Sundew-Inspired Adhesive Hydrogel Threads through Reversible Complexation of Polyphenol and Boronic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of AlBA
2.3. AlBA/TA Hydrogel Thread Formation
2.4. Rheological Characterization
2.5. Measurement of Mechanical Properties
2.6. Release of TA from the AlBA/TA Hydrogel Thread
2.7. Statistical Analysis
3. Results and Discussion
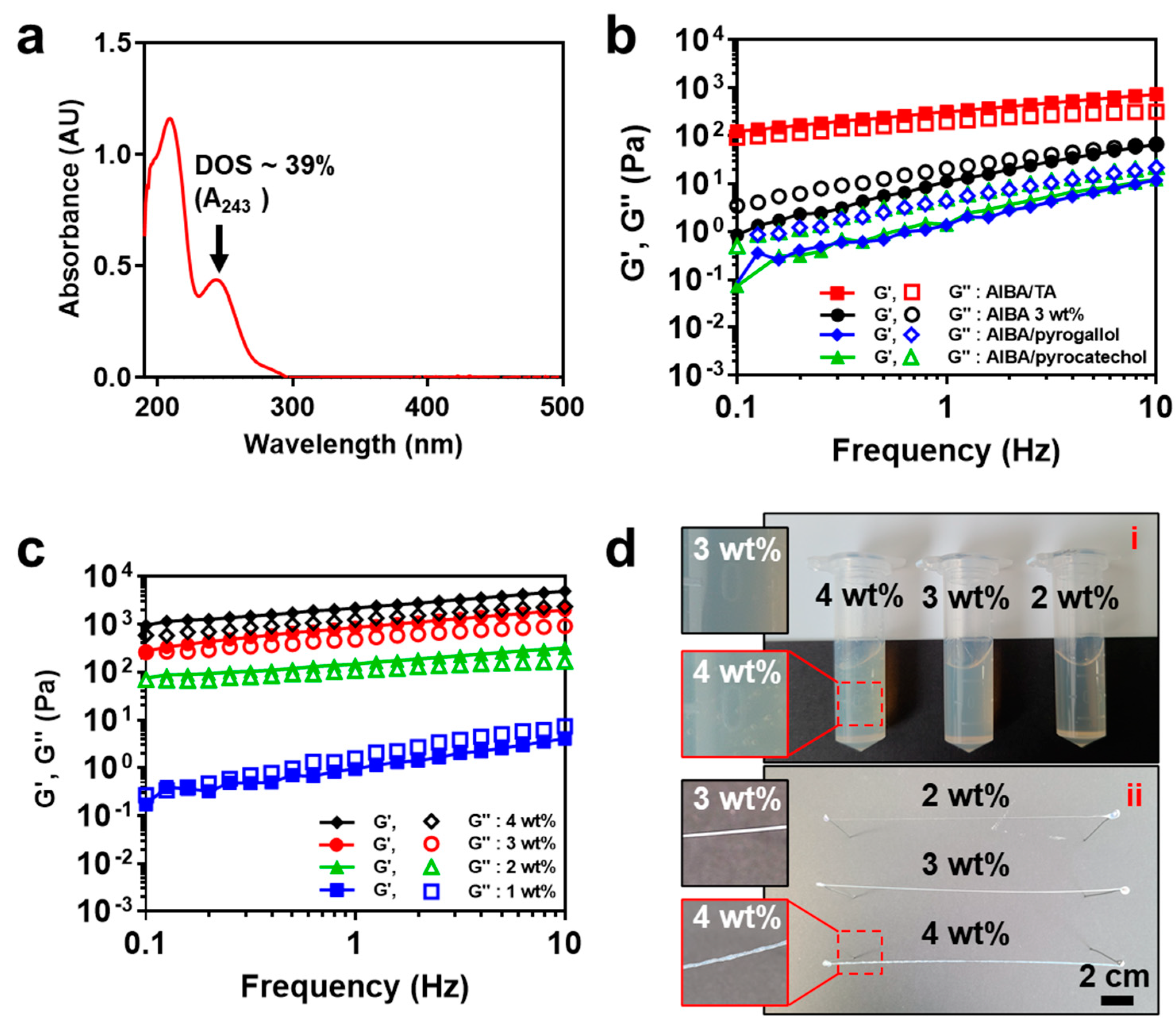
3.1. Characterization of AlBA
3.2. Rheological Behavior of AlBA/TA Hydrogel Thread
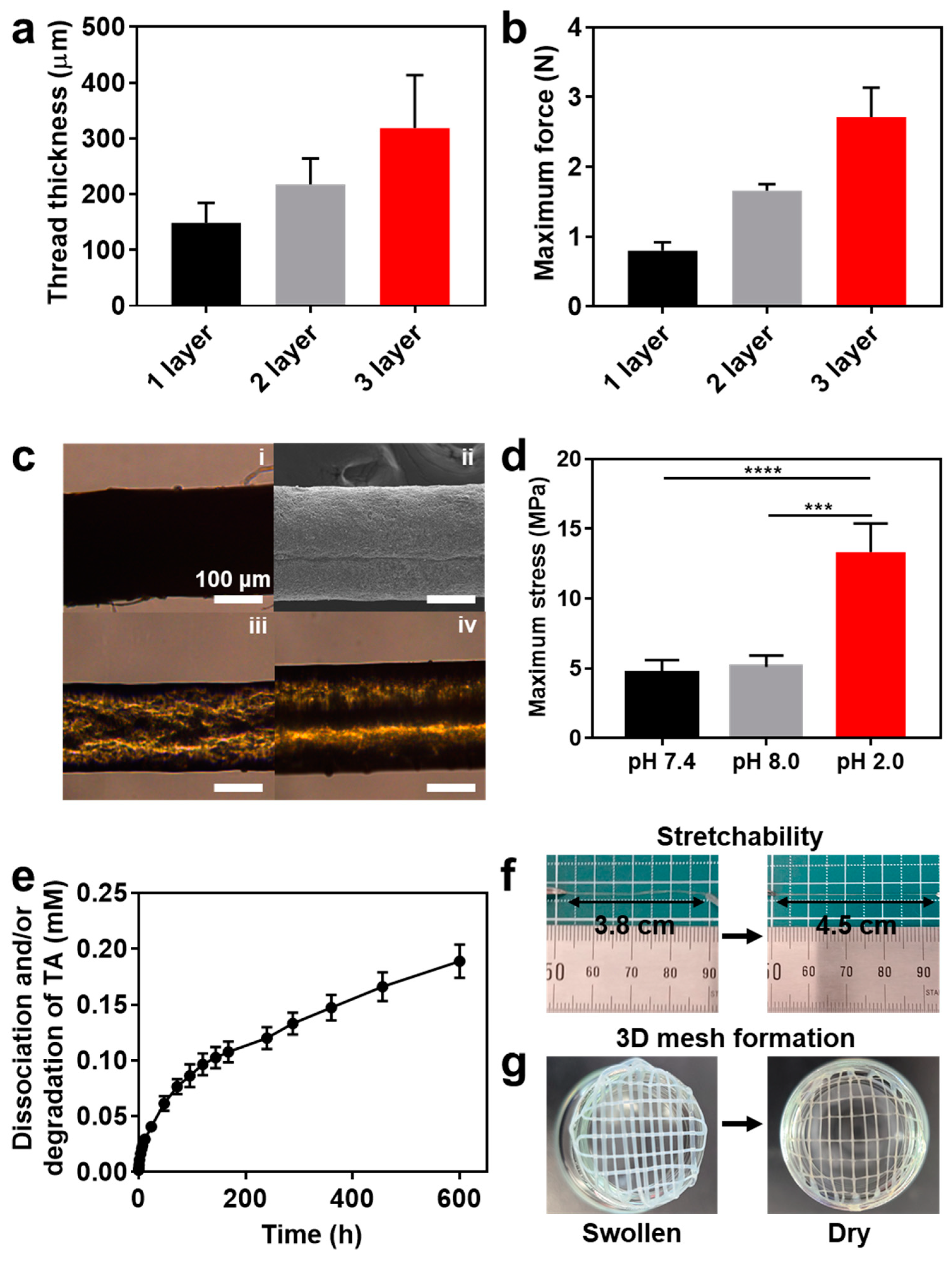
3.3. Characterization of AlBA/TA Hydrogel Thread
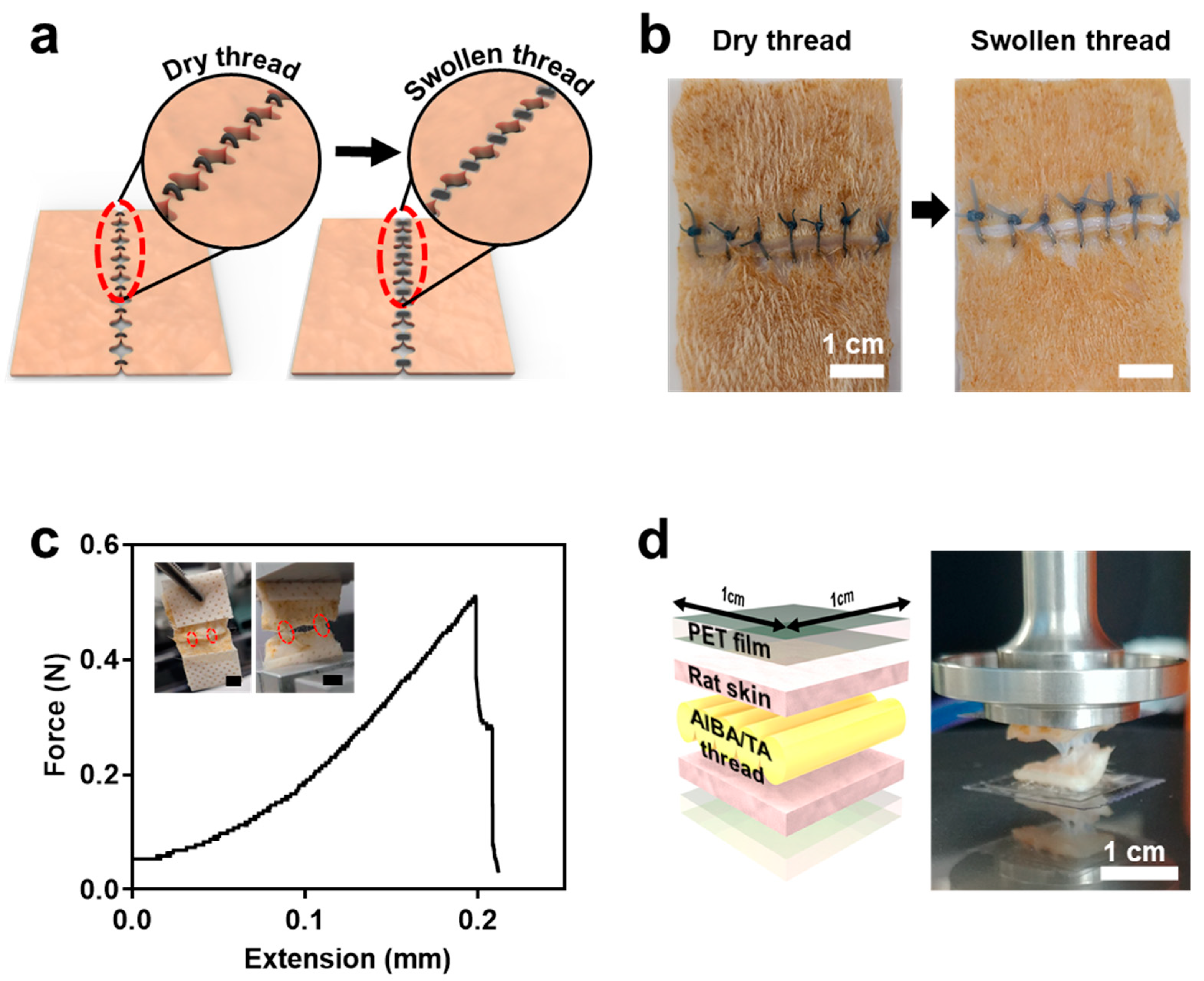
3.4. AlBA/TA Hydrogel Thread as a Tissue Suturing Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.; Shin, M.; Koh, M.Y.; Ryu, J.H.; Lee, M.S.; Hong, S.; Lee, H. TAPE: A medical adhesive inspired by a ubiquitous compound in plants. Adv. Funct. Mater. 2015, 25, 2402–2410. [Google Scholar] [CrossRef]
- Grabowski, N.; Hillaireau, H.; Vergnaud, J.; Santiago, L.A.; Kerdine-Romer, S.; Pallardy, M.; Tsapis, N.; Fattal, E. Toxicity of surface-modified PLGA nanoparticles toward lung alveolar epithelial cells. Int. J. Pharm. 2013, 454, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Luo, J.; Gulgunje, P.V.; Kumar, S. Structural and functional fibers. Annu. Rev. Mater. Res. 2017, 47, 331–359. [Google Scholar] [CrossRef]
- Nary Filho, H.; Matsumoto, M.A.; Batista, A.C.; Lopes, L.C.; de Góes, F.C.; Consolaro, A. Comparative study of tissue response to polyglecaprone 25, polyglactin 910 and polytetrafluorethylene suture materials in rats. Braz. Dent. J. 2002, 13, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, S.R.; Jaber, M.; Aboufanas, S.; Thomas, S.; Al Hujailan, R.G.; Al Qaoud, S.K. Evaluating tensile strengths of absorbable suture materials in herbal solutions: An In vitro study. J. Int. Oral Health 2019, 11, 148. [Google Scholar] [CrossRef]
- Meng, H.; Li, Y.; Faust, M.; Konst, S.; Lee, B.P. Hydrogen peroxide generation and biocompatibility of hydrogel-bound mussel adhesive moiety. Acta Biomater. 2015, 17, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Mi, H.Y.; Lin, Y.J.; Enriquez, E.; Peng, X.F.; Turng, L.S. Highly stretchable and biocompatible strain sensors based on mussel-inspired super-adhesive self-healing hydrogels for human motion monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, J.; Chen, L.; Yu, L.; Xu, J.; Ding, J. Biodegradable and thermoreversible PCLA–PEG–PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials 2011, 32, 4725–4736. [Google Scholar] [CrossRef]
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L.; Wu, Q.; Wei, Y.; Gong, C. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar] [CrossRef]
- Jung, H.; Kim, M.K.; Lee, J.Y.; Choi, S.W.; Kim, J. Adhesive hydrogel patch with enhanced strength and adhesiveness to skin for transdermal drug delivery. Adv. Funct. Mater. 2020, 30, 2004407. [Google Scholar] [CrossRef]
- Abebe, M.W.; Appiah-Ntiamoah, R.; Kim, H. Gallic acid modified alginate self-adhesive hydrogel for strain responsive transdermal delivery. Int. J. Biol. Macromol. 2020, 163, 147–155. [Google Scholar] [CrossRef]
- Do, M.; Im, B.G.; Park, J.P.; Jang, J.H.; Lee, H. Functional polysaccharide sutures prepared by wet fusion of interfacial polyelectrolyte complexation fibers. Adv. Funct. Mater. 2017, 27, 1702017. [Google Scholar] [CrossRef]
- Mirabedini, A.; Foroughi, J.; Romeo, T.; Wallace, G.G. Development and characterization of novel hybrid hydrogel fibers. Macromol. Mater. Eng. 2015, 300, 1217–1225. [Google Scholar] [CrossRef] [Green Version]
- Naficy, S.; Le, T.Y.L.; Oveissi, F.; Lee, A.; Hung, J.C.; Wise, S.G.; Winlaw, D.S.; Dehghani, F. Highly porous, biocompatible tough hydrogels, processable via gel fiber spinning and 3D gel printing. Adv. Mater. Interfaces 2020, 7, 1901770. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Shah, D.U.; Liu, C.; Yu, Z.; Liu, J.; Ren, X.; Rowland, M.J.; Abell, C.; Ramage, M.H.; Scherman, O.A. Bioinspired supramolecular fibers drawn from a multiphase self-assembled hydrogel. Proc. Natl. Acad. Sci. USA 2017, 114, 8163–8168. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wang, Y.; Sun, L.; Agrawal, R.; Zhang, M. Sundew adhesive: A naturally occurring hydrogel. J. R. Soc. Interface J. R. Soc. Interface 2015, 12, 20150226. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, M.; Nue, V.; Birkedal, H. Mussel-inspired materials: Self-healing through coordination chemistry. Chem.–Eur. J. 2016, 22, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, S.; Park, J.P.; Shin, M.; Kim, K.; Ryu, J.H.; Lee, H. Dynamic bonds between boronic acid and alginate: Hydrogels with stretchable, self-healing, stimuli-responsive, remoldable, and adhesive properties. Biomacromolecules 2018, 19, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Blandino, A.; Macias, M.; Cantero, D. Formation of calcium alginate gel capsules: Influence of sodium alginate and CaCl2 concentration on gelation kinetics. J. Biosci. Bioeng. 1999, 88, 686–689. [Google Scholar] [CrossRef]
- Hong, S.H.; Shin, M.; Park, E.; Ryu, J.H.; Burdick, J.A.; Lee, H. Alginate-boronic acid: pH-triggered bioinspired glue for hydrogel assembly. Adv. Funct. Mater. 2020, 30, 1908497. [Google Scholar] [CrossRef]
- Ovesen, L.; Bendtsen, F.; Tage-Jensen, U.; Pedersen, N.T.; Gram, B.R.; Rune, S.J. Intraluminal pH in the stomach, duodenum, and proximal jejunum in normal subjects and patients with exocrine pancreatic insufficiency. Gastroenterology 1986, 90, 958–962. [Google Scholar] [CrossRef]
- IIbekwe, V.C.; Fadda, H.M.; McConnell, E.L.; Khela, M.K.; Evans, D.F.; Basit, A.W. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm. Res. 2008, 25, 1828–1835. [Google Scholar] [CrossRef]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater. Sci. 2020, 8, 1148–1159. [Google Scholar] [CrossRef]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannic acids against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef]
- Edmiston, C.E., Jr.; Krepel, C.J.; Marks, R.M.; Rossi, P.J.; Sanger, J.; Goldblatt, M.; Graham, M.B.; Rothenburger, S.; Collier, J.; Seabrook, G.R. Microbiology of explanted suture segments from infected and noninfected surgical patients. J. Clin. Microbiol. 2013, 51, 417–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.H.; Son, D.; Shin, M. Sundew-Inspired Adhesive Hydrogel Threads through Reversible Complexation of Polyphenol and Boronic Acid. Appl. Sci. 2021, 11, 8591. https://doi.org/10.3390/app11188591
Choi JH, Son D, Shin M. Sundew-Inspired Adhesive Hydrogel Threads through Reversible Complexation of Polyphenol and Boronic Acid. Applied Sciences. 2021; 11(18):8591. https://doi.org/10.3390/app11188591
Chicago/Turabian StyleChoi, Jae Hyuk, Donghee Son, and Mikyung Shin. 2021. "Sundew-Inspired Adhesive Hydrogel Threads through Reversible Complexation of Polyphenol and Boronic Acid" Applied Sciences 11, no. 18: 8591. https://doi.org/10.3390/app11188591
APA StyleChoi, J. H., Son, D., & Shin, M. (2021). Sundew-Inspired Adhesive Hydrogel Threads through Reversible Complexation of Polyphenol and Boronic Acid. Applied Sciences, 11(18), 8591. https://doi.org/10.3390/app11188591







