Abstract
The aim of this study is to analyze surgical and functional outcomes in order to verify the applicability of surgical treatment guidelines as foreseen by MASCC/ISOO/ASCO 2019. Patients affected by stage 2 MRONJ refractory to conservative management were grouped if underwent surgical resection (Group A) or debridement (Group B). Health-related quality of life was evaluated by using the European Organization for Research and Treatment of Cancer questionnaires, QLQ-C30 and H&N35. Statistical analysis was performed using Wilcoxon/Mann–Whitney test, Kaplan–Meier test, Cox regression model and Cox multivariate regression. Group A showed higher complete healing cases vs. group B. Recurrence rate difference in group A vs. group B was statistically significant. Debridement is an unfavorable prognostic factor when compared to surgical resection (p = 0.0032, HR 4.9). Quality of life mean values showed a marked improvement in Group A and a slight improvement in Group B. Debridement has 4.9 times more risk to develop recurrence when compared to surgical resection. A more satisfactory quality of life was shown in patients subject to resective surgery with an improving trend from baseline. Debridement patients showed more variability of results and an overall negative trend at the end of the 6-month follow-up.
1. Introduction
Medication-related osteonecrosis of the jaws (MRONJ) is a severe adverse event that may arise in patients affected by solid tumors or osteoporosis treated with antiresorptive or antiangiogenetic drugs. According to the American Association of Oral and Maxillofacial Surgeons (AAOMS), diagnosis of MRONJ could be considered in patients under current or previous treatment with antiresorptive and/or antiangiogenetic medications, presenting exposed bone or intra-/extraoral fistulas that allow the bone to be probed, persisting for more than 8 weeks, without history of radiation therapy to the jaws or clear bony metastases to the jaws [1,2].
As this phenomenon was initially observed in patients treated with bisphosphonates, MRONJ was firstly defined as bisphosphonates-related osteonecrosis of the jaws (BRONJ) [3,4]. Further evidences demonstrated that other medications (such as denosumab and antiangiogenetic agents, e.g., bevacizumab, sunitinib) might produce the same adverse events, leading to the current definition of Medication Related Osteonecrosis of the Jaws (MRONJ) [5,6].
Incidence of MRONJ ranges between 0.2 and 6.7% according to drug type, underlying disease (i.e., cancer or osteoporosis), dose, treatment duration and protocol (i.e., single drug or association treatments), and route of administration [7,8,9,10]. Appraisal of the United States Food and Drug Administration’s Adverse Event Reposting System (FAERS) revealed the highest risk for intravenous bisphosphonates, followed by denosumab and oral bisphosphonates, while bevacizumab showed a more favorable profile [7,11].
Etiopathogenesis is multifactorial: age, sex, genetics, comorbidities, metabolic status, obesity, steroids assumption, xerostomia, poor oral hygiene, infection, and inflammation status of the oral cavity have been advocated to play a role [12,13,14]. Although it is still debated in the literature, dental infections are believed to impact more than other factors [15]. Presence of bacteria and inflammation have been demonstrated to stimulate bony resorption via stimulation of osteoclastic activity, thus producing a favorable context for the evolution toward MRONJ [16,17,18].
The AAOMS proposed in 2014 a classification that differentiates MRONJ into five stages according to disease severity [1]. Although it has been questioned, this classification remains the most commonly used among surgeons and clinicians all over the world. Based on the AAOMS classification, the Multinational Association of Supportive Care in Cancer (MASCC), the International Society of Oral Oncology (ISOO), and the American Society of Clinical Oncology (ASCO) released a joined practice guideline to standardize the management of patients developing MRONJ [19]. As clearly stated by the experts, poor evidence has been reported in the published literature and proposed recommendations are mainly based on authors’ formal consensus.
Focusing on treatment recommendations proposed for stage 2 disease acc. AAOMS classification, MASCC/ISOO/ASCO guidelines advise to carry out a strict follow-up, trying to educate patients on optimal oral hygiene and the use of topical medications (such as antimicrobial oral rinses) to minimize avoidable risk factors and to use systemic antibiotic therapy. However, it has been observed that following necrotic bone exposure, bacterial contamination may produce biofilms and membranes that affect the efficacy of systemic therapies [18,20].
The aim of this study is to compare surgical and functional outcomes of patients affected by stage 2 MRONJ acc. AAOMS staging system, receiving surgical debridement or surgical resection and to verify the applicability of treatment guidelines as foreseen by MASC/ISOO/ASCO 2019 in patients refractory to conservative management.
2. Materials and Methods
The authors performed a retrospective comparative study that conformed to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines of patients diagnosed with MRONJ attended to by our Maxillo-Facial Surgery Department, from January 2014 to March 2019. Patient where selected according to the following inclusion criteria: oncologic patients with confirmed stage 2 MRONJ diagnosis, ongoing or previous therapy with zoledronic acid or denosumab, no MRONJ healing after at least 8 weeks of conservative antibiotic therapy and oral hygiene, at least 12 moths of available follow-up data. Patients were excluded if staged as 0, 1 or 3 according to the AAOMS staging system, underwent radiotherapy treatments in the head and neck area, were MRONJ diagnosed non-oncologic patients, treated with different antiresorptive and/or antiangiogenetic drugs than zoledronic acid and denosumab, and presented severe contraindications to local/general anesthesia and surgery.
The following data were retrieved from each included subject: age, gender, type of primary tumor, type of antiresorptive therapy received, MRONJ location. Collected data were organized using Microsoft Excel, version 14.0.7104.5000 (Microsoft Excel 2010 by Microsoft Corporation, Albuquerque, NM, USA) and anonymized. The patients were then grouped in two: group A patients who underwent surgical resection, group B patients who underwent debridement. Studied cohorts were homogeneous with respect of the received antiresorptive therapy (zoledronate or denosumab), age, and sex. All patients were studied by orthopantomography and CT or cone beam CT of the facial skeleton and mandible at baseline. Patients presenting lesions of the jaws of uncertain nature were studied by PET scan.
2.1. Treatment Protocol
All included subjects followed the same conservative treatment protocol during the first 8 weeks since diagnosis, according with recommendations of the Canadian Association of Oral and Maxillofacial Surgeons (CAOMS) and the American Association of Oral and Maxillofacial Surgeons (AAOMS) [1,21]. Careful counseling was carried out to educate patients to the best home oral hygiene (using antimicrobial oral rinses with 0.2% chlorhexidine solutions and tooth brushing) and to follow regular appointment for professional oral hygiene. All included subjects received the same systemic antibiotic therapy consisting of amoxicillin 1.75 g, clavulanic acid 250 mg and metronidazole 1.5 g daily, three days prior surgery, and at least 10 days after surgery.
Failure of conservative therapy was assessed by clinic evaluation. The evolution pattern of the disease was classified as stable or progressive (acc. with MASCC/ISOO/ASCO guidelines [19]) and pre-surgical cone beam CT was taken before surgery. Surgical procedures were carried out according to surgeon’s intraoperative assessment during general anesthesia or local anesthesia with sedation.
Patients enrolled in group A underwent marginal resection procedure. It consisted of necrotic bone removal achieving macroscopically healthy margins, bleeding of the surrounding bone and preserving bony contiguity. Primary closure of mucous lining was achieved after removal of sharp bony margins to minimize traumas to the overlying soft tissues [22,23].
Patients enrolled in group B underwent surgical debridement procedure, which consisted only of removal of sharp bony margins and the eradication of bone sequestrum, if present [22,23]. Systemic antibiotic therapy as previously described was administered in all patients for at least 10 days postoperatively. Surgical specimens underwent histopathological examination to ensure the absence of bone metastasis from the primary tumor.
During the follow-up period, clinical evaluations were carried out at 1, 3, 7, 30 days, 3, 6, 9, 12, 18 months post-surgery. Orthopantomography were taken 1, 3, 6 months and cone beam CT were taken 12 months post-operatively. Clinical symptoms and radiological signs of focal osteosclerosis or osteolysis guided early recurrence diagnosis. All subjects were evaluated to detect local or general post-operative complications.
2.2. Assessment of Clinical Outcome
The primary outcome of the present study was to evaluate differences in healing rate between the studied protocols. The secondary outcome was to assess the achieved primary closure and complete healing of the oral mucous lining using the surgical approach.
Post-operative complications (wound dehiscence, presence of purulent fluids, development of fistulas, recurrence) were detected during the entire follow-up period and charted. All patients were staged according to MASCC/ISOO/ASCO guidelines, as follows:
- -
- Complete healing (stage 0 following treatment): intact mucosal lining, absence of pain, no sign of inflammation for at least 3 months postoperatively
- -
- Improving MRONJ (post-operative stage < pre-operative stage)
- -
- Stable MRONJ (post-operative stage = pre-operative stage)
- -
- Progressive MRONJ (post-operative stage > pre-operative stage)
Patients with improving, stable or progressive MRONJ were grouped together in the incomplete healing group.
2.3. Assessment of Functional Status and Health-Related Quality of Life (HRQOL)
Health-related quality of life (HRQOL) was evaluated using the European Organization for Research and Treatment of Cancer (EORTC) questionnaires (QLQ-C30 and H&N35 modules) [24,25]. Raw scores were converted following a linear transformation according to the EORTC Scoring Manual [26]. Questionnaires and functional assessment were carried out at baseline (T0), 30 days (T1), 3 months (T2), and 6 months (T3) after surgery.
2.4. Statistical Analysis
Statistical analysis was performed using MedCalc Statistical Software version 19.1 (MedCalc Software by, Ostend, Belgium). The following variables were analyzed: drug used, mucosal healing, complication, recurrence. Wilcoxon/Mann-Whitney test for independent non-parametric variables was used; statistical significance was set as p < 0.05. In order to assess recurrence endpoint, Kaplan–Meier test was used, and the different categories were normalized through the long-rank Mantel–Haenszel test. The Cox regression model was used to analyze the simultaneous contribution of different factors to the recurrence risk; univariate analysis variables were: surgical treatment (resection vs. debridement), complete mucosal healing and drug (zoledronic acid vs. denosumab). Cox multivariate regression was then elaborated using the significative results of the univariate analysis (surgical treatment and complete mucosal healing) in order to set the hazard ratio (HR). For each test, significance was set as p > 0.05.
Raw score in accordance with the EORTC Manual Scoring (3rd edition, 2001) was used for questionnaire data assessment. A linear transformation was then applied to the results in order to obtain the score, that measures the multiple items scales and single statements with a percentage from 0 to 100 in order to allow the comparison among similar scores used in literature. Raw data were transformed in a 0 to 100 score, multiplying for 20 the points of each question. The mean value was calculated for the items from 1 to 7, then the final score mean value between the two questionnaires was assessed. In order to evaluate questionnaires reliability, Cronbach’s α coefficient was used. A high α level indicate that the subjects expressed coherence towards each item; acceptable levels were set as α > 0.70. Mean, range and standard deviation were obtained using Microsoft Excel, version 14.0.7104.5000 (Microsoft Excel 2010 by Microsoft Corporation, Albuquerque, NM, USA).
3. Results
Patients’ demographics are summarized in Table 1.

Table 1.
Patient demographics.
Group A showed higher complete healing cases when compared to group B (Figure 1, Figure 2, Figure 3 and Figure 4).
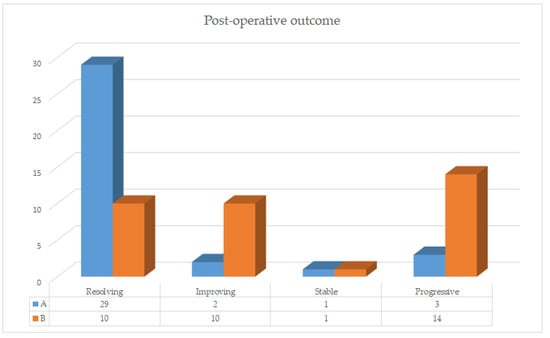
Figure 1.
Post-operative outcome histograms group A vs. group B.
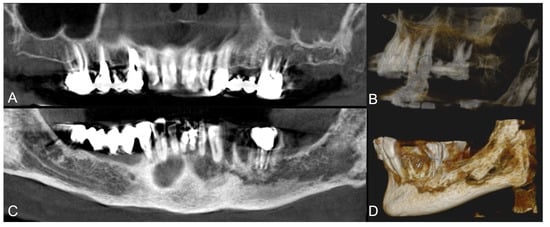
Figure 2.
Group A patient. Radiological pre-operative assessment through cone beam computed tomography of a stage 2 maxillary and mandibular MRONJ. Maxillary view (A) and 3D reconstruction (B); mandibular view (C) and 3D reconstruction (D).
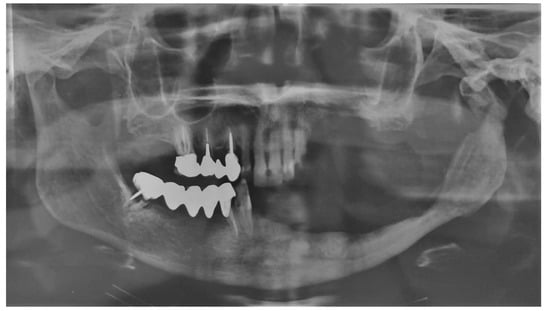
Figure 3.
Post-operative follow-up at 6 months through orthopantomography.
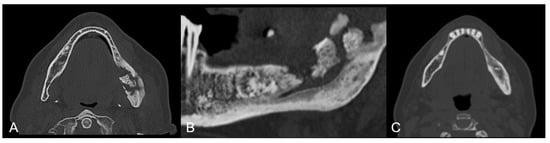
Figure 4.
Group B patient. Radiological pre-operative assessment through cone beam computed tomography of a stage 2 maxillary and mandibular MRONJ. Axial view (A) and mandibular view (B). Twelve months follow-up axial view (C).
Summary of the observed complication is reported in Figure 5.
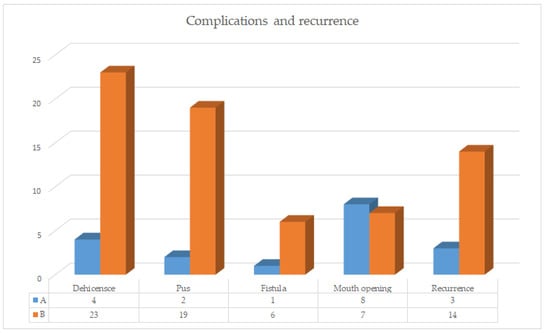
Figure 5.
Complication and recurrence histograms group A vs. group B.
Among those patients who developed a wound dehiscence, 1 in group A and 15 in group B showed persistence of the disease, while the others were healed by secondary intention. Surgical revision was necessary in 3 cases among those patients in group B who developed a postoperative fistula. Temporary difficulty in mouth opening was observed in 8 patients in group A and 7 patients in group B, which resolved spontaneously at the resolution of the acute event. Impairments in mastication force and pain during chewing, swallowing and palpation of the surgical site solved in all patients according to the ongoing healing process, except for those who developed persistence of the disease. Recurrence was observed in 3 patients within group A and 14 patients in group B (p = 0.0045).
Via Kaplan–Meier method, we confronted group A and B setting the recurrence as endpoint. The results were significant on recurrence risk of surgical resection vs. debridement. Chi-square 8.056, DF 1, p = 0.0045, HR 0.2015, CI 95% 0.1020 to 0.6584 (Figure 6).
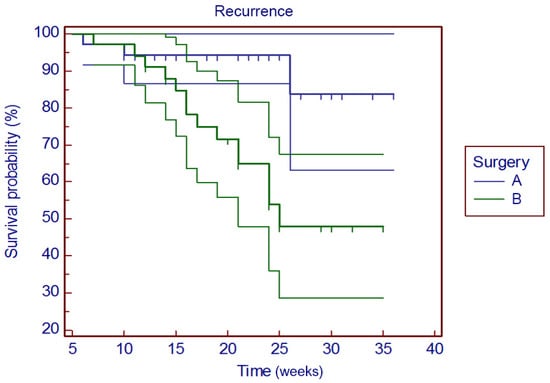
Figure 6.
Kaplan–Meier curve analyzes recurrence risk of group A and B.
Univariate Cox analysis of the surgical treatment points debridement as unfavorable prognostic factor when compared to surgical resection (p = 0.0032, HR 4.9). Similarly, complete mucosal healing in group B represents a prognostic factor related to recurrence rate p < 0.0001. The drug used (zoledronic acid vs. denosumab) seems not to be related to recurrence rate (p = 0.2434) (Table 2).

Table 2.
Univariate Cox regression: surgical treatment (resection vs. debridement) and mucosal healing are statistically correlated to recurrence.
Multivariate Cox analysis shows how the surgical treatment is an independent prognostic factor related to recurrence p < 0.0001, Chi-square 18, DF 2; 1.3274 HR (Table 3).

Table 3.
Multivariate Cox regression: surgical treatment (resection vs. debridement) is an independent prognostic factor to recurrence.
The EORTC QLQ C30 questionnaire evaluates oncologic patients’ quality of life. Answers among the items, both related to the matter of subject and the undergoing primitive tumor, was set in the preoperative stage on a mid-high score level (50–75%) in both groups. The perceived disability is mostly related to food both in its alimentary and social role.
The EORTC QLQ H&N35 questionnaire specifically addresses the oncologic issue of the head and neck district. Even in this case, the answers among the items set in the preoperative stage on a mid-high score level (50–75%) in both groups. Additionally, in this case, the items directly related to the pathology essentially underlined feeding related issues.
Cronbach’s α coefficient for the EORTC QLQ C30 questionnaire was 0.95 at T0, 0.87 at T1, 0.91 at T2 and 0.97 at T3. Cronbach’s α coefficient for the EORTC QLQ H&N35 questionnaire was 0.93 at T0, 0.88 at T1, 0.82 at T2 and 0.94 at T3. Internal coherence and reliability of the questionnaires was high since the coefficient values were α > 0.70. QoL was evaluated for each group individually.
Therefore, we assessed the global QoL between the two groups. The group homogeneity is confirmed by the numerical data of the two questionnaires (group A: 62.68%, range 52–75; group B: 62.37%, range 52–75). At T1 a variation is noted between group A (52.54%, range 40–70) vs. group B (60.83% range 40–70) further increased at T2 group A (42.43%, range 29–58) vs. group B (56.17%, range 31–78). Keeping in mind that a lower score correspond to a better perceived health status by the patient, at T3 the values between group A (33.06%, range 25–77) vs. group B (51.97%, range 25–80) are statistically significant, further stressing the difference (Figure 7).
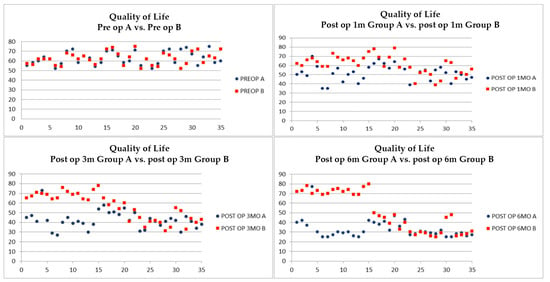
Figure 7.
Quality of life assessment at T0 (upper-left), T1 (upper-right), T2 (lower-left) and T3 (lower-right).
When confronting the two groups the mean values shows a marked QoL improvement in group A and a slight improvement in group B (Figure 8).
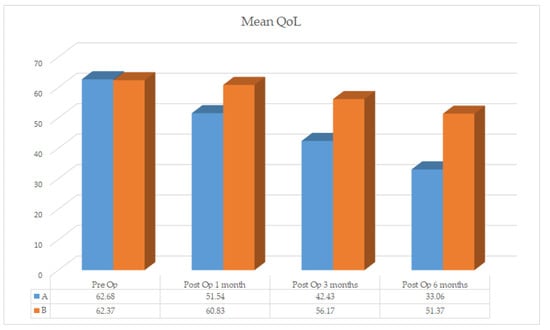
Figure 8.
Global quality of life group A vs. group B.
4. Discussion
MRONJ treatment is an exciting and divisive challenge since its definition in 2003 [1,4,27]. Despite MASCC/ISOO/ASCO 2019 guidelines suggesting the debridement conservative treatment, a universally accepted behavior is lacking [2,12,15,28,29].
The discontinuation of therapy role before the surgical intervention was often debated since therapy suspension time was not significantly associated to post-surgical prognosis. The results described by Stanton et al. suggest that the interruption of bisphosphonate therapy does not represent an optimal protocol [30]. Hayashida et al. did not show a statistically significant relation between the discontinuation of therapy and treatment outcomes [31]. Nonetheless, more studies are required to identify the optimal time when the drug should be discontinued in MRONJ patients.
Conservative treatments include analgesic drugs, antiseptic mouthwashes, regular dental treatments, dental and periodontal diseases treatment and antibiotic therapy and the removal of sharp bone edges that may cause soft tissue inflammation [30,32,33].
During a literature review, two studies did not report any significant differences in healing rate between surgical and non-surgical treatments [34,35]. In a wide retrospective study on 337 patients, Ruggiero et al. stated that patients who underwent surgery were 28 times more likely to have a positive outcome then non-surgical patients [36]. Systematic reviews of literature confronted surgical vs. non-surgical treatments and showed better results after the surgical treatment [37,38,39]. Few more studies highlighted how a less aggressive surgical treatment produces better results when compared to demolitive surgery, although no statistical significance was shown [40].
Fertlito et al. suggest a support therapy based upon antiseptic mouthwash and antibiotic drugs. In their research, 91 out of 94 patients developed sequestrum that was later removed with a minimally invasive approach [41]. Pharmacologic aid was further reported to help the mucous healing above the osteonecrosis in 50–57% of cases thanks to amoxicillin/clavulanic acid plus mouthwashes without the need for surgery [42,43].
The 2010 Japanese position paper on the subject of matter recommended a non-surgical treatment associated, in case of need, with debridement [44]. Similarly, the AAOMS 2014 position paper suggested the same line of treatment [1]. The AAOMS 2014 recommendation, further confirmed in 2019 edition, discourage a surgical approach, suggesting to continue indefinitely a conservative treatment until disease progression [1].
Nonetheless, the necrotic bone never heals spontaneously, therefore the non-surgical treatment goal is to improve MRONJ symptoms rather than achieve complete healing.
Hayashida et al. results showed how complete healing rate was significantly higher when the patients underwent surgical treatment (94.6% of patients) rather than non-surgical treatment [31]. Therefore, the authors believe that the surgical treatment should be the first line of therapy, since long-term non-surgical treatment reduces patients’ quality of life and can lead to disease progression. Published literature suggests that resective surgical treatment is more effective in MRONJ patients. Furthermore, early intervention with adequate resection margins of the necrotic areas and primary closure is strongly suggested due to a better outcome and to be preferred over less invasive but multiple surgical treatments [45,46]. Non-responders to the conservative therapy should be addressed to surgery, including resection osteotomies of the affected area with margins extending to macroscopically sound bone tissue. Osteotomies should be performed with piezosurgery rather than rotary burs and soft tissue suture should be tensionless and without sharp bony edges that may damage the mucosa [47,48,49]. Marx et al. underlined how debridements and refinements attempts are usually counterproductive and lead to a further bone exposure and worsen of the symptomatology [15].
According to Hyashida et al., major surgery is more effective than mini-invasive surgery [31]. Carlson et al. removed the necrotic bone during the surgical treatment and extended the osteotomy reaching the surrounding bone bleeding within a safety margin of 1 cm [50]. Bone resection has to be extended horizontally in order to reach healthy, bleeding bone, although the reliability of this vitality sign remain controversial. Pautke et al. developed a technique where bone resection in guided by tetracycline induced fluorescence, administered 7–10 days prior surgery in order to selectively resect the necrotic bone and allowing a more conservative approach [51].
Lopes and Stockmann independently reported a success rate over 80% in MRONJ patients treated with sequestrectomy [52,53]. Based on Carlson and Basile experience, when MRONJ recurrence is diagnosed after a marginal resection, the surgeon should perform a segmentary resection: when a surgical retreatment is needed, is not advisable to repeat the marginal resection [50]. Consistent with Carlson, many authors underline the radicality obtained through the “one-shot” resective surgery [29,36,46,54,55,56,57,58,59,60,61]. Moreover, the systematic review performed by Fliefel et al. showed how better results are obtained in patients that underwent major surgery vs. mini-invasive surgery [62].
In our study, in order to verify the outcome differences between surgical resection and debridement, we composed two homogeneous groups of 35 randomized patients each, affected from stage 2 MRONJ. Contrary to what is foresaw by MRONJ stage 2 surgical guidelines, our work highlights a superior functional outcome in Group A treated with resective surgery, when compared to Group B treated with debridement only.
Hayashida et al. reported complete healing in 25.2% of patients subject to non-surgical conservative treatments and in 76.7% of the surgically treated patients [31]. The Japanese Task Force reports a 44.7% of complete healing rate with conservative treatment, while a significantly higher rate is shown for wide surgical interventions (86.8% of patients) [44]. Klingelhoffer et al. use debridement, with the goal to reduce pain and infection [63]. Therefore, complete closure is achieved in 27.6% of cases vs. 72.3% of cases of non-closure, 34.2% of which presented exposed bone since the first days, while 28.9% of cases showed bone exposure in the first 3 months after an initial mucosal healing. Mucke et al. used debridement in 102 patients as first treatment: 69.6% benefitted of a single treatment, 16.7% underwent 2 treatments, 8.8% underwent 3 treatments, 4.9% 4 debridement [46]. Debridement was chosen as single therapy in 83% of cases, 17% of cases were further treated with wider resections. Shin obtained complete healing with surgical therapy in 63.5% and a lack of closure in 36.5% of cases [64].
In our study complete healing was achieved in 82.86% of group A patients vs. 28.57% of group B, a statically significant difference (p = 0.0136). Group A patients showed in 82.86% of cases absence of lesions, 5.71% improvement, 2.86% unchanged, 8.57% worsening; group B showed in 28.57% of cases absence of lesion, 28.57% improvement, 2.86% unchanged, and 40% worsening.
Literature reviews regarding complications are scarce: Kim et al. reported a 29.6% rate of complication following surgical treatment without analyzing each complication, while Mucke et al. reported a 9.4% of pathological fractures [46,65].
The complication analyzed in our work were the followings: suture dehiscence (11.43% in group A, 65.71% in group B), dehiscence persistence (2.86% in group A, 42.86% in group B), purulent discharge (5.71% in group A, 54.28 in group B), fistulae (2.86% in group A vs. 17.14 in group B), mouth opening impairment (22.86% in group A vs. 20% in group B). Mucke states that the pre-operatory duration of the disease represents a negative prognostic factor towards the recurrence of disease, while, according to other studies, therapy duration with bisphosphonate or antiresorptive drugs, MRONJ staging or necrosis localization were not considered relevant recurrence prognostic factors [46]. Recurrence analysis shows a superior percentage in debridement treatments (28.7% of Mucke and 9.2% of Klingelhoffer) compared to resective treatments (8.4% Carlson et al. and 11.1% Kim et al.) [46,50,54,63]. Analyzing our clinical records, we recorded the following recurrence rates: 8.57% in group A, 40% in group B, with statistically significant difference (p = 0.0136).
Mucke et al. used a multiple linear regression to analyze the influencing factors of the recurrence development. The authors fund a statically significant difference base on the treatment performed (conservative vs. surgical, p = 0.001) and among the various surgical treatments, showing better outcome in wide surgical resections (p < 0.0001). Moreover, debridement shows 39.07 odd ratio with p < 0.0001, representing a highly unfavorable prognostic factor [46]. Multivariate analysis performed by Hayashida on 159 patients subject to surgical treatment further showed how the extension of the surgical area is an independent factor related to a better outcome when compared to debridement, further underlining the protective value of the surgical treatment (p < 0.001; OR 0.051; IC 95%, from 0.017 to 0.152) [31].
During our statistical analysis, univariate Cox analysis of the surgical treatment demonstrates how debridement represent an unfavorable prognostic factor in recurrence development compared to surgical resection (p = 0.0032 Hazard Ration HR 4.9); similarly, complete mucosal healing represents in group B a prognostic factor related to recurrence development (p < 0.0001). The drug used (zoledronic acid vs. denosumab) is not correlated to recurrence (p = 0.2434). Cox multivariate analysis showed how the surgical treatment is an independent prognostic factor correlated to recurrence development (p < 0.0001, 1.3 Hazard Ratio HR).
Quality of life was investigated in few studies: Yoneda et al. underlined how non-surgical treatments, in addition to occasionally cause disease progression during treatment, often diminish the quality of life [44]. Of the same opinion, Hayashida et al. underlined how the need of a continuous pharmacological treatment lowers the quality of life [31].
In our study we quantified QoL with EORTC and calculated the considerable increase obtained in patients subject to surgical resection (group A) compared to slight improvements in patients subject to surgical debridement (group B). Despite the fact that underlying oncological condition of the patients has an impact on their overall quality of life, the same starting condition in the preoperative phase (62.68% vs. 62.37%) let us analyze the differences that appeared during the concomitant MRONJ treatment [66]. Since lower scores corresponds to better perceived quality of life by the patients, the difference between the two groups is evident.
At one month follow up group A gain a mean improvement (52.42% vs. 60.83%) that becomes more pronounced after three months (42.43% vs. 56.17%) and that at the six months follow-up is 18.31% superior then group B (33.06% vs. 51.37%). This evidence shows how surgical resection, although considered a more aggressive treatment in already debilitated patients for their underlying oncologic condition, improves QoL by almost 20%.
5. Conclusions
Our research, in a broader vision, and with respect of the international directives, aimed to verify the surgical and functional outcomes described, and to quantitatively evaluate the QoL in a disease stage considered as borderline. The results, which need to be validated by a broader casuistry, showed how the intervention of choosing influences mucosal healing, which is the goal of clinical success. The type of surgical treatment is related to recurrence rate: debridement has 4.9 times more risk to develop recurrence when compared to surgical resection. Such a risk when corrected with the other significative concomitant factor, mucosal healing, drops to 1.3, nonetheless remains an independent negative prognostic factor. The statistically validated data we obtained confirm our hypothesis, namely the choice of resective surgery for stage 2 MRONJ.
Data regarding QOL showed a more satisfactory quality of life in patients subject to resective surgery with an improving trend from baseline to 1-, 3- and 6-months follow-up after the treatment. Debridement patients showed more variability of results but the questionnaires showed fluctuating trend and an overall negative trend at the end of the 6-month follow-up.
In light of our results regarding mucosal healing, recurrence, and quality of life, this study ambitiously proposes the revision of the international MASCC/ISOO/ASCO 2019 guidelines on stage 2 MRONJ treatments, underlining the need to expand the choice of surgical resection in those patients where the general condition could benefit from “one shot” therapy.
Author Contributions
Conceptualization, M.R.; methodology, A.T.; data curation, S.D.; writing—original draft preparation, S.D.; writing—review and editing, G.L.G. and D.D.C.; supervision, G.T.; project administration, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of University of Campania “Luigi Vanvitelli” (prot. N°319, 23 October 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) prevention and diagnosis: Italian consensus update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef]
- Migliorati, C.A. Bisphosphanates and oral cavity avascular bone necrosis. J. Clin. Oncol. 2003, 21, 4253–4254. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Mehrotra, B.; Rosenberg, T.J.; Engroff, S.L. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J. Oral Maxillofac. Surg. 2004, 62, 527–534. [Google Scholar] [CrossRef]
- Aragon-Ching, J.B.; Ning, Y.M.; Chen, C.C.; Latham, L.; Guadagnini, J.P.; Gulley, J.L.; Arlen, P.M.; Wright, J.J.; Parnes, H.; Figg, W.D.; et al. Higher incidence of Osteonecrosis of the Jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Investig. 2009, 27, 221–226. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Fizazi, K.; Body, J.J.; Brown, J.E.; Carducci, M.; Diel, I.; Fujiwara, Y.; Martín, M.; Paterson, A.; Tonkin, K.; et al. Erratum to: Safety of long-term denosumab therapy: Results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support. Care Cancer 2016, 24, 457–458. [Google Scholar] [CrossRef]
- Guarneri, V.; Miles, D.; Robert, N.; Diéras, V.; Glaspy, J.; Smith, I.; Thomssen, C.; Biganzoli, L.; Taran, T.; Conte, P. Bevacizumab and osteonecrosis of the jaw: Incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res. Treat. 2010, 122, 181–188. [Google Scholar] [CrossRef]
- Qi, W.X.; Tang, L.N.; He, A.N.; Yao, Y.; Shen, Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: A meta-analysis of seven randomized controlled trials. Int. J. Clin. Oncol. 2014, 19, 403–410. [Google Scholar] [CrossRef]
- Boquete-Castro, A.; Gómez-Moreno, G.; Calvo-Guirado, J.L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin. Oral Implant. Res. 2016, 27, 367–375. [Google Scholar] [CrossRef]
- Mauri, D.; Valachis, A.; Polyzos, I.P.; Polyzos, N.P.; Kamposioras, K.; Pesce, L.L. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: A meta-analysis. Breast Cancer Res. Treat. 2009, 116, 433–439. [Google Scholar] [CrossRef]
- Zhang, X.; Hamadeh, I.S.; Song, S.; Katz, J.; Moreb, J.S.; Langaee, T.Y.; Lesko, L.J.; Gong, Y. Osteonecrosis of the Jaw in the United States Food and Drug Administration’s Adverse Event Reporting System (FAERS). J. Bone Miner. Res. 2016, 31, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Badros, A.; Weikel, D.; Salama, A.; Goloubeva, O.; Schneider, A.; Rapoport, A.; Fenton, R.; Gahres, N.; Sausville, E.; Ord, R.; et al. Osteonecrosis of the jaw in multiple myeloma patients: Clinical features and risk factors. J. Clin. Oncol. 2006, 24, 945–952. [Google Scholar] [CrossRef]
- Hoff, A.O.; Toth, B.B.; Altundag, K.; Johnson, M.M.; Warneke, C.L.; Hu, M.; Nooka, A.; Sayegh, G.; Guarneri, V.; Desrouleaux, K.; et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J. Bone Miner. Res. 2008, 23, 826–836. [Google Scholar] [CrossRef]
- Thumbigere-Math, V.; Tu, L.; Huckabay, S.; Dudek, A.Z.; Lunos, S.; Basi, D.L.; Hughes, P.J.; Leach, J.W.; Swenson, K.K.; Gopalakrishnan, R. A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. Am. J. Clin. Oncol. 2012, 35, 386–392. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Meghji, S.; Crean, S.J.; Hill, P.A.; Sheikh, M.; Nair, S.P.; Heron, K.; Henderson, B.; Mawer, E.B.; Harris, M. Surface-associated protein from Staphylococcus aureus stimulates osteoclastogenesis: Possible role in S. aureus-induced bone pathology. Br. J. Rheumatol. 1998, 37, 1095–1101. [Google Scholar] [CrossRef]
- Nair, S.P.; Meghji, S.; Wilson, M.; Reddi, K.; White, P.; Henderson, B. Bacterially induced bone destruction: Mechanisms and misconceptions. Infect. Immun. 1996, 64, 2371–2380. [Google Scholar] [CrossRef]
- Lesclous, P.; Abi Najm, S.; Carrel, J.P.; Baroukh, B.; Lombardi, T.; Willi, J.P.; Rizzoli, R.; Saffar, J.L.; Samson, J. Bisphosphonate-associated osteonecrosis of the jaw: A key role of inflammation? Bone 2009, 45, 843–852. [Google Scholar] [CrossRef]
- Yarom, N.; Shapiro, C.L.; Peterson, D.E.; Van Poznak, C.H.; Bohlke, K.; Ruggiero, S.L.; Migliorati, C.A.; Khan, A.; Morrison, A.; Anderson, H.; et al. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2270–2290. [Google Scholar] [CrossRef]
- Sedghizadeh, P.P.; Kumar, S.K.; Gorur, A.; Schaudinn, C.; Shuler, C.F.; Costerton, J.W. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J. Am. Dent. Assoc. 2009, 140, 1259–1265. [Google Scholar] [CrossRef]
- Khan, A.A.; Sandor, G.K.; Dore, E.; Morrison, A.D.; Alsahli, M.; Amin, F.; Peters, E.; Hanley, D.A.; Chaudry, S.R.; Dempster, D.W.; et al. Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J. Rheumatol. 2008, 35, 1391–1397. [Google Scholar]
- Maurer, P.; Sandulescu, T.; Kriwalsky, M.S.; Rashad, A.; Hollstein, S.; Stricker, I.; Hölzle, F.; Kunkel, M. Bisphosphonate-related osteonecrosis of the maxilla and sinusitis maxillaris. Int. J. Oral Maxillofac. Surg. 2011, 40, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Heufelder, M.; Winter, K.; Hendricks, J.; Frerich, B.; Schramm, A.; Hemprich, A. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 153–163. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Bjordal, K.; de Graeff, A.; Fayers, P.M.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.J.; Meyza, J.W.; Brédart, A.; et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur. J. Cancer 2000, 36, 1796–1807. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organization for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Schubert, M.M.; Peterson, D.E.; Seneda, L.M. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: An emerging oral complication of supportive cancer therapy. Cancer 2005, 104, 83–93. [Google Scholar] [CrossRef]
- Rupel, K.; Ottaviani, G.; Gobbo, M.; Contardo, L.; Tirelli, G.; Vescovi, P.; Di Lenarda, R.; Biasotto, M. A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosis of the jaw (BRONJ). Oral Oncol. 2014, 50, 1049–1057. [Google Scholar] [CrossRef]
- Stanton, D.C.; Balasanian, E. Outcome of surgical management of bisphosphonate-related osteonecrosis of the jaws: Review of 33 surgical cases. J. Oral Maxillofac. Surg. 2009, 67, 943–950. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the treatment strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the factors affecting treatment outcome: A multicenter retrospective study with propensity score matching analysis. J. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef]
- Hewitt, C.; Farah, C.S. Bisphosphonate-related osteonecrosis of the jaws: A comprehensive review. J. Oral Pathol Med. 2007, 36, 319–328. [Google Scholar] [CrossRef]
- Leite, A.F.; Figueiredo, P.T.; Melo, N.S.; Acevedo, A.C.; Cavalcanti, M.G.; Paula, L.M.; Paula, A.P.; Guerra, E.N. Bisphosphonate-associated osteonecrosis of the jaws. Report of a case and literature review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 14–21. [Google Scholar] [CrossRef]
- Elad, S.; Yarom, N.; Hamed, W.; Ayalon, S.; Yahalom, R.; Regev, E. Osteomylelitis and necrosis of the jaw in patients treated with bisphosphonates: A comparative study focused on multiple myeloma. Clin. Lab. Haematol. 2006, 28, 393–398. [Google Scholar] [CrossRef]
- Scoletta, M.; Arduino, P.G.; Dalmasso, P.; Broccoletti, R.; Mozzati, M. Treatment outcomes in patients with bisphosphonate-related osteonecrosis of the jaws: A prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Kohn, N. Disease stage and mode of therapy are important determinants of treatment outcomes for medication-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2015, 73, S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Zadik, Y.; Benoliel, R.; Fleissig, Y.; Casap, N. Painful trigeminal neuropathy induced by oral bisphosphonate-related osteonecrosis of the jaw: A new etiology for the numb-chin syndrome. Quintessence Int. 2012, 43, 97–104. [Google Scholar] [PubMed]
- Otto, S.; Hafner, S.; Grotz, K.A. The role of inferior alveolar nerve involvement in bisphosphonate-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2009, 67, 589–592. [Google Scholar] [CrossRef]
- Ferlito, S.; Puzzo, S.; Liardo, C. Preventive protocol for tooth extractions in patients treated with zoledronate: A case series. J. Oral Maxillofac. Surg. 2011, 69, e1–e4. [Google Scholar] [CrossRef]
- Lesclous, P.; Grabar, S.; Abi Najm, S.; Carrel, J.P.; Lombardi, T.; Saffar, J.L.; Samson, J. Relevance of surgical management of patients affected by bisphosphonate-associated osteonecrosis of the jaws. A prospective clinical and radiological study. Clin. Oral Investig. 2014, 18, 391–399. [Google Scholar] [CrossRef]
- Ferlito, S.; Puzzo, S.; Palermo, F.; Verzi, P. Treatment of bisphosphonate-related osteonecrosis of the jaws: Presentation of a protocol and an observational longitudinal study of an Italian series of cases. Br. J. Oral Maxillofac. Surg. 2012, 50, 425–429. [Google Scholar] [CrossRef]
- Saussez, S.; Javadian, R.; Hupin, C.; Magremanne, M.; Chantrain, G.; Loeb, I.; Decaestecker, C. Bisphosphonate-related osteonecrosis of the jaw and its associated risk factors: A Belgian case series. Laryngoscope 2009, 119, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Van den Wyngaert, T.; Claeys, T.; Huizing, M.T.; Vermorken, J.B.; Fossion, E. Initial experience with conservative treatment in cancer patients with osteonecrosis of the jaw (ONJ) and predictors of outcome. Ann. Oncol. 2009, 20, 331–336. [Google Scholar] [CrossRef]
- Yoneda, T.; Hagino, H.; Sugimoto, T.; Ohta, H.; Takahashi, S.; Soen, S.; Taguchi, A.; Toyosawa, S.; Nagata, T.; Urade, M. Bisphosphonate-related osteonecrosis of the jaw: Position paper from the Allied Task Force Committee of Japanese Society for Bone and Mineral Research, Japan Osteoporosis Society, Japanese Society of Periodontology, Japanese Society for Oral and Maxillofacial Radiology, and Japanese Society of Oral and Maxillofacial Surgeons. J. Bone Miner. Metab. 2010, 28, 365–383. [Google Scholar] [CrossRef]
- Allen, M.R.; Ruggiero, S.L. A review of pharmaceutical agents and oral bone health: How osteonecrosis of the jaw has affected the field. Int. J. Oral Maxillofac. Implants 2014, 29, e45–e57. [Google Scholar] [CrossRef]
- Mucke, T.; Koschinski, J.; Deppe, H.; Wagenpfeil, S.; Pautke, C.; Mitchell, D.A.; Wolff, K.D.; Holzle, F. Outcome of treatment and parameters influencing recurrence in patients with bisphosphonate-related osteonecrosis of the jaws. J. Cancer Res. Clin. Oncol. 2011, 137, 907–913. [Google Scholar] [CrossRef]
- Ngamphaiboon, N.; Frustino, J.L.; Kossoff, E.B.; Sullivan, M.A.; O’Connor, T.L. Osteonecrosis of the jaw: Dental outcomes in metastatic breast cancer patients treated with bisphosphonates with/without bevacizumab. Clin. Breast Cancer 2011, 11, 252–257. [Google Scholar] [CrossRef]
- Vandone, A.M.; Donadio, M.; Mozzati, M.; Ardine, M.; Polimeni, M.A.; Beatrice, S.; Ciuffreda, L.; Scoletta, M. Impact of dental care in the prevention of bisphosphonate-associated osteonecrosis of the jaw: A single-center clinical experience. Ann. Oncol. 2012, 23, 193–200. [Google Scholar] [CrossRef]
- Lo Giudice, R.; Puleio, F.; Rizzo, D.; Alibrandi, A.; Lo Giudice, G.; Centofanti, A.; Fiorillo, L.; Di Mauro, D.; Nicita, F. Comparative investigation of cutting devices on bone blocks: An SEM morphological analysis. Appl. Sci. 2019, 9, 351. [Google Scholar] [CrossRef]
- Carlson, E.R.; Basile, J.D. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2009, 67, 85–95. [Google Scholar] [CrossRef]
- Pautke, C.; Bauer, F.; Otto, S.; Tischer, T.; Steiner, T.; Weitz, J.; Kreutzer, K.; Hohlweg-Majert, B.; Wolff, K.D.; Hafner, S.; et al. Fluorescence-guided bone resection in bisphosphonate-related osteonecrosis of the jaws: First clinical results of a prospective pilot study. J. Oral Maxillofac. Surg. 2011, 69, 84–91. [Google Scholar] [CrossRef]
- Lopes, R.N.; Rabelo, G.D.; Rocha, A.C.; Carvalho, P.A.; Alves, F.A. Surgical therapy for bisphosphonate-related osteonecrosis of the jaw: Six-year experience of a single institution. J. Oral Maxillofac. Surg. 2015, 73, 1288–1295. [Google Scholar] [CrossRef]
- Stockmann, P.; Vairaktaris, E.; Wehrhan, F.; Seiss, M.; Schwarz, S.; Spriewald, B.; Neukam, F.W.; Nkenke, E. Osteotomy and primary wound closure in bisphosphonate-associated osteonecrosis of the jaw: A prospective clinical study with 12 months follow-up. Support. Care Cancer 2010, 18, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Seo, W.G.; Koo, C.H.; Lee, J.H. Evaluation of the predisposing factors and involved outcome of surgical treatment in bisphosphonate-related osteonecrosis of the jaw cases including bone biopsies. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 193–204. [Google Scholar] [CrossRef]
- Schubert, M.; Klatte, I.; Linek, W.; Muller, B.; Doring, K.; Eckelt, U.; Hemprich, A.; Berger, U.; Hendricks, J. The saxon bisphosphonate register—Therapy and prevention of bisphosphonate-related osteonecrosis of the jaws. Oral Oncol. 2012, 48, 349–354. [Google Scholar] [CrossRef]
- Nisi, M.; La Ferla, F.; Karapetsa, D.; Gennai, S.; Ramaglia, L.; Graziani, F.; Gabriele, M. Conservative surgical management of patients with bisphosphonate-related osteonecrosis of the jaws: A series of 120 patients. Br. J. Oral Maxillofac. Surg. 2016, 54, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, P.; Merigo, E.; Meleti, M.; Manfredi, M.; Guidotti, R.; Nammour, S. Bisphosphonates-related osteonecrosis of the jaws: A concise review of the literature and a report of a single-centre experience with 151 patients. J. Oral Pathol. Med. 2012, 41, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Vescovi, P.; Campisi, G.; Favia, G.; Gabriele, M.; Gaeta, G.M.; Gennai, S.; Goia, F.; Miccoli, M.; Peluso, F.; et al. Resective surgical approach shows a high performance in the management of advanced cases of bisphosphonate-related osteonecrosis of the jaws: A retrospective survey of 347 cases. J. Oral Maxillofac. Surg. 2012, 70, 2501–2507. [Google Scholar] [CrossRef]
- Jacobsen, C.; Metzler, P.; Obwegeser, J.A.; Zemann, W.; Graetz, K.W. Osteopathology of the jaw associated with bone resorption inhibitors: What have we learned in the last 8 years? Swiss Med. Wkly. 2012, 142, w13605. [Google Scholar] [CrossRef][Green Version]
- Vescovi, P.; Campisi, G.; Fusco, V.; Mergoni, G.; Manfredi, M.; Merigo, E.; Solazzo, L.; Gabriele, M.; Gaeta, G.M.; Favia, G.; et al. Surgery-triggered and non surgery-triggered Bisphosphonate-related Osteonecrosis of the Jaws (BRONJ): A retrospective analysis of 567 cases in an Italian multicenter study. Oral Oncol. 2011, 47, 191–194. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef]
- Fliefel, R.; Troltzsch, M.; Kuhnisch, J.; Ehrenfeld, M.; Otto, S. Treatment strategies and outcomes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) with characterization of patients: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef]
- Klingelhoffer, C.; Zeman, F.; Meier, J.; Reichert, T.E.; Ettl, T. Evaluation of surgical outcome and influencing risk factors in patients with medication-related osteonecrosis of the jaws. J. Craniomaxillofac. Surg. 2016, 44, 1694–1699. [Google Scholar] [CrossRef]
- Shin, W.J.; Kim, C.H. Prognostic factors for outcome of surgical treatment in medication-related osteonecrosis of the jaw. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Hinkmann, F.M.; Lell, M.M.; Fenner, M.; Vairaktaris, E.; Neukam, F.W.; Nkenke, E. Panoramic radiograph, computed tomography or magnetic resonance imaging. Which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin. Oral Investig. 2010, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, D.; Tartaro, G.; Ciardiello, F.; Fasano, M.; Rauso, R.; Fiore, F.; Spuntarelli, C.; Troiano, A.; Lo Giudice, G.; Colella, G. Health-Related Quality of Life in Oral Cancer Patients: Scoping Review and Critical Appraisal of Investigated Determinants. Cancers 2021, 13, 4398. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).