Identification of Bioactive Plant Volatiles for the Carob Moth by Means of GC-EAD and GC-Orbitrap MS
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Identification of Bioactive Compounds
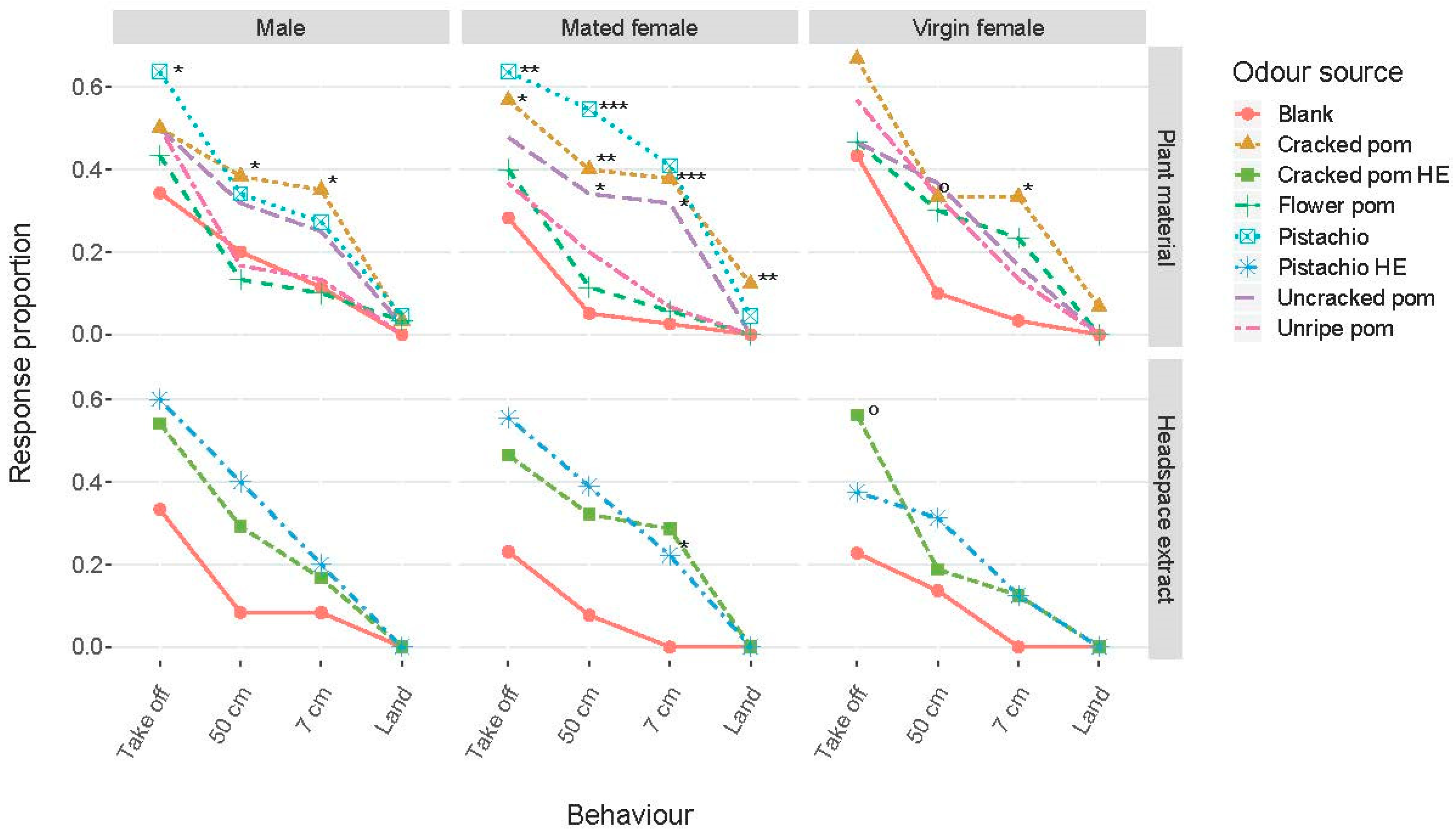
3.2. Biological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Kooner, R.; Arora, R. Insect Pests and Crop Losses. In Breeding Insect Resistant Crops for Sustainable Agriculture; Arora, R., Sandhu, S., Eds.; Springer: Singapore, 2017; pp. 45–66. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, S.; Demissew, S.; Carabias, J.; Joly, C.; Lonsdale, M.; Ash, N.; Larigauderie, A.; Adhikari, J.R.; Arico, S.; Báldi, A.; et al. The IPBES Conceptual Framework—Connecting nature and people. Curr. Opin. Environ. Sustain. 2014, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M. IPBES Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Visseren-Hamakers, I.J., Willis, K.J., Zayas, C.N., Eds.; IPBES Secretariat: Bonn, Germany, 2019; 56p. [Google Scholar]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Gut, L.J. Mating Disruption for the 21st Century: Matching Technology with Mechanism. Environ. Èntomol. 2015, 44, 427–453. [Google Scholar] [CrossRef] [Green Version]
- Cusumano, A.; Harvey, J.; E Bourne, M.; Poelman, E.H.; De Boer, J.G. Exploiting chemical ecology to manage hyperparasitoids in biological control of arthropod pests. Pest Manag. Sci. 2019, 76, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Caswell, H. Matrix Population Models: Construction, Analysis, and Interpretation, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Preti, M.; Knight, A.L.; Mujica, M.V.; Basoalto, E.; Herrera, S.L.; Tasin, M.; Angeli, S. Development of multi-component non-sex pheromone blends to monitor both sexes of Cydia pomonella (Lepidoptera: Tortricidae). J. Appl. Èntomol. 2021, 145, 822–830. [Google Scholar] [CrossRef]
- Isman, M.B. Insect Chemical Ecology—A Physiological Perspective; Chapman & Hall: New York, NY, USA, 1992; pp. 156–176. [Google Scholar]
- Szendrei, Z.; Rodriguez-Saona, C. A meta-analysis of insect pest behavioral manipulation with plant volatiles. Èntomol. Exp. Appl. 2010, 134, 201–210. [Google Scholar] [CrossRef]
- Wink, M. Quinolizidine and Pyrrolizidine Alkaloid Chemical Ecology—A Mini-Review on Their Similarities and Differences. J. Chem. Ecol. 2018, 45, 109–115. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M. Evolution of Insect Olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Shuttleworth, A.; Johnson, S.D. Using two confluent capillary columns for improved gas chromatography-electroantennographic detection (GC-EAD). Èntomol. Exp. Appl. 2020, 168, 191–197. [Google Scholar] [CrossRef]
- Sapozhnikova, Y. Non-targeted screening of chemicals migrating from paper-based food packaging by GC-Orbitrap mass spectrometry. Talanta 2021, 226, 122120. [Google Scholar] [CrossRef]
- Domínguez, I.; Arrebola, F.J.; Vidal, J.L.M.; Frenich, A.G. Assessment of wastewater pollution by gas chromatography and high resolution Orbitrap mass spectrometry. J. Chromatogr. A 2020, 1619, 460964. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, H.; Liu, R.; Xiang, P.; Deng, K.; Zhang, J.; Tuo, Y.; Wang, Z.; Huang, P. Exploring metabolic alterations associated with death from asphyxia and the differentiation of asphyxia from sudden cardiac death by GC-HRMS-based untargeted metabolomics. J. Chromatogr. B 2021, 1171, 122638. [Google Scholar] [CrossRef]
- Guo, P.; Lin, E.Z.; Koelmel, J.P.; Ding, E.; Gao, Y.; Deng, F.; Dong, H.; Liu, Y.; Cha, Y.; Fang, J.; et al. Exploring personal chemical exposures in China with wearable air pollutant monitors: A repeated-measure study in healthy older adults in Jinan, China. Environ. Int. 2021, 156, 106709. [Google Scholar] [CrossRef] [PubMed]
- Nay, J.E.; Boyd, E.A.; Perring, T.M. Reduction of carob moth in ‘Deglet Noor’ dates using a bunch cleaning tool. Crop. Prot. 2006, 25, 758–765. [Google Scholar] [CrossRef]
- Vetter, R.; Millar, J.; Vickers, N.; Baker, T. Mating disruption of carob moth, Ectomyelois ceratoniae, with a sex pheromone analog. Southwest. Entomol. 2006, 31, 33–47. [Google Scholar]
- Madge, D. Carob moth Eating your profits? In Proceedings of the 16th Australian Almond Conference, Glenelg, Australia, 28–30 October 2014; p. 33. [Google Scholar]
- Carter, D.J. Pest Lepidoptera of Europe: With Special Reference to the British Isles; Springer: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Kashkuli, A.; Eghtedar, E. Biologie und oekologie von specterobates ceratoniae (lep. pyralidae) in der provinz fars. Entomol. Phytopathol. Appl. 1975, 41, 21–32. [Google Scholar]
- Shakeri, M. A review on investigations on pomegranate neck worm in Iran. In A proceeding on Evaluation of Finding and Current Problems Associated with Spectrobates ceratoniae Management in Pomegranate; Shakeri, M., Ed.; Ministry of Ja-had-e-Keshavarzi, Organization of Research and Education, Yazd Agriculture and Natural Resources Research Center: Tehran, Iran, 2014; p. 18. [Google Scholar]
- Sobhani, M.; Goldansaz, S.H.; Hatami, B.; Hosseini, S.A. A field screening of pomegranate cultivars for resistance to the carob moth, Ectomyelois ceratoniae, and compatibility with its larval parasitoids. Int. J. Pest Manag. 2015, 61, 346–352. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Goldansaz, S.H.; Menken, S.B.J.; Van Wijk, M.; Roessingh, P.; Groot, A.T. Field Attraction of Carob Moth to Host Plants and Conspecific Females. J. Econ. Èntomol. 2017, 110, 2076–2083. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Goldansaz, S.H.; Fotoukkiaii, S.M.; Menken, S.B.; Groot, A.T. Seasonal pattern of infestation by the carob moth Ectomyelois ceratoniae in pomegranate cultivars. Crop. Prot. 2017, 102, 19–24. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Goldansaz, S.H.; Sadeghhasani, S.; Mousavi, S.G. A field screening of 10 high yield pomegranate cultivars for resistance to the carob moth, Ectomyelois ceratoniae, in the climate condition of Karaj, Alborz, Iran. In Proceedings of the 21st Iranian Plant Protection Congress, Urmia, Iran, 23–26 August 2014; p. 754. [Google Scholar]
- Galindo, A.; Rodríguez, P.; González, M.C.; Cruz, Z.; Torrecillas, E.; Ondoño, S.; Corell, M.; Moriana, A. Rainfall intensifies fruit peel cracking in water stressed pomegranate trees. Agric. For. Meteorol. 2014, 194, 29–35. [Google Scholar] [CrossRef]
- Saei, H.; Sharifani, M.M.; Dehghani, A.; Seifi, E.; Akbarpour, V. Description of biomechanical forces and physiological parameters of fruit cracking in pomegranate. Sci. Hortic. 2014, 178, 224–230. [Google Scholar] [CrossRef]
- Guo, H.; Wang, C.-Z. The ethological significance and olfactory detection of herbivore-induced plant volatiles in interactions of plants, herbivorous insects, and parasitoids. Arthropod-Plant Interact. 2019, 13, 161–179. [Google Scholar] [CrossRef]
- Dool, H.V.D.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Kwiecien, N.W.; Bailey, D.J.; Rush, M.J.P.; Cole, J.S.; Ulbrich, A.; Hebert, A.S.; Westphall, M.S.; Coon, J.J. High-Resolution Filtering for Improved Small Molecule Identification via GC/MS. Anal. Chem. 2015, 87, 8328–8335. [Google Scholar] [CrossRef] [Green Version]
- Vaniya, A.; Fiehn, O. Using fragmentation trees and mass spectral trees for identifying unknown compounds in metabolomics. TrAC Trends Anal. Chem. 2015, 69, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochmutt. Reproducibility of Retention Indices. Available online: https://massfinder.com/wiki/Retention_index_guide#Retention_Times2012 (accessed on 19 May 2021).
- Weissbecker, B.; Holighaus, G.; Schütz, S. Gas chromatography with mass spectrometric and electroantennographic detection: Analysis of wood odorants by direct coupling of insect olfaction and mass spectrometry. J. Chromatogr. A 2004, 1056, 209–216. [Google Scholar] [CrossRef]
- Boeker, P.; Leppert, J.; Mysliwietz, B.; Lammers, P.S. Comprehensive Theory of the Deans’ Switch as a Variable Flow Splitter: Fluid Mechanics, Mass Balance, and System Behavior. Anal. Chem. 2013, 85, 9021–9030. [Google Scholar] [CrossRef]
- Li, C.; Cao, J.; Wang, X.; Xu, P.; Wang, X.; Ren, G. Efficacy of an improved method to screen semiochemicals of insect. PeerJ 2021, 9, e11510. [Google Scholar] [CrossRef] [PubMed]
- Beghè, D.; Cirlini, M.; Beneventi, E.; Miroslav, Č.; Tatjana, P.; Ganino, T.; Petruccelli, R.; Dall’Asta, C. Volatile profile of Italian and Montenegrine pomegranate juices for geographical origin classification. Eur. Food Res. Technol. 2020, 247, 211–220. [Google Scholar] [CrossRef]
- Güler, Z.; Gül, E. Volatile organic compounds in the aril juices and seeds from selected five pomegranate (Punica granatum L.) cultivars. Int. J. Food Prop. 2016, 20, 281–293. [Google Scholar] [CrossRef]
- Reidel, R.V.B.; Cioni, P.L.; Pistelli, L. Volatiles from different plant parts of Punica granatum grown in Tuscany (Italy). Sci. Hortic. 2018, 231, 49–55. [Google Scholar] [CrossRef]
- Pawlowski, S.P.; Sweeney, J.D.; Hillier, N.K. Electrophysiological Responses of the Beech Leaf-Mining Weevil, Orchestes fagi, to Seasonally-Variant Volatile Organic Compounds Emitted by American Beech, Fagus grandifolia. J. Chem. Ecol. 2020, 46, 935–946. [Google Scholar] [CrossRef]
- Silva, D.B.; Weldegergis, B.T.; Van Loon, J.J.; Bueno, V.H.P. Qualitative and Quantitative Differences in Herbivore-Induced Plant Volatile Blends from Tomato Plants Infested by Either Tuta absoluta or Bemisia tabaci. J. Chem. Ecol. 2017, 43, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2017, 220, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Ståhl, B. Diversity and Distribution of Floral Scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Wang, R.; Yu, L.-F.; Lu, P.-F.; Luo, Y.-Q. Identification of Caraganaplant volatiles, overlapping profiles, and olfactory attraction to Chlorophorus caraganain the laboratory. J. Plant Interact. 2015, 10, 41–50. [Google Scholar] [CrossRef]
- Tasin, M.; Anfora, G.; Ioriatti, C.; Carlin, S.; De Cristofaro, A.; Schmidt, S.; Bengtsson, M.; Versini, G.; Witzgall, P. Antennal and behavioral responses of grapevine moth Lobesia botrana females to volatiles from grapevine. J. Chem. Ecol. 2005, 31, 77–87. [Google Scholar] [CrossRef]
- Xuan, T.D.; Chung, I.M.; Khanh, T.D.; Tawata, S. Identification of Phytotoxic Substances from Early Growth of Barnyard Grass (Echinochloa crusgalli) Root Exudates. J. Chem. Ecol. 2006, 32, 895–906. [Google Scholar] [CrossRef]
- Hassani-Kakhki, M.; Karimi, J.; El Borai, F.; Killiny, N.; Hosseini, M.; Stelinski, L.L.; Duncan, L. Drought Stress Impairs Communication Between Solanum tuberosum (Solanales: Solanaceae) and Subterranean Biological Control Agents. Ann. Èntomol. Soc. Am. 2019, 113, 23–29. [Google Scholar] [CrossRef]
- Christianson, D.W. Unearthing the roots of the terpenome. Curr. Opin. Chem. Biol. 2008, 12, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Mirabella, R.; Diergaarde, P.J.; Van Doorn, A.; Tissier, A.; Kant, M.; Prins, M.; de Vos, M.; Haring, M.A.; Schuurink, R.C. Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA 2012, 109, 20124–20129. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.C.; Osbourn, A. Plant terpenes that mediate below-ground interactions: Prospects for bioengineering terpenoids for plant protection. Pest Manag. Sci. 2019, 75, 2368–2377. [Google Scholar] [CrossRef] [Green Version]
- Damodaram, K.J.P.; Kempraj, V.; Aurade, R.M.; Rajasekhar, S.B.; Venkataramanappa, R.K.; Nandagopal, B.; Verghese, A. Centuries of domestication has not impaired oviposition site-selection function in the silkmoth, Bombyx mori. Sci. Rep. 2014, 4, 7472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvagnin, U.; Malnoy, M.; Thöming, G.; Tasin, M.; Carlin, S.; Martens, S.; Vrhovsek, U.; Angeli, S.; Anfora, G. Adjusting the scent ratio: Using genetically modified Vitis vinifera plants to manipulate European grapevine moth behaviour. Plant Biotechnol. J. 2017, 16, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Piesik, D.; Delaney, K.J.; Wenda-Piesik, A.; Sendel, S.; Tabaka, P.; Buszewski, B. Meligethes aeneus pollen-feeding suppresses, and oviposition induces, Brassica napus volatiles: Beetle attraction/repellence to lilac aldehydes and veratrole. Chemoecology 2013, 23, 241–250. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, A.; Vázquez-Araújo, L.; Hernández, F.; Martínez, J.J.; Legua, P.; Carbonell-Barrachina, A. Volatile Composition of Pomegranates from 9 Spanish Cultivars Using Headspace Solid Phase Microextraction. J. Food Sci. 2011, 76, S114–S120. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Chambers, E.; Adhikari, K.; Carbonell-Barrachina, A. Physico-chemical and sensory properties of pomegranate juices with pomegranate albedo and carpellar membranes homogenate. LWT Food Sci. Technol. 2011, 44, 2119–2125. [Google Scholar] [CrossRef]
- Bachrouch, O.; Jemâa, J.M.-B.; Wissem, A.W.; Talou, T.; Marzouk, B.; Abderrabba, M. Composition and insecticidal activity of essential oil from Pistacia lentiscus L. against Ectomyelois ceratoniae Zeller and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2010, 46, 242–247. [Google Scholar] [CrossRef]
- Góngora, C.E.; Tapias, J.; Jaramillo, J.; Medina, R.; Gonzalez, S.; Casanova, H.; Ortiz, A.; Benavides, P. Evaluation of Terpene-Volatile Compounds Repellent to the Coffee Berry Borer, Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae). J. Chem. Ecol. 2020, 46, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, R.; Caleb, O.J.; Fawole, O.; Opara, U.L. Effects of different maturity stages and growing locations on changes in chemical, biochemical and aroma volatile composition of ‘Wonderful’ pomegranate juice. J. Sci. Food Agric. 2015, 96, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Anfora, G.; Tasin, M.; De Cristofaro, A.; Ioriatti, C.; Lucchi, A. Synthetic Grape Volatiles Attract Mated Lobesia botrana Females in Laboratory and Field Bioassays. J. Chem. Ecol. 2009, 35, 1054–1062. [Google Scholar] [CrossRef]
- Smit, S.J.; Vivier, M.A.; Young, P.R. Linking Terpene Synthases to Sesquiterpene Metabolism in Grapevine Flowers. Front. Plant Sci. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantaye, C.A.; Köpke, D.; Gershenzon, J.; Degenhardt, J. Restoring (E)-β-Caryophyllene Production in a Non-producing Maize Line Compromises its Resistance against the Fungus Colletotrichum graminicola. J. Chem. Ecol. 2015, 41, 213–223. [Google Scholar] [CrossRef]
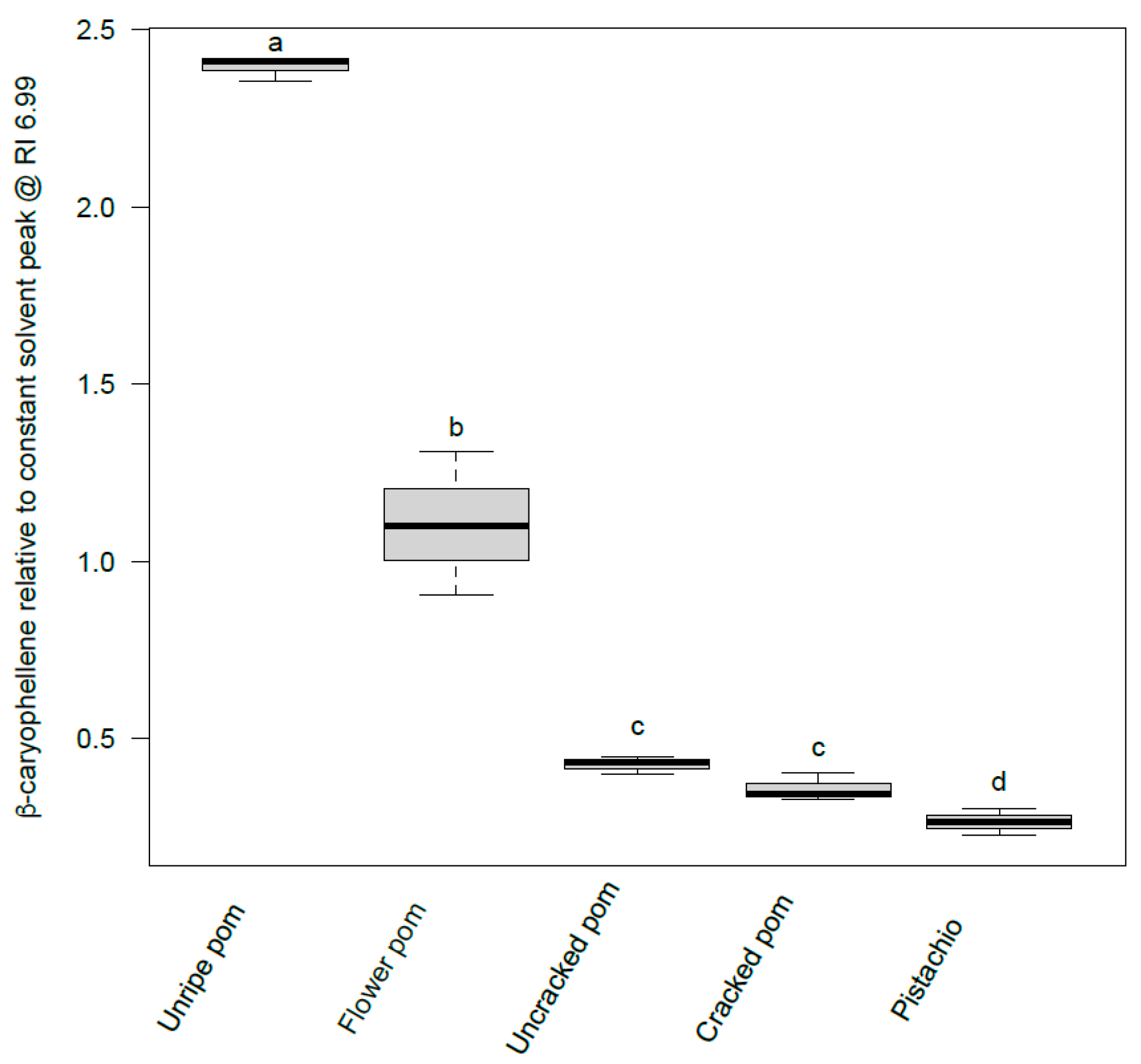
| Peak | RT (min) | EAD | Candidate | TIC (Area/106) | MF 1 | HRF 2 | RI 3 | ΔRI 4 | PCI 5 | Appm 6 | FISh 7 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6.26 | N | Hexanal | 9.79 | C6H12O | 98.8 | n/a * | n/a | + | 0.45 | + |
| 2 | 7.06 | N | trans-3-Hexen-1-ol | 5.69 | C6H12O | 99.3 | 834 | 18 | - | n/a | + |
| 3 | 7.53 | N | cis-3-Hexen-1-ol | 496 | C6H12O | 99.8 | 861 | 4 | - | n/a | + |
| 4 | 9.48 | Y | α-Pinene | 969 | C10H16 | 98.3 | 945 | 3 | + | −0.67 | + |
| 5 | 34.08 | Y | β-Caryophyllene | 244 | C15H24 | 99.9 | 1424 | 5 | + | 0.78 | + |
| 6 | 34.75 | Y | cis-α-Bergamotene | 3110 | C15H24 | 99.7 | 1435 | 20 | + | 0.65 | + |
| 7 | 35.16 | Y | β-Cedrene | 455 | C15H24 | 99.7 | 1442 | 21 | + | 0.57 | + |
| 8 | 35.78 | Y | cis-β-Farnesene | 468 | C15H24 | 99.8 | 1452 | 12 | + | 0.48 | + |
| 9 | 35.92 | Y | Isogermacrene D | 468 | C15H24 | 99.7 | 1455 | 7 | + | 0.09 | + |
| 10 | 36.23 | Y | α-Humulene | 574 | C15H24 | 99.8 | 1460 | 6 | + | 0.49 | + |
| 11 | 37.83 | Y | 9-epi-(E)-Caryophyllene | 478 | C15H24 | 99.8 | 1486 | 8 | + | 0.21 | + |
| 12 | 38.67 | Y | Pentadecane ** | 1380 | C15H32 | n/a | 1500 | 0 | - | n/a | + |
| 13 | 39.18 | Y | Valencene | 438 | C15H24 | 99.5 | 1509 | 9 | + | −0.03 | + |
| 14 | 39.95 | Y | Ethyl-4-ethoxy benzoate | 916 | C11H14O3 | 98.0 | 1523 | 1 | + | −0.28 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, S.A.; Goldansaz, S.H.; Groot, A.T.; Menken, S.B.J.; Van Der Wielen, F.; Wissel, C.; Vercammen, J.; De Rijke, E.; Roessingh, P. Identification of Bioactive Plant Volatiles for the Carob Moth by Means of GC-EAD and GC-Orbitrap MS. Appl. Sci. 2021, 11, 8603. https://doi.org/10.3390/app11188603
Hosseini SA, Goldansaz SH, Groot AT, Menken SBJ, Van Der Wielen F, Wissel C, Vercammen J, De Rijke E, Roessingh P. Identification of Bioactive Plant Volatiles for the Carob Moth by Means of GC-EAD and GC-Orbitrap MS. Applied Sciences. 2021; 11(18):8603. https://doi.org/10.3390/app11188603
Chicago/Turabian StyleHosseini, Seyed Ali, Seyed Hossein Goldansaz, Astrid T. Groot, Steph B. J. Menken, Frans Van Der Wielen, Cedric Wissel, Joeri Vercammen, Eva De Rijke, and Peter Roessingh. 2021. "Identification of Bioactive Plant Volatiles for the Carob Moth by Means of GC-EAD and GC-Orbitrap MS" Applied Sciences 11, no. 18: 8603. https://doi.org/10.3390/app11188603
APA StyleHosseini, S. A., Goldansaz, S. H., Groot, A. T., Menken, S. B. J., Van Der Wielen, F., Wissel, C., Vercammen, J., De Rijke, E., & Roessingh, P. (2021). Identification of Bioactive Plant Volatiles for the Carob Moth by Means of GC-EAD and GC-Orbitrap MS. Applied Sciences, 11(18), 8603. https://doi.org/10.3390/app11188603






