults: A Double-Blind, Randomized, and Placebo-Controlled Pilot Trial. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ahn, H.-S.; Oh, E.-H.; Kim, Y.-L. Effect of Taeeumjoweetang on the body composition, serum lipid level and an-tioxidant enzyme activity of obese female college students. J. Sasang Const. Med. 2011, 23, 391–401. [Google Scholar]
- Park, Y.J.; Do, J.-H.; Kim, H.; Kim, J.Y. Differences in Complexion between Cold- and Heat-Prescription Groups in Sasang Medicine. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Lee, B.J.; Ku, B.; Park, K.; Kim, K.H.; Kim, J.Y. A New Method of Diagnosing Constitutional Types Based on Vocal and Facial Features for Personalized Medicine. J. Biomed. Biotechnol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Noble, D. Recent progress and prospects in Sasang constitutional medicine: A traditional type of physiome-based treatment. Prog. Biophys. Mol. Biol. 2014, 116, 76–80. [Google Scholar] [CrossRef]
| Variables | SBP | DBP | TG | HDL | |||||
|---|---|---|---|---|---|---|---|---|---|
| All | Non-Improvement | Improvement | Non-Improvement | Improvement | Non-Improvement | Improvement | Non-Improvement | Improvement | |
| Number of subjects | 98 | 80 | 18 | 64 | 34 | 48 | 50 | 50 | 48 |
| Age (years) | 44.61 ± 10.49 | 44.64 ± 10.49 | 44.50 ± 10.79 | 45.14 ± 10.30 | 43.62 ± 10.94 | 44.46 ± 10.54 | 44.76 ± 10.55 | 44.5 ± 11.23 | 44.73 ± 9.78 |
| BMI (kg/m2) | 29.30 ± 3.47 | 29.14 ± 2.99 | 30.01 ± 5.14 | 29.16 ± 3.03 | 29.56 ± 4.21 | 29.83 ± 3.32 | 28.79 ± 3.56 | 29.09 ± 3.13 | 29.51 ± 3.81 |
| Height (cm2) | 159.13 ± 5.84 | 159.01 ± 5.99 | 159.70 ± 5.23 | 159.39 ± 6.11 | 158.66 ± 5.37 | 158.09 ± 6.28 | 160.14 ± 5.26 | 158.82 ± 5.89 | 159.46 ± 5.84 |
| Weight (kg) | 74.34 ± 10.92 | 73.76 ± 9.43 | 76.88 ± 16.07 | 74.14 ± 9.31 | 74.70 ± 13.58 | 74.62 ± 9.86 | 74.07 ± 11.94 | 73.49 ± 9.99 | 75.21 ± 11.84 |
| SBP (mmHg) | 129.78 ± 10.65 | 129.15 ± 10.49 | 132.56 ± 11.22 | 128.14 ± 11.04 | 132.85 ± 9.27* | 130.46 ± 10.26 | 129.12 ± 11.08 | 129.00 ± 11.39 | 130.58 ± 9.87 |
| DBP (mmHg) | 82.00 ± 8.31 | 81.31 ± 8.24 | 85.06 ± 8.15 | 80.86 ± 8.61 | 84.15 ± 7.34 | 82.75 ± 7.75 | 81.28 ± 8.83 | 82.22 ± 9.29 | 81.77 ± 7.24 |
| Pulse rate (beats per minute) | 73.96 ± 9.13 | 74.01 ± 9.11 | 73.72 ± 9.45 | 74.23 ± 9.39 | 73.44 ± 8.71 | 74.63 ± 9.57 | 73.32 ± 8.73 | 73.58 ± 8.35 | 74.35 ± 9.94 |
| HbA1c (%) | 5.63 ± 0.54 | 5.67 ± 0.53 | 5.42 ± 0.54 | 5.63 ± 0.54 | 5.62 ± 0.54 | 5.64 ± 0.52 | 5.62 ± 0.55 | 5.69 ± 0.43 | 5.56 ± 0.63 |
| Triglyceride (mg/dL) | 141.36 ± 74.48 | 139.00 ± 72.39 | 151.83 ± 84.57 | 133.27 ± 59.29 | 156.59 ± 96.06 | 139.08 ± 77.06 | 143.54 ± 72.62 | 149.36 ± 79.17 | 133.02 ± 69.09 |
| LDL (mg/dL) | 123.33 ± 31.12 | 123.79 ± 29.73 | 121.26 ± 37.56 | 127.33 ± 31.99 | 115.79 ± 28.34 | 122.39 ± 32.25 | 124.22 ± 30.29 | 125.00 ± 30.72 | 121.58 ± 31.76 |
| HDL (mg/dL) | 55.89 ± 11.19 | 55.70 ± 11.13 | 56.73 ± 11.73 | 55.37 ± 10.46 | 56.88 ± 12.54 | 57.20 ± 10.74 | 54.64 ± 11.57 | 56.39 ± 11.28 | 55.37 ± 11.18 |
| Red Blood Cell (106/µL) | 4.44 ± 0.30 | 4.44 ± 0.30 | 4.39 ± 0.30 | 4.46 ± 0.28 | 4.39 ± 0.33 | 4.39 ± 0.26 | 4.48 ± 0.33 | 4.43 ± 0.32 | 4.45 ± 0.29 |
| Hemoglobin (g/dL) | 13.37 ± 1.04 | 13.36 ± 1.09 | 13.41 ± 0.84 | 13.37 ± 1.06 | 13.36 ± 1.03 | 13.25 ± 0.98 | 13.48 ± 1.09 | 13.17 ± 1.12 | 13.57 ± 0.92 |
| Fastingblood sugar (mg/dL) | 98.85 ± 15.26 | 99.59 ± 16.2 | 95.56 ± 9.78 | 99.63 ± 14.71 | 97.38 ± 16.37 | 98.52 ± 14.25 | 99.16 ± 16.31 | 98.38 ± 13.89 | 99.33 ± 16.7 |
| Free thyroxine (ng/dL) | 1.25 ± 0.16 | 1.26 ± 0.16 | 1.23 ± 0.15 | 1.23 ± 0.16 | 1.29 ± 0.16 | 1.24 ± 0.16 | 1.27 ± 0.16 | 1.24 ± 0.17 | 1.27 ± 0.14 |
| Blood Urea Nitrogen (mg/dL) | 12.08 ± 3.12 | 12.18 ± 2.94 | 11.62 ± 3.90 | 12.27 ± 3.09 | 11.71 ± 3.21 | 11.98 ± 3.31 | 12.17 ± 2.96 | 12.35 ± 2.91 | 11.79 ± 3.34 |
| Creatinine (mg/dL) | 0.73 ± 0.08 | 0.72 ± 0.08 | 0.74 ± 0.08 | 0.73 ± 0.08 | 0.72 ± 0.08 | 0.73 ± 0.08 | 0.72 ± 0.08 | 0.72 ± 0.07 | 0.74 ± 0.09 |
| AST (U/L) | 22.89 ± 8.17 | 22.86 ± 8.32 | 23 ± 7.73 | 22.70 ± 8.66 | 23.24 ± 7.29 | 22.77 ± 8.96 | 23.00 ± 7.43 | 22.92 ± 7.70 | 22.85 ± 8.73 |
| ALT (U/L) | 23.18 ± 12.3 | 23.03 ± 11.52 | 23.89 ± 15.68 | 22.31 ± 11.97 | 24.82 ± 12.93 | 24.23 ± 13.81 | 22.18 ± 10.70 | 24.10 ± 12.92 | 22.23 ± 11.69 |
| Total bilirubin (mg/dL) | 0.59 ± 0.25 | 0.60 ± 0.25 | 0.57 ± 0.23 | 0.59 ± 0.21 | 0.60 ± 0.31 | 0.58 ± 0.19 | 0.61 ± 0.29 | 0.59 ± 0.28 | 0.60 ± 0.22 |
| White blood cell (103/µL) | 6.27 ± 1.51 | 6.32 ± 1.51 | 6.05 ± 1.55 | 6.44 ± 1.56 | 5.95 ± 1.39 | 6.38 ± 1.40 | 6.16 ± 1.61 | 6.16 ± 1.29 | 6.38 ± 1.72 |
| SMOKING | |||||||||
| Never smoked | 93 (94.9) | 76 (95) | 17 (94.44) | 62 (96.88) | 31 (91.18) | 46 (95.83) | 47 (94.00) | 47 (94.00) | 46 (95.83) |
| Smoking | 4 (4.08) | 3 (3.75) | 1 (5.56) | 1 (1.56) | 3 (8.82) | 2 (4.17) | 2 (4.00) | 2 (4.00) | 2 (4.17) |
| Quit smoking | 1 (1.02) | 1 (1.25) | 0 (0) | 1 (1.56) | 0 (0) | 0 (0) | 1 (2.00) | 1 (2.00) | 0 (0) |
| DRINKING | |||||||||
| No | 65 (66.33) | 53 (66.25) | 12 (66.67) | 42 (65.63) | 23 (67.65) | 33 (68.75) | 32 (64.00) | 35 (70.00) | 30 (62.5) |
| Yes | 33 (33.67) | 27 (33.75) | 6 (33.33) | 22 (34.38) | 11 (32.35) | 15 (31.25) | 18 (36.00) | 15 (30.00) | 18 (37.5) |
| EXERCISE | |||||||||
| No | 53 (54.08) | 48 (60) | 5 (27.78) * | 39 (60.94) | 14 (41.18) | 19 (39.58) | 34 (68.00) ** | 29 (58.00) | 24 (50.00) |
| Yes | 45 (45.92) | 32 (40) | 13 (72.22) | 25 (39.06) | 20 (58.82) | 29 (60.42) | 16 (32.00) | 21 (42.00) | 24 (50.00) |
| SBP_efficacy | −3.30 ± 7.89 | −0.76 ± 6.06 | −14.56 ± 4.46 † | −1.63 ± 7.26 | −6.44 ± 8.15 ** | −3.06 ± 8.22 | −3.52 ± 7.63 | −2.82 ± 8.21 | −3.79 ± 7.59 |
| DBP_efficacy | −2.35 ± 6.65 | −1.45 ± 6.14 | −6.33 ± 7.51 * | 1.33 ± 4.23 | −9.26 ± 4.49 † | −1.75 ± 7.48 | −2.92 ± 5.75 | −3.14 ± 6.60 | −1.52 ± 6.66 |
| TG_efficacy | −1.00 ± 40.62 | 0.68 ± 42.43 | −8.47 ± 31.31 | 1.02 ± 43.55 | −4.80 ± 34.75 | 30.06 ± 35.34 | −30.82 ± 14.72 † | 7.69 ± 39.11 | −10.06 ± 40.59 * |
| HDL_efficacy | 2.51 ± 13.26 | 1.94 ± 13.28 | 5.03 ± 13.24 | 3.27 ± 14.51 | 1.06 ± 10.54 | −1.54 ± 13.00 | 6.39 ± 12.42 ** | −7.99 ± 7.40 | 13.44 ± 8.14 † |
| Model | Variables | Description |
|---|---|---|
| SBP-efficacy | FDV_52_50 | Vertical distance between 52 and 50 in a frontal face image |
| FVV_47_52_81_50 | Vertical distance between 47 and 52/vertical distance between 81 and 50 in a frontal face image | |
| FVD_81_50_94_194 | Vertical distance between 81 and 50/distance between 94 and 194 in a frontal face image | |
| FVD_81_51_94_194 | Vertical distance between 81 and 51/distance between 94 and 194 in a frontal face image | |
| DBP-efficacy | BodyCenter_a | The mean value of the CIE a* color at the center of the tongue body |
| FurCenter_a | The mean value of the CIE a* color at the center of the coated tongue | |
| FurRoot_a | The mean value of the CIE a* color at the root of the coated tongue | |
| Tip_a | The mean value of the CIE a* color at the tip of the tongue | |
| TotalRatio | Coated ratio of the tongue | |
| Q_10 | Do you express or hide your opinions? | |
| Express | ||
| Moderate | ||
| Hide | ||
| TG-efficacy | FHD_25_125_53_153 | Horizontal distance between 25 and 125/distance between 53 and 153 in a frontal face image |
| ChLD_a_avg | Average value of a* (L*a*b* color space) in the lower left cheek region | |
| R83_84 | Axillary-to-Chest circumference ratio | |
| sMFCC4 | 4th coefficient of Mel-frequency cepstral coefficients | |
| Q_23 | What does your excrement look like? Select an image that is closest in appearance. | |
| Sausage-shaped with a hard and uneven surface | ||
| Sausage-shaped with cracks | ||
| Soft chocolate bar | ||
| Mushy pasta | ||
| Bits of gruel | ||
| HDL-efficacy | FHD_33_133_43_143 | Horizontal distance between 33 and 133/distance between 43 and 143 in a frontal face image |
| FA_53_94 | Angle between the line through 53 and 94 and a horizontal line in a frontal face image | |
| FA_94_43 | Angle between the line through 94 and 43 and a horizontal line in a frontal face image | |
| PAi_72_73 | Angle between the line through 72 and 73 and a horizontal line in a frontal face image | |
| FHD_43_143_94_194 | Horizontal distance between 43 and 143/distance between 94 and 194 in a frontal face image | |
| ChRD_L_avg | Average value of L* (L*a*b* color space) in the lower right cheek region | |
| ChRD_b_avg | Average value of b* (L*a*b* color space) in the lower right cheek region | |
| Q_5 | Are you an extrovert or introvert? | |
| Extrovert | ||
| Moderate | ||
| Introvert | ||
| Q_6 | Are you energetic or quiet? | |
| Energetic | ||
| Moderate | ||
| Quiet | ||
| Q_18 | How much do you perspire? | |
| A lot | ||
| Moderate | ||
| A little | ||
| None |
| Model | Variables | Non-Improvement | Improvement | OR (95% CI) | p-Value | AUC (95%) |
|---|---|---|---|---|---|---|
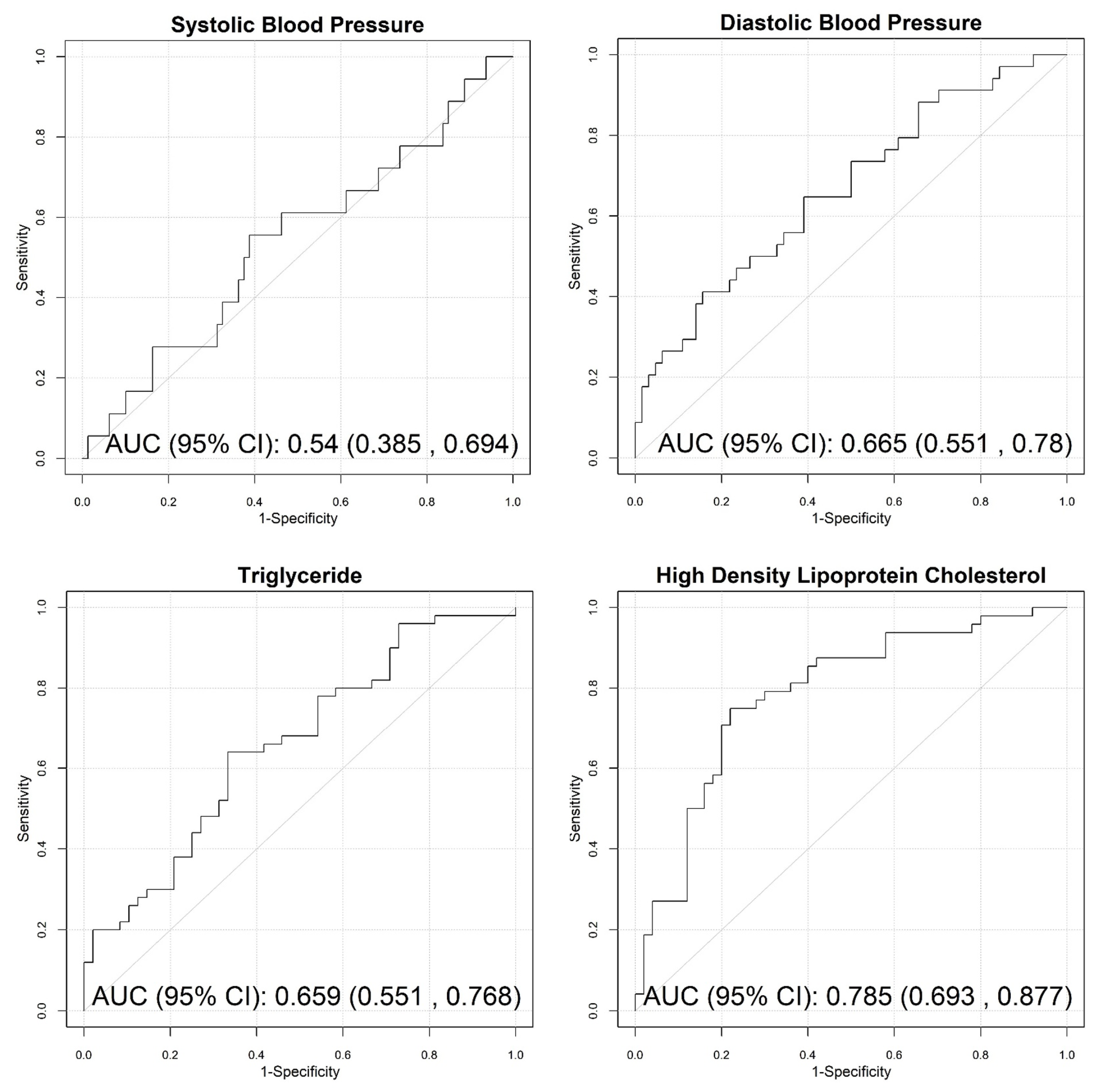
| SBP-efficacy | FDV_52_50 | 71.68 ± 3.81 | 73.96 ± 4.07 | 1.83 (1.06, 3.17) | 0.031 | 0.61 (0.45, 0.76) |
| FVV_47_52_81_50 | 5.42 ± 1.50 | 4.76 ± 1.07 | 0.57 (0.30, 1.09) | 0.090 | 0.55 (0.42, 0.69) | |
| FVD_81_50_94_194 | 0.17 ± 0.01 | 0.18 ± 0.01 | 2.03 (1.15, 3.58) | 0.014 | 0.66 (0.51, 0.82) | |
| FVD_81_51_94_194 | 0.52 ± 0.03 | 0.54 ± 0.02 | 2.10 (1.16, 3.80) | 0.014 | 0.63 (0.50, 0.77) | |
| DBP-efficacy | BodyCenter_a | 28.42 ± 1.94 | 27.44 ± 1.61 | 0.54 (0.33, 0.90) | 0.017 | 0.60 (0.48, 0.72) |
| FurCenter_a | 19.43 ± 0.78 | 18.97 ± 0.98 | 0.59 (0.38, 0.91) | 0.018 | 0.6 (0.48, 0.72) | |
| FurRoot_a | 17.83 ± 1.16 | 17.19 ± 1.35 | 0.59 (0.38, 0.92) | 0.019 | 0.59 (0.47, 0.72) | |
| Tip_a | 30.69 ± 4.12 | 28.75 ± 4.26 | 0.62 (0.40, 0.97) | 0.035 | 0.60 (0.48, 0.72) | |
| TotalRatio | 28.39 ± 12.33 | 36.69 ± 16.56 | 1.83 (1.16, 2.90) | 0.010 | 0.61 (0.49, 0.72) | |
| Q_10 | 0.003 | 0.51 (0.38, 0.64) | ||||
| Express | 29 (45.31) | 6 (17.65) | 1 | |||
| Moderate | 30 (46.88) | 18 (52.94) | 2.90 (1.01, 8.33) | 0.048 | ||
| Hide | 5 (7.81) | 10 (29.41) | 9.67 (2.41, 38.71) | 0.001 | ||
| TG-efficacy | FHD_25_125_53_153 | 0.68 ± 0.04 | 0.67 ± 0.03 | 0.62 (0.40, 0.95) | 0.030 | 0.58 (0.47, 0.69) |
| ChLD_a_avg | 149.72 ± 1.90 | 148.93 ± 2.07 | 0.66 (0.43, 1.01) | 0.053 | 0.59 (0.48, 0.71) | |
| R83_84 | 0.93 ± 0.04 | 0.96 ± 0.07 | 3.01 (1.37, 6.61) | 0.006 | 0.64 (0.53, 0.75) | |
| sMFCC4 | −7.77 ± 3.02 | −6.29 ± 3.48 | 1.60 (1.04, 2.45) | 0.031 | 0.61 (0.49, 0.72) | |
| Q_23 | 0.016 | 0.48 (0.35, 0.60) | ||||
| Sausage-shaped with a hard and uneven surface | 2 (4.17) | 5 (10.00) | 1 | |||
| Sausage-shaped with cracks | 4 (8.33) | 12 (24.00) | 1.2 (0.16, 8.8) | 0.858 | ||
| Soft chocolate bar | 28 (58.33) | 29 (58.00) | 0.41 (0.07, 2.31) | 0.315 | ||
| Mushy pasta | 7 (14.58) | 3 (6.00) | 0.17 (0.02, 1.44) | 0.104 | ||
| Bits of gruel | 7 (14.58) | 1 (2.00) | 0.06 (0, 0.82) | 0.035 | ||
| HDL-efficacy | FHD_33_133_43_143 | 1.13 ± 0.04 | 1.11 ± 0.03 | 0.59 (0.37, 0.94) | 0.027 | 0.59 (0.48, 0.70) |
| FA_53_94 | 86.08 ± 2.56 | 87.37 ± 2.58 | 1.69 (1.09, 2.61) | 0.018 | 0.60 (0.49, 0.71) | |
| FA_94_43 | 80.66 ± 2.80 | 81.82 ± 2.89 | 1.53 (1.00, 2.35) | 0.049 | 0.57 (0.45, 0.68) | |
| PAi_72_73 | 71.39 ± 4.40 | 68.69 ± 5.03 | 0.54 (0.34, 0.86) | 0.009 | 0.61 (0.5, 0.73) | |
| FHD_43_143_94_194 | 0.94 ± 0.02 | 0.95 ± 0.02 | 1.57 (1.02, 2.41) | 0.039 | 0.59 (0.48, 0.70) | |
| ChRD_L_avg | 146.24 ± 8.79 | 142.11 ± 10.37 | 0.64 (0.41, 0.98) | 0.041 | 0.55 (0.43, 0.66) | |
| ChRD_b_avg | 109.81 ± 1.99 | 111.08 ± 1.74 | 2.08 (1.3, 3.35) | 0.002 | 0.67 (0.56, 0.78) | |
| Q_5 | 0.003 | 0.53 (0.41, 0.65) | ||||
| Extrovert | 25 (50.00) | 10 (20.83) | 1 | |||
| Moderate | 18 (36.00) | 20 (41.67) | 2.78 (1.05, 7.34) | 0.039 | ||
| Introvert | 7 (14.00) | 18 (37.50) | 6.43 (2.06, 20.10) | 0.001 | ||
| Q_6 | 0.001 | 0.54 (0.41, 0.66) | ||||
| Energetic | 31 (62.00) | 12 (25.00) | 1 | |||
| Moderate | 13 (26.00) | 23 (47.92) | 4.57 (1.76, 11.84) | 0.002 | ||
| Quiet | 6 (12.00) | 13 (27.08) | 5.6 (1.73, 18.12) | 0.004 | ||
| Q_18 | 0.031 | 0.50 (0.37, 0.62) | ||||
| A lot | 10 (20.00) | 21 (43.75) | 1 | |||
| Moderate | 23 (46.00) | 16 (33.33) | 0.33 (0.12, 0.89) | 0.028 | ||
| A little | 15 (30.00) | 7 (14.58) | 0.22 (0.07, 0.72) | 0.012 | ||
| None | 2 (4.00) | 4 (8.33) | 0.95 (0.15, 6.10) | 0.959 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.J.; Yim, M.H.; Jeon, Y.; Jang, J.S.; So, J.H.; Kim, J.I.; Choi, W.; Kim, J.; Yoon, J.; Kim, M.J.; et al. Prediction of Efficacy of Taeumjowi-Tang for Treatment of Metabolic Risk Factors Based on Machine Learning. Appl. Sci. 2021, 11, 8741. https://doi.org/10.3390/app11188741
Lee BJ, Yim MH, Jeon Y, Jang JS, So JH, Kim JI, Choi W, Kim J, Yoon J, Kim MJ, et al. Prediction of Efficacy of Taeumjowi-Tang for Treatment of Metabolic Risk Factors Based on Machine Learning. Applied Sciences. 2021; 11(18):8741. https://doi.org/10.3390/app11188741
Chicago/Turabian StyleLee, Bum Ju, Mi Hong Yim, Youngju Jeon, Jun Su Jang, Ji Ho So, Joong Il Kim, Woosu Choi, Jihye Kim, Jiwon Yoon, Min Ji Kim, and et al. 2021. "Prediction of Efficacy of Taeumjowi-Tang for Treatment of Metabolic Risk Factors Based on Machine Learning" Applied Sciences 11, no. 18: 8741. https://doi.org/10.3390/app11188741
APA StyleLee, B. J., Yim, M. H., Jeon, Y., Jang, J. S., So, J. H., Kim, J. I., Choi, W., Kim, J., Yoon, J., Kim, M. J., Kim, Y. M., Ahn, T. W., Kim, J. Y., & Do, J. H. (2021). Prediction of Efficacy of Taeumjowi-Tang for Treatment of Metabolic Risk Factors Based on Machine Learning. Applied Sciences, 11(18), 8741. https://doi.org/10.3390/app11188741







