Facile Fabrication of Polyaniline/Graphene Composite Fibers as Electrodes for Fiber-Shaped Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
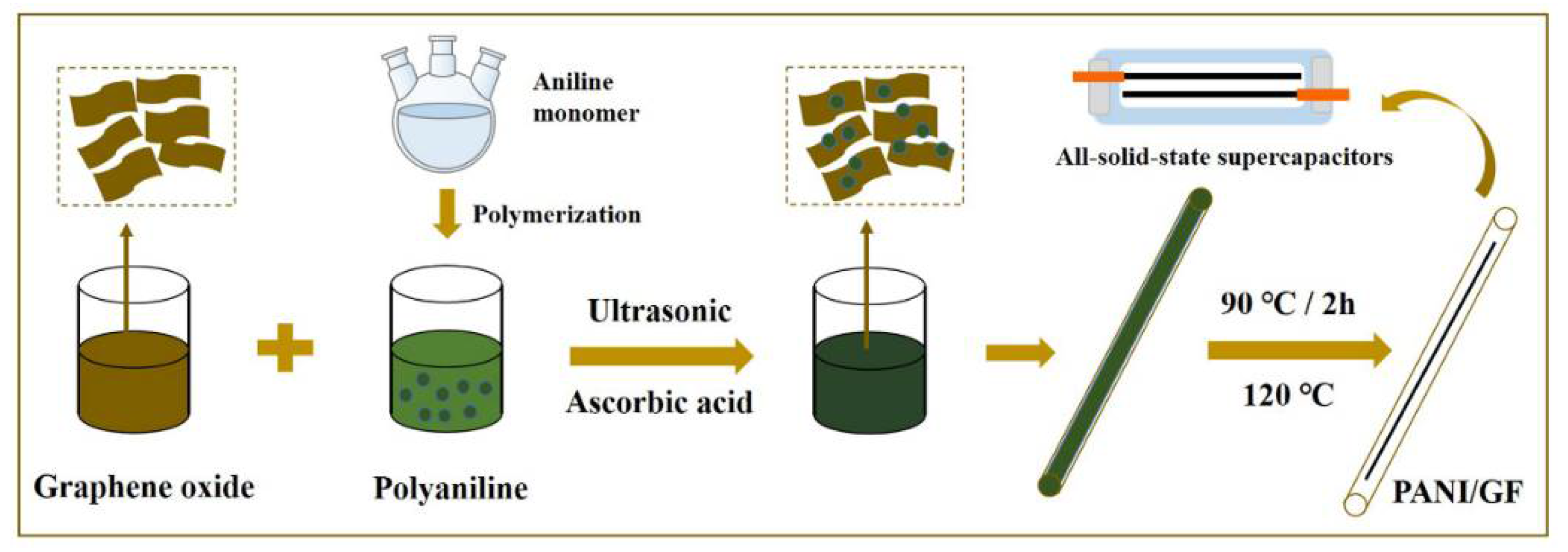
2.1. Synthesis of Materials
2.1.1. Preparation of Polyaniline
2.1.2. Preparation of Polyaniline/Graphene Composite Fibers
2.2. Characterization
2.3. Construction of Fiber-Shaped Supercapacitors and Electrochemical Measurements
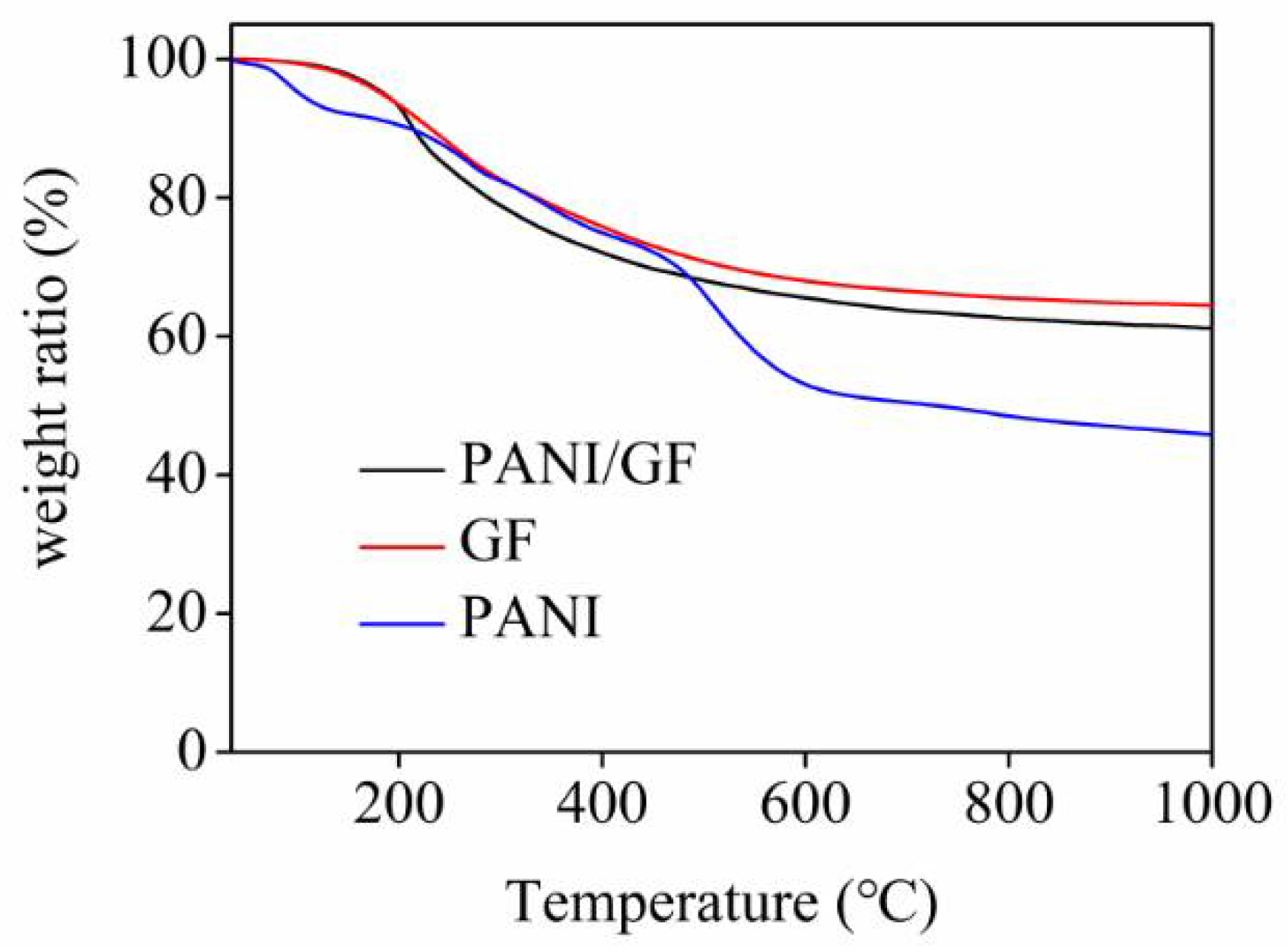
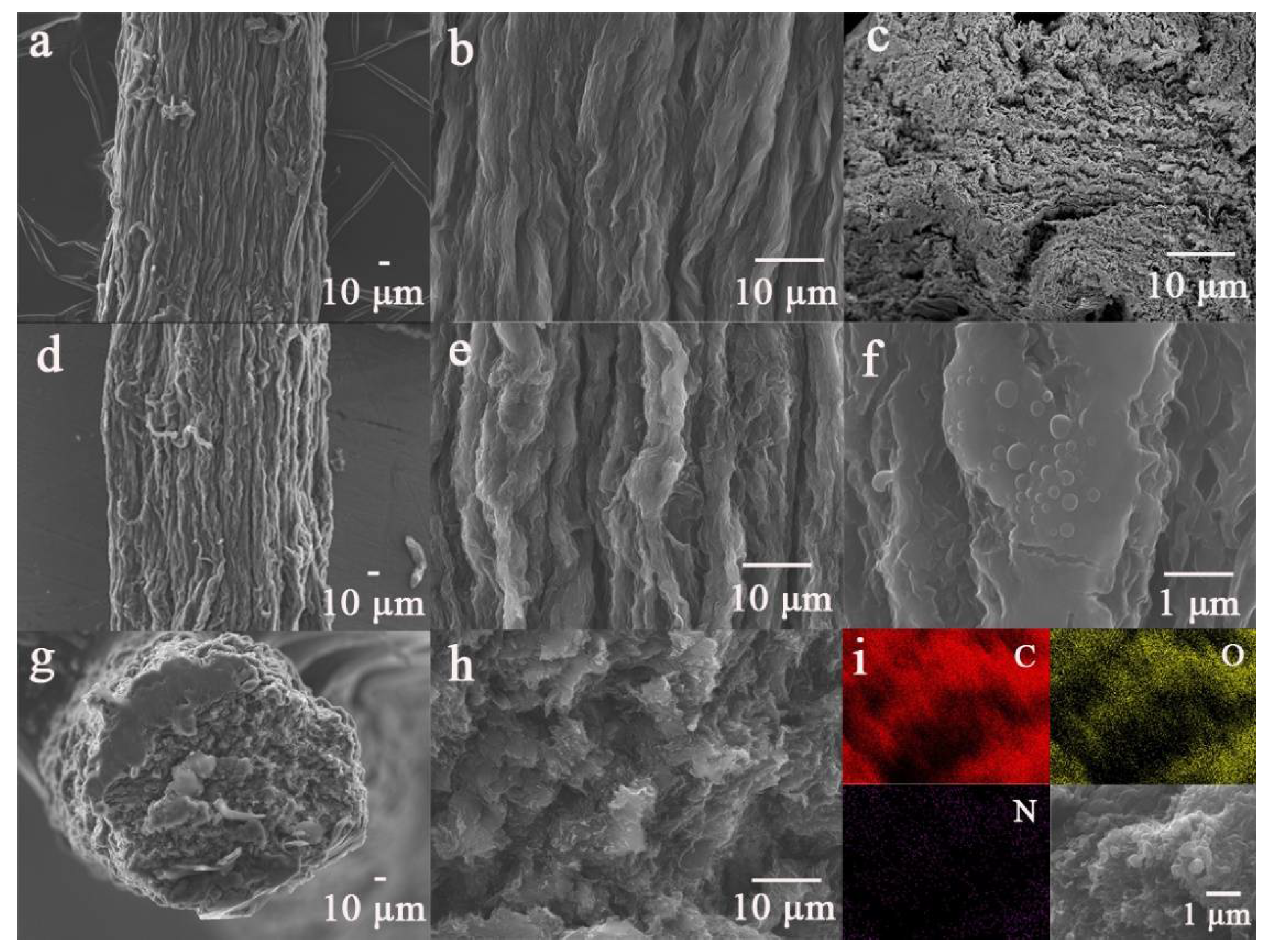
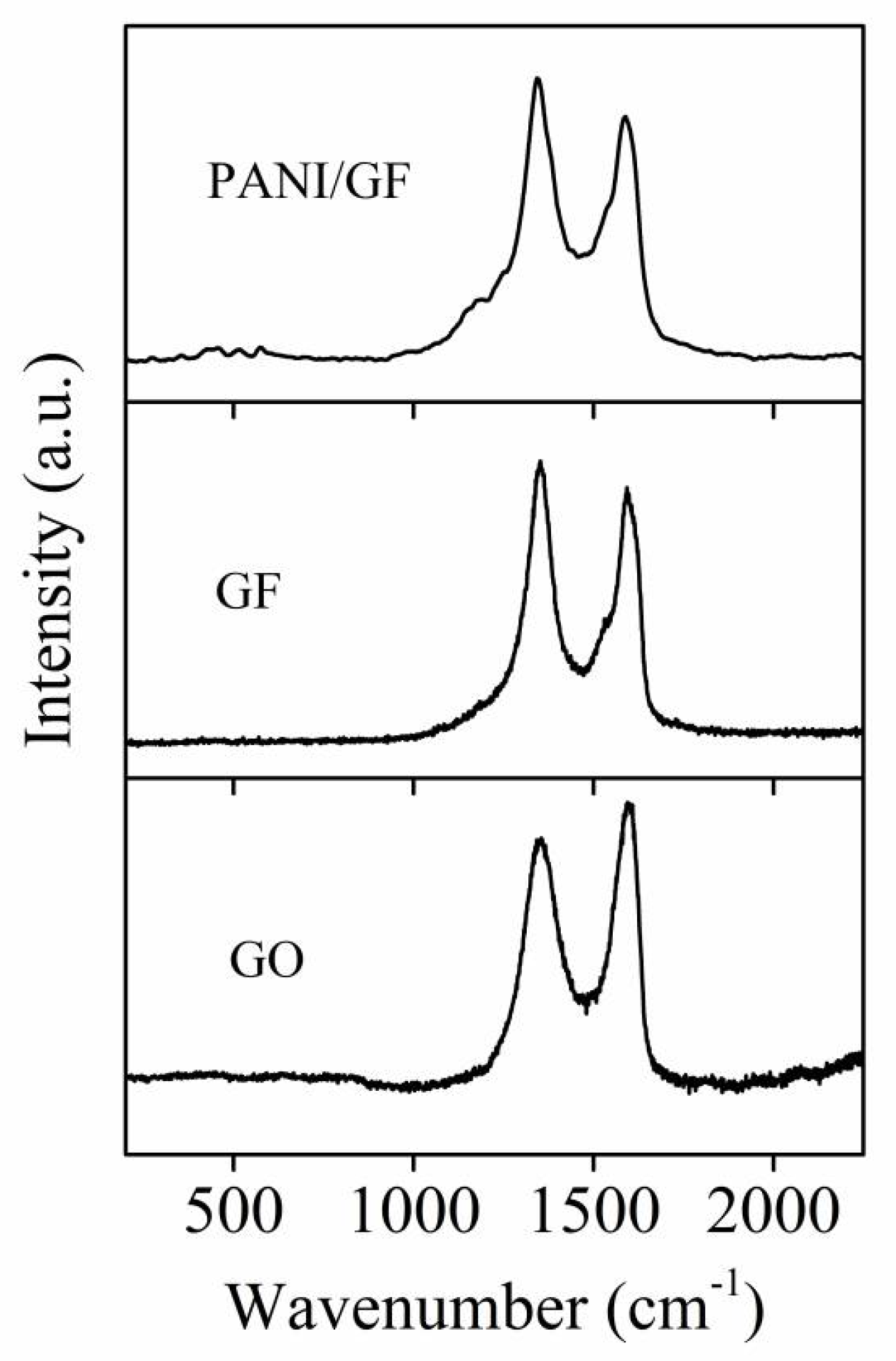
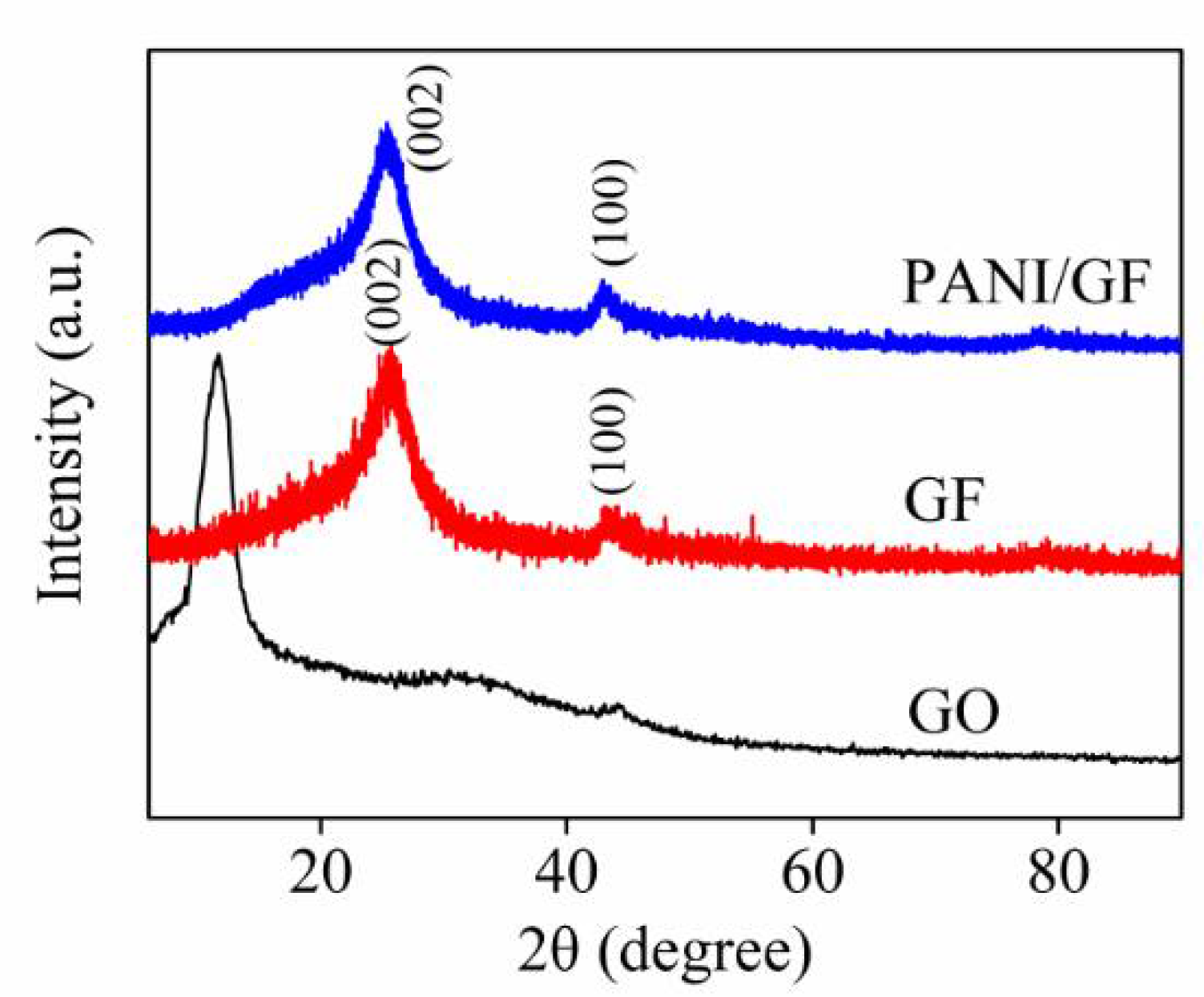
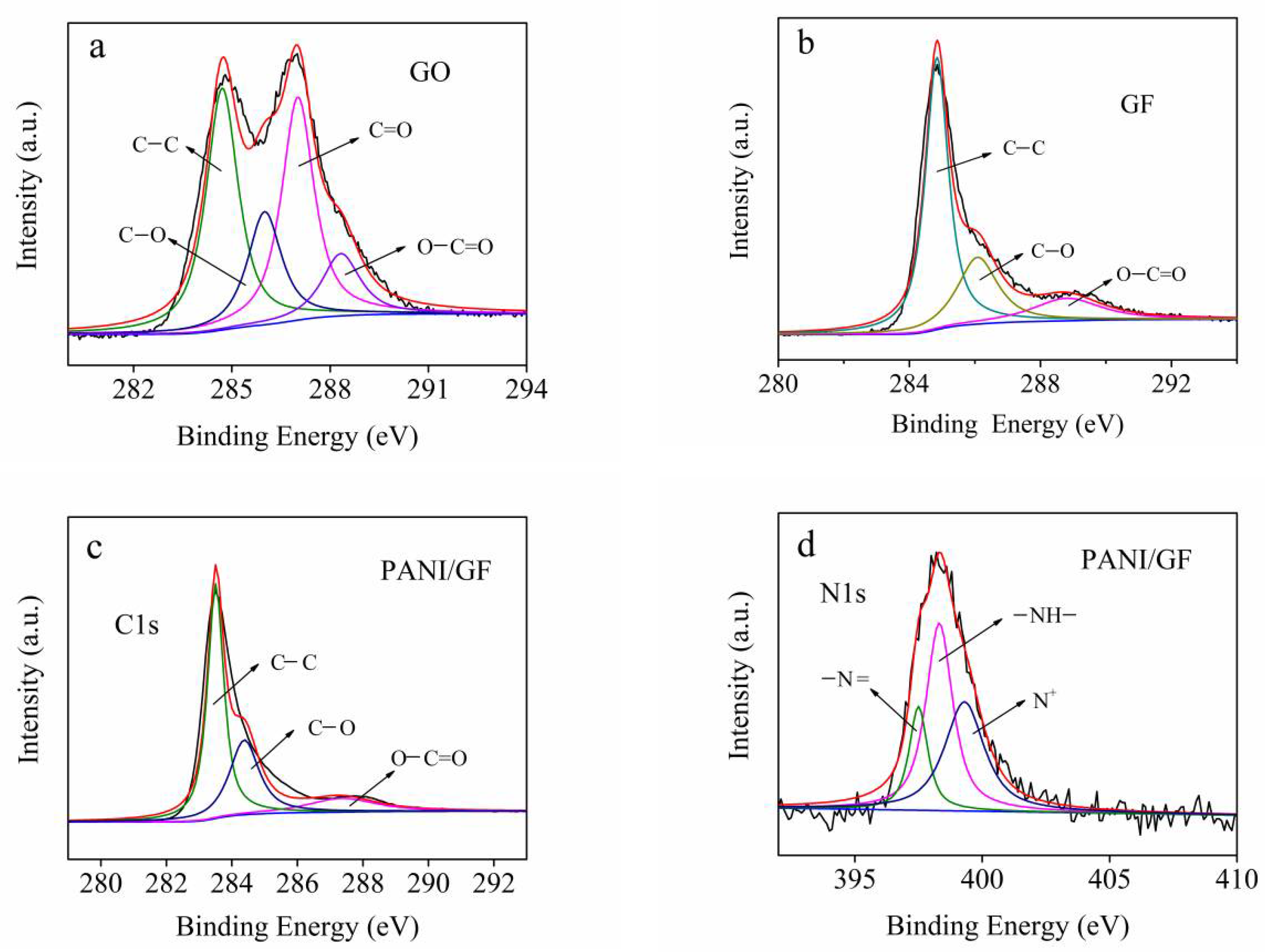
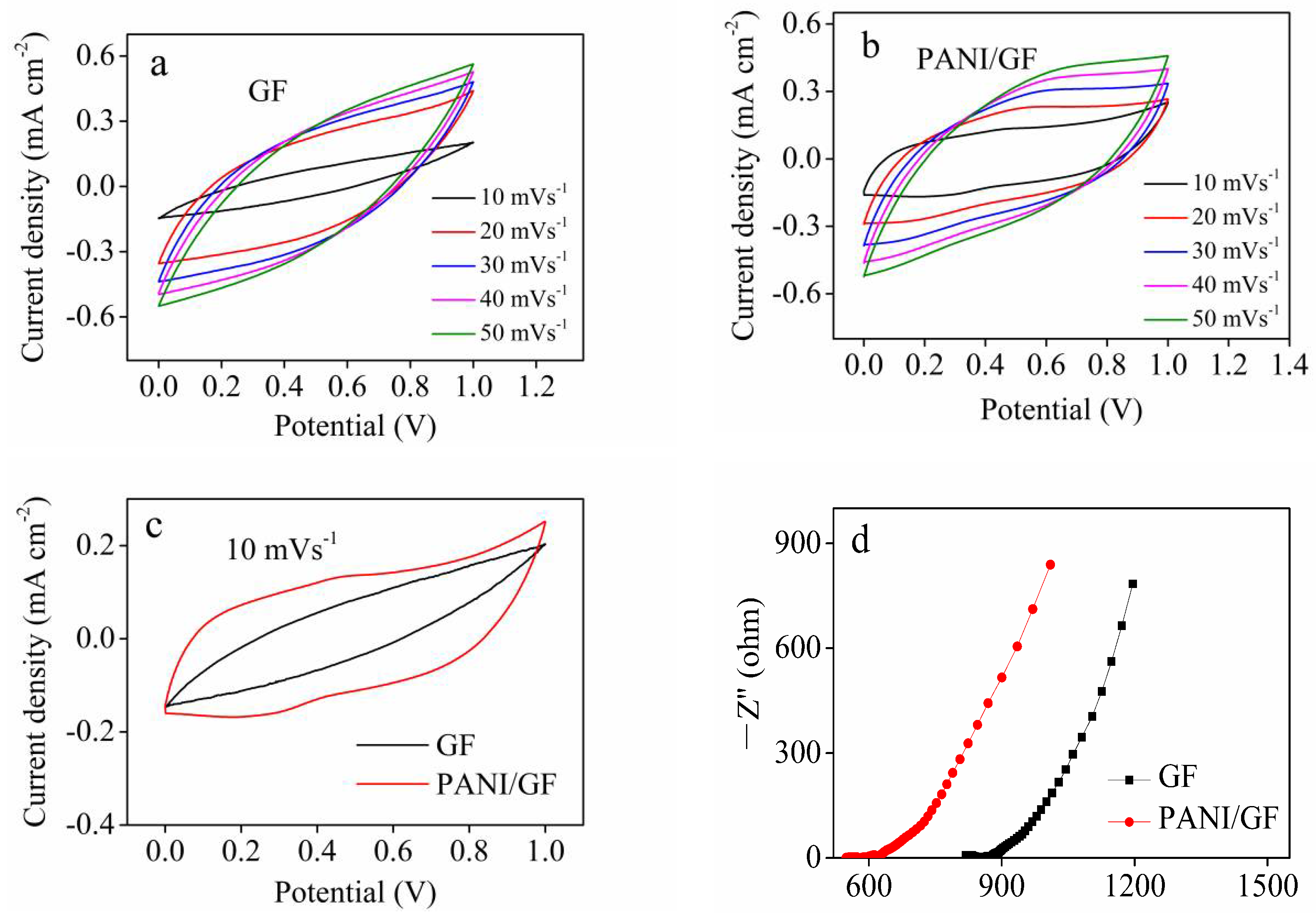
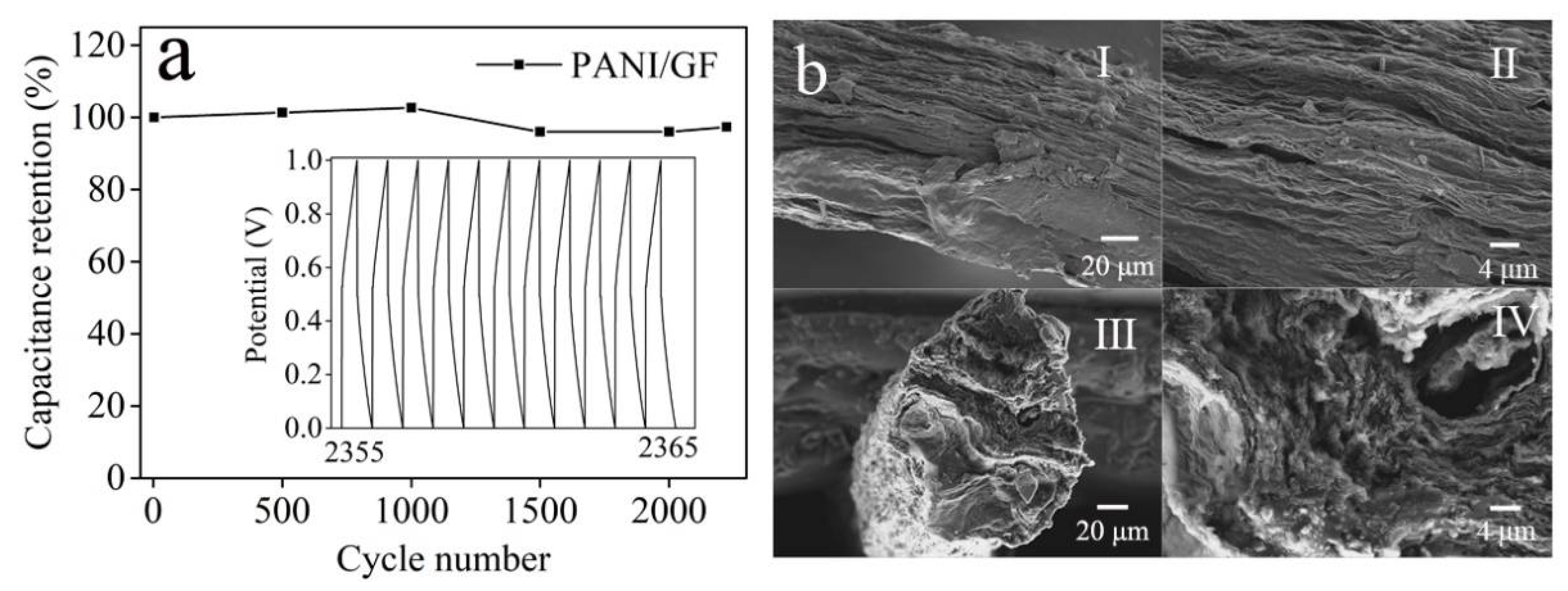
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Wang, C.H.; Lu, W.; Dai, L. Recent Advances in Fiber-Shaped Supercapacitors and Lithium-Ion Batteries. Adv. Mater. 2020, 32, e1902779. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Ye, L.; Zhang, Y.; Chen, T.; Peng, H. The Recent Advance in Fiber-Shaped Energy Storage Devices. Adv. Electron. Mater. 2019, 5, 1800456. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lou, Z.; Chen, D.; Jiang, K.; Han, W.; Shen, G. Recent Advances in Flexible/Stretchable Supercapacitors for Wearable Electronics. Small 2018, 14, e1702829. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, K.; Huang, T.; Shen, G. Recent Advances in Fiber Supercapacitors: Materials, Device Configurations, and Applications. Adv. Mater. 2020, 32, e1901806. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Karahan, H.E.; Wang, C.; Pei, Z.; Wei, L.; Chen, Y. 1D Supercapacitors for Emerging Electronics: Current Status and Future Directions. Adv. Mater. 2019, 32, e1902387. [Google Scholar] [CrossRef] [PubMed]
- Tebyetekerwa, M.; Marriam, I.; Xu, Z.; Yang, S.; Zhang, H.; Zabihi, F.; Jose, R.; Peng, S.; Zhu, M.; Ramakrishna, S. Critical insight: Challenges and requirements of fibre electrodes for wearable electrochemical energy storage. Energy Environ. Sci. 2019, 12, 2148–2160. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Yang, X.Y.; Liu, T.; Zhang, X.B. Flexible 1D Batteries: Recent Progress and Prospects. Adv. Mater. 2020, 32, e1901961. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Zhao, Y.; Chen, N.; Qu, L. Graphene-based fibers for supercapacitor applications. Nanotechnology 2016, 27, 032001. [Google Scholar] [CrossRef]
- Qu, G.; Cheng, J.; Li, X.; Yuan, D.; Chen, P.; Chen, X.; Wang, B.; Peng, H. A Fiber Supercapacitor with High Energy Density Based on Hollow Graphene/Conducting Polymer Fiber Electrode. Adv. Mater. 2016, 28, 3646–3652. [Google Scholar] [CrossRef]
- Cheng, H.; Meng, J.; Wu, G.; Chen, S. Hierarchical Micro-Mesoporous Carbon-Framework-Based Hybrid Nanofibres for High-Density Capacitive Energy Storage. Angew. Chem. Int. Ed. Engl. 2019, 58, 17465–17473. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Yang, T.; Zhang, P.; Wei, X.; Zhang, L.; Li, H. Polyaniline/graphene hybrid fibers as electrodes for flexible supercapacitors. Synth. Met. 2020, 268, 116484. [Google Scholar] [CrossRef]
- Qiu, H.; Cheng, H.; Meng, J.; Wu, G.; Chen, S. Magnetothermal Microfluidic-Assisted Hierarchical Microfibers for Ultrahigh-Energy-Density Supercapacitors. Angew. Chem. Int. Ed. Engl. 2020, 59, 7934–7943. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. Graphene chiral liquid crystals and macroscopic assembled fibres. Nat. Commun. 2011, 2, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Shao, Y.; Jiang, P.; Zhang, Q.; Hou, C.; Li, Y.; Wang, H. 1T-Molybdenum disulfide/reduced graphene oxide hybrid fibers as high strength fibrous electrodes for wearable energy storage. J. Mater. Chem. A 2019, 7, 3143–3149. [Google Scholar] [CrossRef]
- Meng, J.; Nie, W.; Zhang, K.; Xu, F.; Ding, X.; Wang, S.; Qiu, Y. Enhancing Electrochemical Performance of Graphene Fiber-Based Supercapacitors by Plasma Treatment. ACS Appl. Mater. Interfaces 2018, 10, 13652–13659. [Google Scholar] [CrossRef]
- Li, Z.; Wei, J.; Ren, J.; Wu, X.; Wang, L.; Pan, D.; Wu, M. Hierarchical construction of high-performance all-carbon flexible fiber supercapacitors with graphene hydrogel and nitrogen-doped graphene quantum dots. Carbon 2019, 154, 410–419. [Google Scholar] [CrossRef]
- Gao, L.; Song, J.; Surjadi, J.U.; Cao, K.; Han, Y.; Sun, D.; Tao, X.; Lu, Y. Graphene-Bridged Multifunctional Flexible Fiber Supercapacitor with High Energy Density. ACS Appl. Mater. Interfaces 2018, 10, 28597–28607. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yang, D.; Fan, Z.; Li, X.; Liu, Y.; Guo, C.; Zhang, M.; Yu, Z.Z. Reduced graphene oxide/carbon nanotube hybrid fibers with narrowly distributed mesopores for flexible supercapacitors with high volumetric capacitances and satisfactory durability. Carbon 2019, 152, 134–143. [Google Scholar] [CrossRef]
- Zhai, S.; Wang, C.; Karahan, H.E.; Wang, Y.; Chen, X.; Sui, X.; Huang, Q.; Liao, X.; Wang, X.; Chen, Y. Nano-RuO2-Decorated Holey Graphene Composite Fibers for Micro-Supercapacitors with Ultrahigh Energy Density. Small 2018, 14, e1800582. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, X.; Xu, J.; Zou, L.; Nie, W.; Hu, X.; Dai, S.; Qiu, Y.; Yuan, N. Highly stretchable CNT/MnO2 nanosheets fiber supercapacitors with high energy density. J. Mater. Sci. 2020, 55, 8251–8263. [Google Scholar] [CrossRef]
- Ramesh, S.; Lee, Y.J.; Shin, K.; Sanjeeb, L.; Karuppasamy, K.; Vikraman, D.; Kathalingam, A.; Kim, H.S.; Kim, H.S.; Kim, J.H. Effect of ruthenium oxide on the capacitance and gas-sensing performances of cobalt oxide @nitrogen-doped graphene oxide composites. Int. J. Energy Res. 2021, 45. [Google Scholar] [CrossRef]
- Ramesh, S.; Karuppasamy, K.; Vikraman, D.; Kim, E.; Sanjeeb, L.; Lee, Y.J.; Kim, H.S.; Kim, J.H.; Kim, H.S. Hierarchical Co3O4 decorated nitrogen-doped graphene oxide nanosheets for energy storage and gas sensing applications. J. Ind. Eng. Chem. 2021, 101, 253–261. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, S.; Yuan, Z.; Chen, J.; Zhang, X.; Huang, Q.; Wang, Y.; Liao, X.; Wei, L.; Chen, Y. A core-sheath holey graphene/graphite composite fiber intercalated with MoS2 nanosheets for high-performance fiber supercapacitors. Electrochim. Acta 2019, 305, 493–501. [Google Scholar] [CrossRef]
- Zhou, C.; Hong, M.; Yang, Y.; Yang, C.; Hu, N.; Zhang, L.; Yang, Z.; Zhang, Y. Laser-induced bi-metal sulfide/graphene nanoribbon hybrid frameworks for high-performance all-in-one fiber supercapacitors. J. Power Sources 2019, 438, 227044. [Google Scholar] [CrossRef]
- Vikraman, D.; Karuppasamy, K.; Hussain, S.; Kathalingam, A.; Sanmugam, A.; Jung, J.; Kim, H.S. One-pot facile methodology to synthesize MoS2-graphene hybrid nanocomposites for supercapacitors with improved electrochemical capacitance. Compos. Part B 2019, 161, 555–563. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, L.; Wang, W.; Ouyang, L.; Xue, H. Polyaniline-Modified Hierarchical Graphene Fiber for Ultrahigh-Performance Electrochemical Supercapacitor with Carbon Fiber in Core as Current Collector. Energy Technol. 2019, 7, 1900522. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Peng, L.; Zhao, F.; Xiao, D.; Jin, Y.; Yu, G. Stretchable All-Gel-State Fiber-Shaped Supercapacitors Enabled by Macromolecularly Interconnected 3D Graphene/Nanostructured Conductive Polymer Hydrogels. Adv. Mater. 2018, 30, e1800124. [Google Scholar] [CrossRef]
- Ramesh, S.; Yadav, H.M.; Karuppasamy, K.; Vikraman, D.; Kim, H.S.; Kim, J.H.; Kim, H.S. Fabrication of manganese oxide@nitrogen doped graphene oxide/polypyrrole (MnO2@NGO/PPy) hybrid composite electrodes for energy storage devices. J. Mater. Res. Technol. 2019, 8, 4227–4238. [Google Scholar] [CrossRef]
- Zheng, J.; Miao, F.; Peng, Y.; Miao, F. The fabrication of hierarchical nanostructured graphene/PPy fiber composites and its electrochemical properties. Ionics 2020, 26, 2667–2671. [Google Scholar] [CrossRef]
- Almeida, D.A.L.; Couto, A.B.; Ferreira, N.G. Flexible polyaniline/reduced graphene oxide/carbon fiber composites applied as electrodes for supercapacitors. J. Alloy. Compd. 2019, 788, 453–460. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, H.; Wu, X.; Wang, C.F.; Wu, G.; Chen, S. Enriched carbon dots/graphene microfibers towards high-performance micro-supercapacitors. J. Mater. Chem. A 2018, 6, 14112–14119. [Google Scholar] [CrossRef]
- Wu, X.; Wu, G.; Tan, P.; Cheng, H.; Hong, R.; Wang, F.; Chen, S. Construction of microfluidic-oriented polyaniline nanorod arrays/graphene composite fibers for application in wearable micro-supercapacitors. J. Mater. Chem. A 2018, 6, 8940–8946. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Huang, T.; Cai, S.; Chen, H.; Jiang, Y.; Wang, S.; Gao, C. Continuous fabrication of the graphene-confined polypyrrole film for cycling stable supercapacitors. J. Mater. Chem. A 2017, 5, 8255–8260. [Google Scholar] [CrossRef]
- Fan, T.; Tong, S.; Zeng, W.; Niu, Q.; Liu, Y.; Kao, C.Y.; Liu, J.; Huang, W.; Min, Y.; Epstein, A.J. Self-assembling sulfonated graphene/polyaniline nanocomposite paper for high performance supercapacitor. Synth. Met. 2015, 199, 79–86. [Google Scholar] [CrossRef]
- Zhao, H.B.; Yang, J.; Lin, T.T.; Lu, Q.F.; Chen, G. Nanocomposites of sulfonic polyaniline nanoarrays on graphene nanosheets with an improved supercapacitor performance. Chemistry 2015, 21, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Hu, K.; Chen, X.; Yin, H. High conductivity graphene-like MoS2/polyaniline nanocomposites and its application in supercapacitor. J. Alloy. Compd. 2015, 619, 38–43. [Google Scholar] [CrossRef]
- Guo, F.; Kim, F.; Han, T.H.; Shenoy, V.B.; Huang, J.X.; Hurt, R.H. Hydration-Responsive Folding and Unfolding in Graphene Oxide Liquid Crystal Phases. ACS Nano 2011, 5, 8019–8025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Jia, M. High rate capability and long cycle stability Fe3O4–graphene nanocomposite as anode material for lithium ion batteries. J. Alloy. Compd. 2013, 551, 53–60. [Google Scholar] [CrossRef]
- Liu, H.; Du, H.L.; Zhao, W.; Qiang, X.J.; Zheng, B.; Li, Y.; Cao, B. Fast potassium migration in mesoporous carbon with ultrathin framework boosting superiorrateper formance for high-power potassium storage. Energy Stor. Mater. 2021, 40, 490–498. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Jia, M. Three-Dimensional Reduced Graphene Oxides Hydrogel Anchored with Ultrafine CoO Nanoparticles as Anode for Lithium Ion Batteries. Electrochim. Acta 2014, 129, 425–432. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.; Chen, Y.; Li, C.L.; Tang, J.; Li, Q. Synthesis of three-dimensional nitrogen-doped graphene/polyaniline hydrogels for high performance supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 10674–10683. [Google Scholar] [CrossRef]
- Feng, X.; Chen, N.; Zhou, J.; Li, Y.; Huang, Z.; Zhang, L.; Ma, Y.; Wang, L.; Yan, X. Facile synthesis of shape-controlled graphene–polyaniline composites for high performance supercapacitor electrode materials. New J. Chem. 2015, 39, 2261–2268. [Google Scholar] [CrossRef]
- Li, H.; Song, J.; Wang, L.; Feng, X.; Liu, R.; Zeng, W.; Huang, Z.; Ma, Y.; Wang, L. Flexible all-solid-state supercapacitors based on polyaniline orderly nanotubes array. Nanoscale 2017, 9, 193–200. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, M.; Li, H.; Wang, J.; Guan, F. Controllable synthesis and electrochemical performance of hierarchically structured graphene fibers. Mater. Chem. Phys. 2017, 193, 35–41. [Google Scholar] [CrossRef]
- Li, X.; Zhao, T.; Chen, Q.; Li, P.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Wei, B.; Zhu, H. Flexible all solid-state supercapacitors based on chemical vapor deposition derived graphene fibers. Phys. Chem. Chem. Phys. 2013, 15, 17752–17757. [Google Scholar] [CrossRef]
- Cai, S.Y.; Huang, T.Q.; Chen, H.; Salman, M.; Gopalsamy, K.; Gao, C. Wet-spinning of ternary synergistic coaxial fibers for high performance yarn supercapacitors. J. Mater. Chem. A 2017, 5, 22489–22494. [Google Scholar] [CrossRef]
- Zheng, X.; Yao, L.; Qiu, Y.; Wang, S.; Zhang, K. Core–Sheath Porous Polyaniline Nanorods/Graphene Fiber-Shaped Supercapacitors with High Specific Capacitance and Rate Capability. ACS Appl. Energy Mater. 2019, 2, 4335–4344. [Google Scholar] [CrossRef]
- Huang, T.; Zheng, B.; Kou, L.; Gopalsamy, K.; Xu, Z.; Gao, C.; Meng, Y.; Wei, Z. Flexible high performance wet-spun graphene fiber supercapacitors. RSC Adv. 2013, 3, 23957. [Google Scholar] [CrossRef]
- Chen, S.; Ma, W.; Cheng, Y.; Weng, Z.; Sun, B.; Wang, L.; Chen, W.; Li, F.; Zhu, M.; Cheng, H.M. Scalable non-liquid-crystal spinning of locally aligned graphene fibers for high-performance wearable supercapacitors. Nano Energy 2015, 15, 642–653. [Google Scholar] [CrossRef]
- Li, B.; Cheng, J.; Wang, Z.; Li, Y.; Ni, W.; Wang, B. Highly-wrinkled reduced graphene oxide-conductive polymer fibers for flexible fiber-shaped and interdigital-designed supercapacitors. J. Power Sources 2018, 376, 117–124. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, Y.; Hu, C.; Hu, Y.; Dong, Z.; Chen, N.; Zhang, Z.; Qu, L. Spinning fabrication of graphene/polypyrrole composite fibers for all-solid-state, flexible fibriform supercapacitors. J. Mater. Chem. A 2014, 2, 12355. [Google Scholar] [CrossRef]

| Electrode Materials | Electrolyte | Areal Capacitance or Volumetric Capacitance | Power Density | Energy Density | Ref. |
|---|---|---|---|---|---|
| GF or PANI/GF | PVA/H3PO4 | 3.3 or 66.6 mF cm−2 | - | - | [49] |
| Graphene fiber | PVA/H2SO4 | 226 mF cm−3 | 57.7 mW cm−3 | 7.03 mW h cm−3 | [50] |
| Graphene fiber | PVA/H2SO4 or PVDF/ EMIMBF4 | 36.25 mF cm−2 | 0.02 mW cm−2 | 0.8 μW h cm−2 or 18.12 μW h cm−2 | [15] |
| RGO/PEDOT fiber | PVA/H3PO4 | 304.5 mF cm−2 | 66.5 μW cm−2 | 27.1 μW h cm−2 | [9] |
| rGO/PEDOT fiber | PVA/H3PO4 | 131 mF cm−2 | 125 μW cm−2 | 4.55 μW h cm−2 | [51] |
| PPy/GF | PVA/H2SO4 | 95–105 mF cm−2 | - | 6.6–9.7 μW h cm−2 | [52] |
| PANI/G fiber | PVA/H3PO4 or EMITFSI/PVDF-HFP | 230 mF cm−2 | 15 mW cm−2 | 37.2 μW h cm−2 | [32] |
| PANI/GF | PVA/H2SO4 | 357.1 mF cm−2 | 0.23 mW cm−2 | 7.93 μW h cm−2 | [48] |
| PANI/GF | PVA/H3PO4 or EMITFSI/PVDF-HFP | 87.8 mF cm−2 | 0.23 mW cm−2 | 12.2 μW h cm−2 | [11] |
| PANI/GF | PVA/H2SO4 | 370.2 mF cm−2 | 25.3 μW cm−2 | 12.9 μW h cm−2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Qiu, Y.; Zhang, M.; Zhang, L.; Li, H. Facile Fabrication of Polyaniline/Graphene Composite Fibers as Electrodes for Fiber-Shaped Supercapacitors. Appl. Sci. 2021, 11, 8690. https://doi.org/10.3390/app11188690
Yang X, Qiu Y, Zhang M, Zhang L, Li H. Facile Fabrication of Polyaniline/Graphene Composite Fibers as Electrodes for Fiber-Shaped Supercapacitors. Applied Sciences. 2021; 11(18):8690. https://doi.org/10.3390/app11188690
Chicago/Turabian StyleYang, Xuefei, Yihan Qiu, Mei Zhang, Liangjing Zhang, and Hongwei Li. 2021. "Facile Fabrication of Polyaniline/Graphene Composite Fibers as Electrodes for Fiber-Shaped Supercapacitors" Applied Sciences 11, no. 18: 8690. https://doi.org/10.3390/app11188690
APA StyleYang, X., Qiu, Y., Zhang, M., Zhang, L., & Li, H. (2021). Facile Fabrication of Polyaniline/Graphene Composite Fibers as Electrodes for Fiber-Shaped Supercapacitors. Applied Sciences, 11(18), 8690. https://doi.org/10.3390/app11188690





