Study on a New Type of Composite Powder Explosion Inhibitor Used to Suppress Underground Coal Dust Explosion
Abstract
:1. Introduction
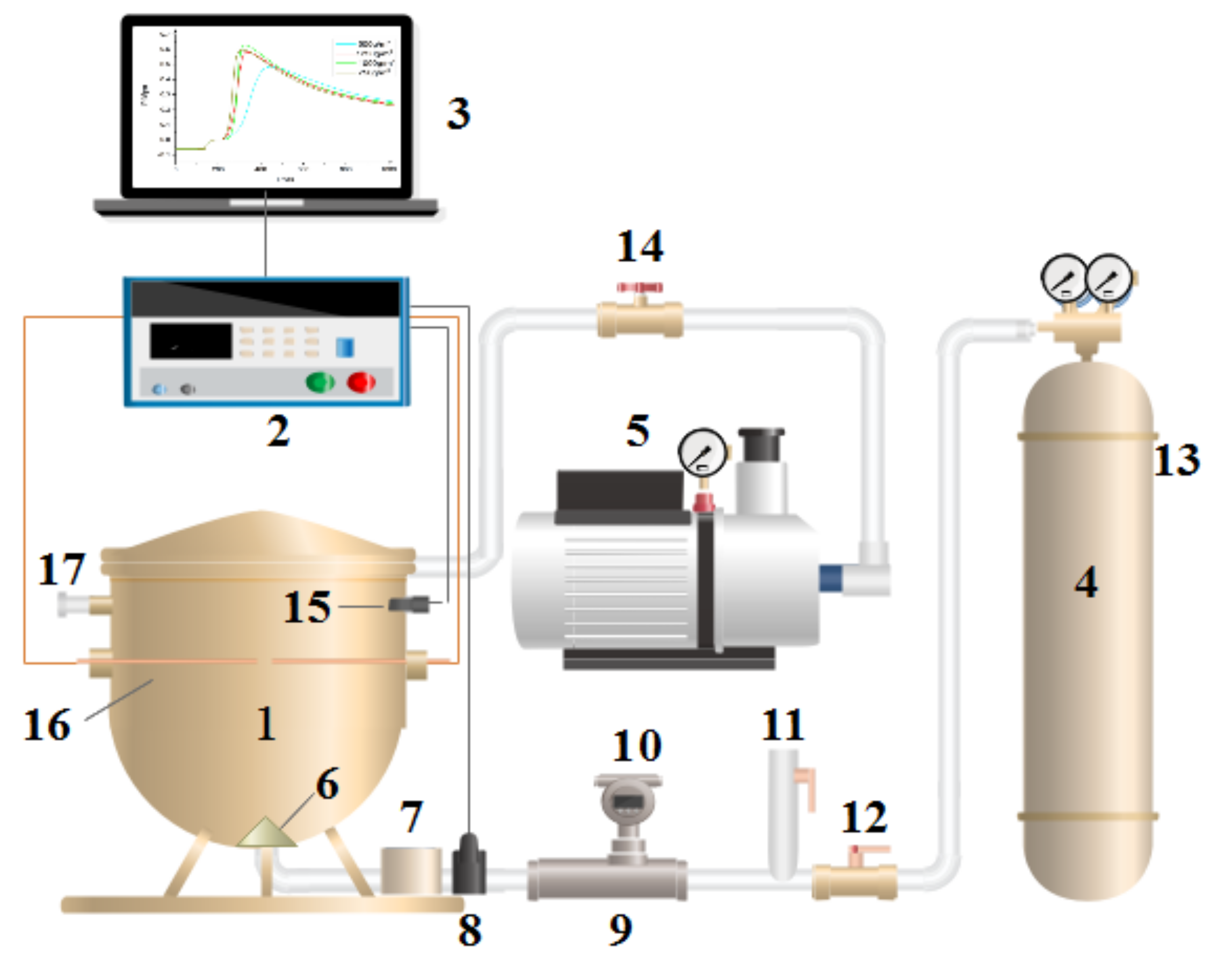
2. Materials and Methods
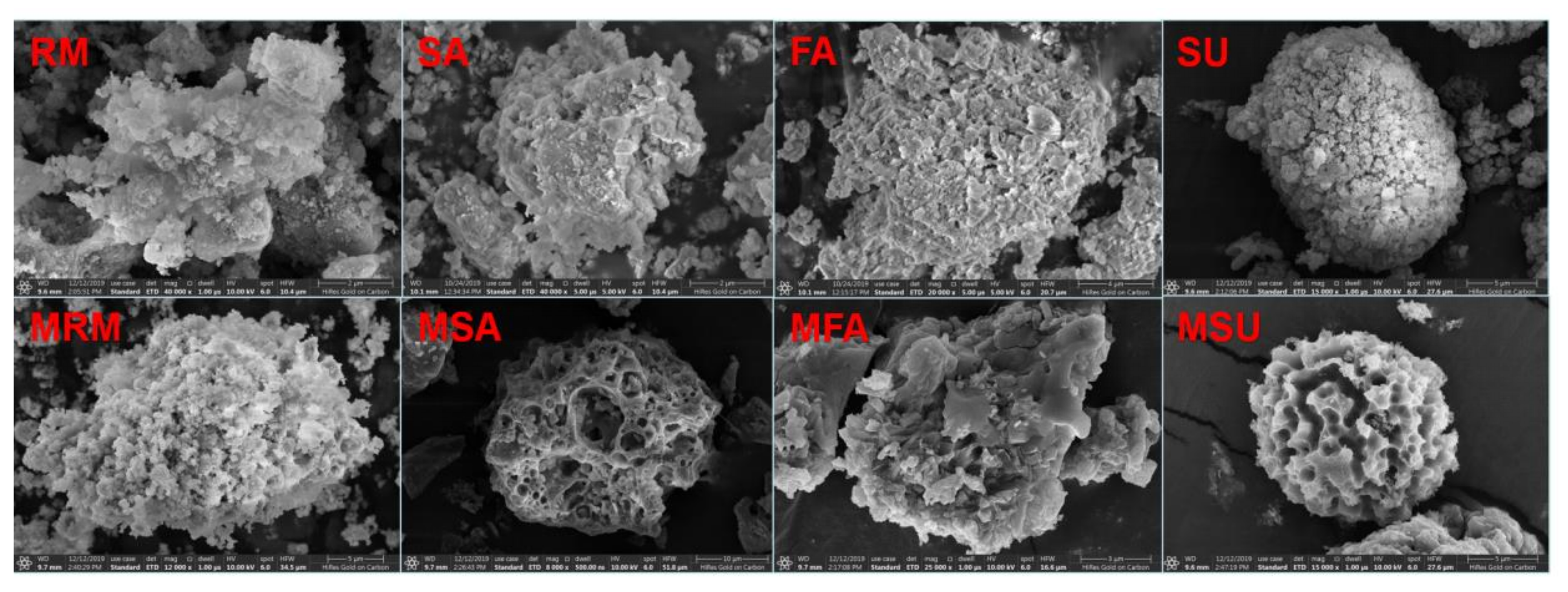
2.1. Modification of Industrial Solid Wastes
- (1)
- Into 100 mL deionized water, 30 g CN was added. To this, 6 mol/L HCl was added dropwise, and the metal salt solution was obtained by stirring at 90 °C for 1.5 h with a magnetic stirrer.
- (2)
- Ammonia water was added to the metal salt solution to the pH value of 7.8; then, 150 mL of Absolute ethanol (C2H6O) was added, and the mixture was stirred at 60 °C for 1 h.
- (3)
- After the substance in the solution was precipitated, it was washed and filtered with non-ionic water using a vacuum pump. The filtered mud was dried in vacuum drying oven for 24 h, and the modified red mud (MRM) with particle size less than 75 μm was obtained by grinding with ball mill.
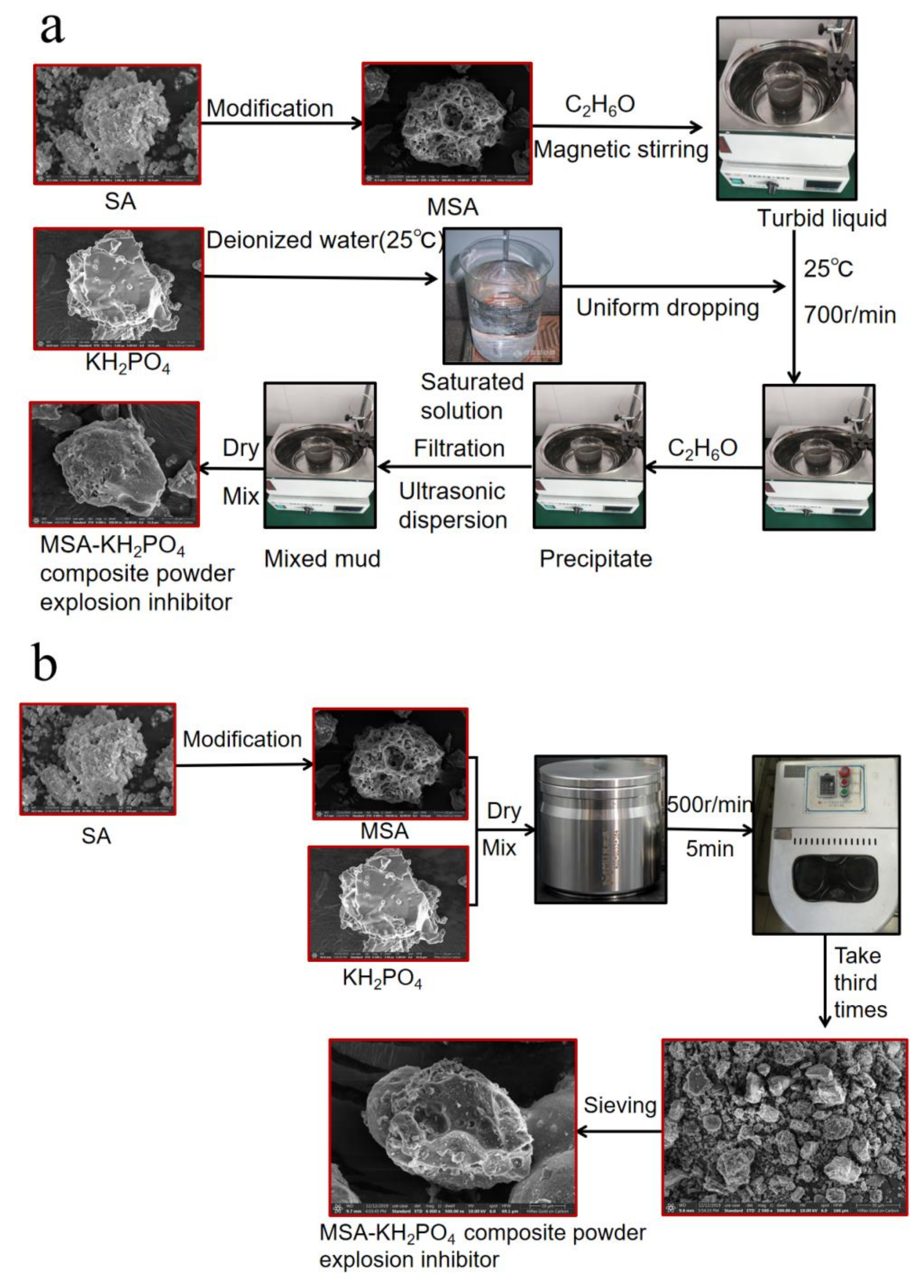
2.2. Compounding Process of Industrial Solid-Waste Based Composite Powder Explosion Inhibitors
- (1)
- All three active powder inhibitors decompose thermally under heating conditions and absorb large amounts of heat in the explosive environment, thereby suppressing explosion by thermolysis endothermic cooling.
- (2)
- The Al2O3 from thermal decomposition of Al(OH)3 can attach to exploding particles during explosion suppression, thereby isolating thermodynamic activity.
- (3)
- All three inhibitors can decompose thermally to generate gases or vapors such as CO2, P2O5 and H2O. The reaction product can dilute the volatile gases and oxygen, thereby inhibiting explosion.
- (4)
- All three inhibitors contain substances that can reduce the activity of free radicals (e.g., O, OH, HCO· and OHP·), which participate in the explosion reaction, thereby inhibiting the combustion–explosion reaction rate.
3. Result and Discussion
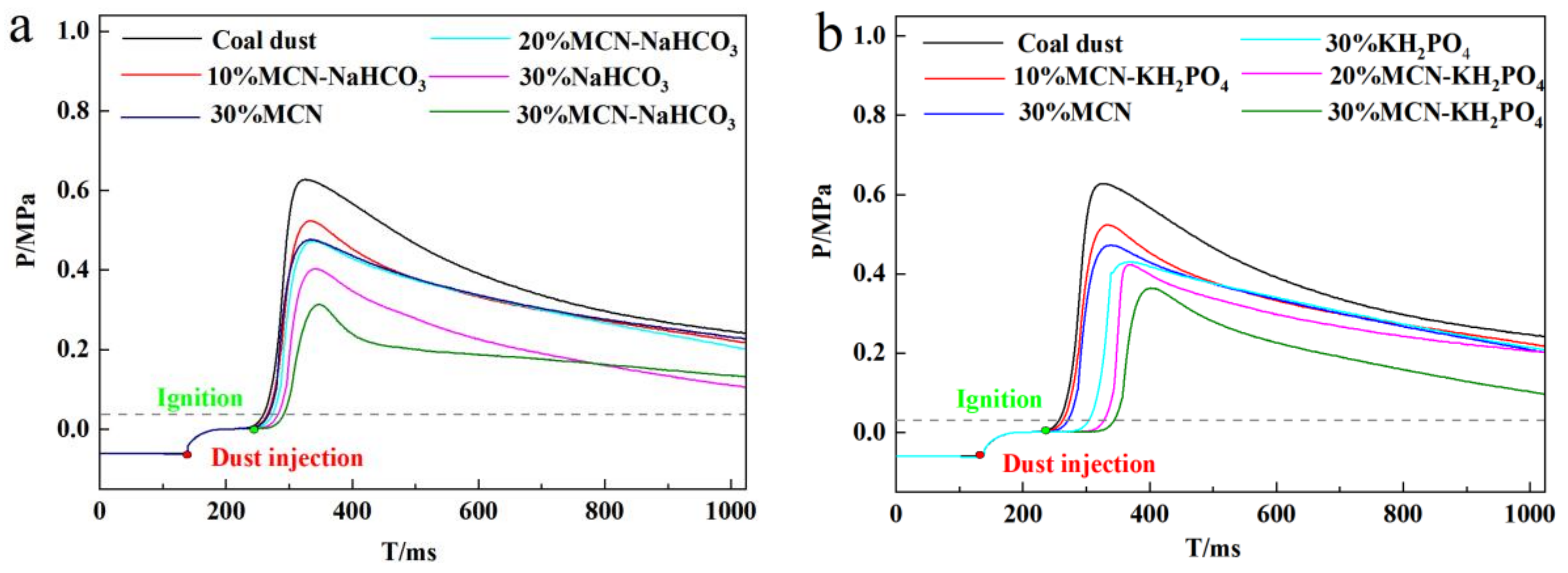
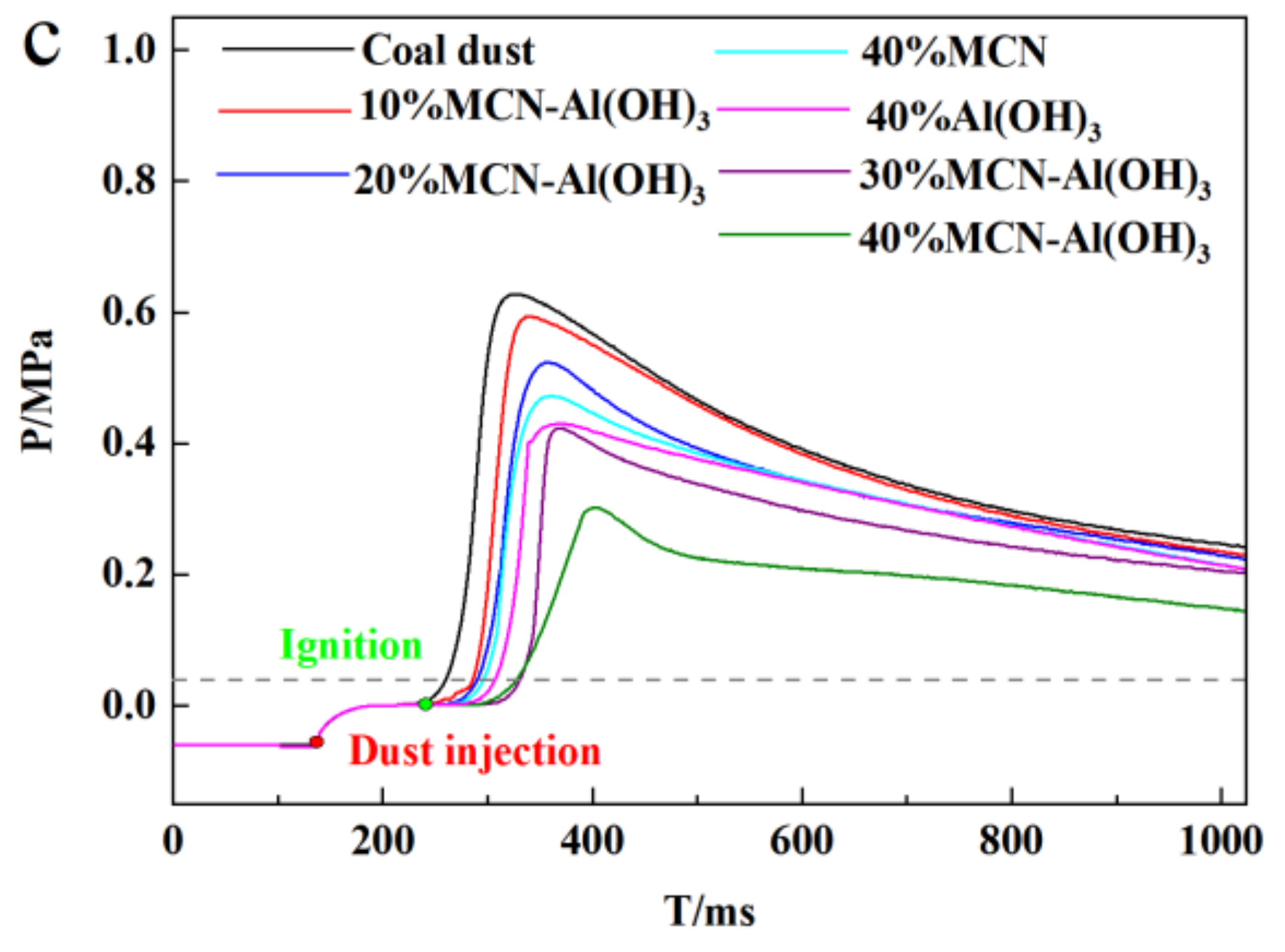
3.1. Suppression Effect of Industrial Solid Waste-Based Composite Powder Explosion Inhibitors on Coal Dust Explosion
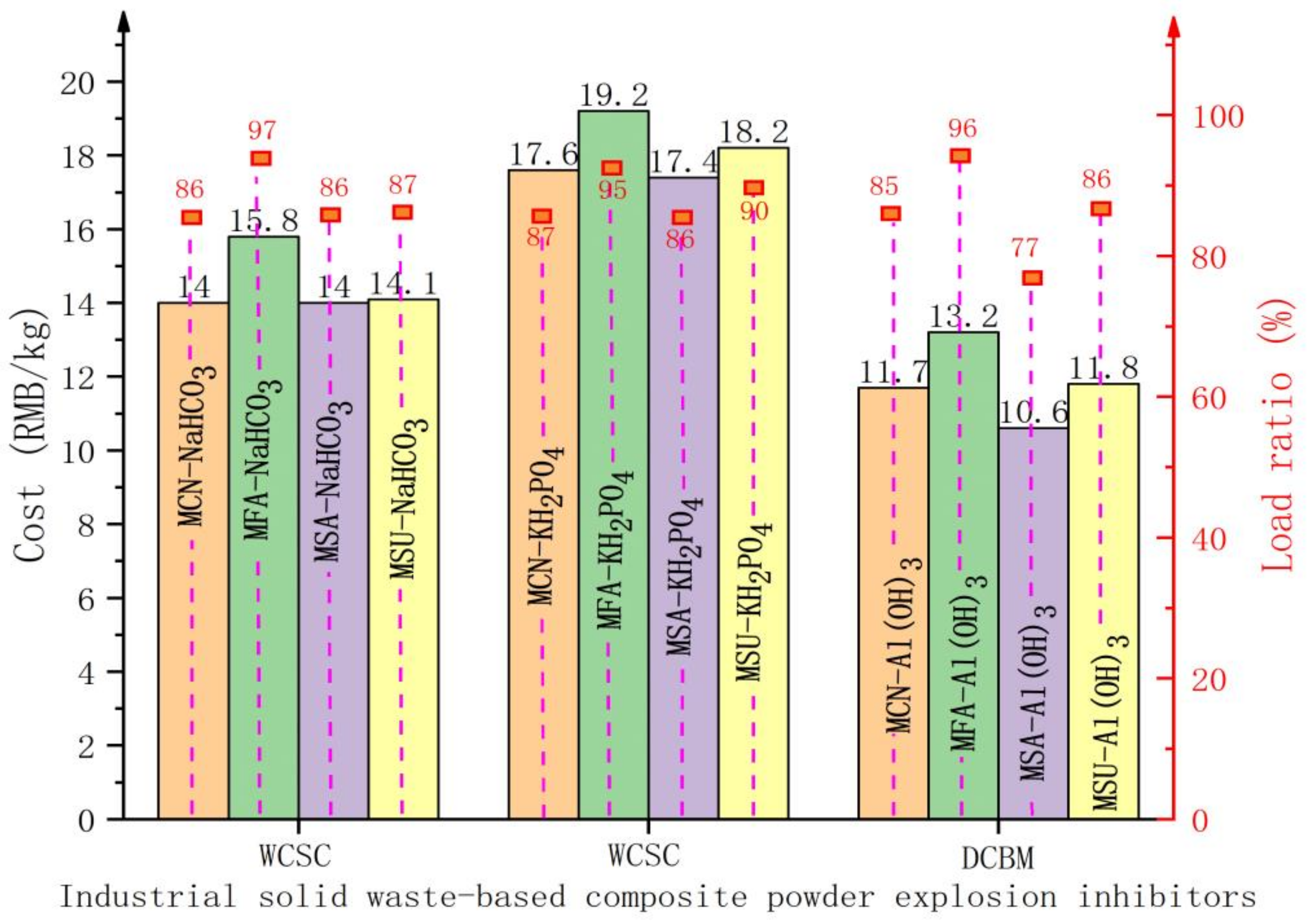
3.2. Cost of Industrial Solid Waste-Based Composite Powder Explosion Inhibitors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Chen, H. Steam jet mill—A prospective solution to industrial exhaust steam and solid waste. Environ. Sci. Pollut. Res. 2018, 25, 17842–17854. [Google Scholar] [CrossRef] [PubMed]
- Angelis-Dimakis, A.; Arampatzis, G.; Pieri, T.; Solomou, K.; Dedousis, P.; Apostolopoulos, G. SWAN platform: A web-based tool to support the development of industrial solid waste reuse business models. Waste Manag. Res. 2021, 39, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, K.; Li, M.; Liu, B.; Wang, B.; Li, J.; Pei, X. Hydrogen inhibition in wet dust removal systems by using L-aspartic: A feasible way of hydrogen explosion control measures. J. Loss Prev. Process. Ind. 2021, 73, 104612. [Google Scholar] [CrossRef]
- Kang, D.; Zhao, Z.; Chen, X.; Wang, X.; Li, J. Characteristics and impacts of solid waste on giant panda habitat in Wanglang Nat. Reserve. Sci. Total Environ. 2020, 724, 138210. [Google Scholar]
- Mukherjee, C.; Denney, J.; Mbonimpa, E.; Slagley, J.; Bhowmik, R. A review on municipal solid waste-to-energy trends in the USA. Renew. Sustain. Energy Rev. 2020, 119, 109512. [Google Scholar] [CrossRef]
- Selvakumar, P.; Sivashanmugam, P. Studies on the extraction of polyphenolic compounds from pre-consumer organic solid waste. J. Eng. Chem. 2020, 82, 130–137. [Google Scholar]
- Caprai, V.; Schollbach, K.; Florea, M.; Brouwers, H. Evaluation of municipal solid waste incineration filter cake as supplementary cementitious material. Constr. Build. Mater. 2020, 250, 118833. [Google Scholar] [CrossRef]
- Li, B.; Wu, H.; Liu, X.; Zhu, T.; Liu, F.; Zhao, X. Simultaneous removal of SO2 and NO using a novel method with red mud as absorbent combined with O3 oxidation. J. Hazard. Mater. 2020, 392, 122270. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Zhang, Y.; Liu, J.; Xiao, X.; Meng, K.; Chu, B.; Chu, P. Multiple flocculant pre-pared with dealkalized red mud and fly ash: Properties and characterization. J. Water Process Eng. 2020, 34, 101–173. [Google Scholar] [CrossRef]
- Yu, M.; Kong, J.; Wang, Y.; Zheng, K.; Zheng, L. Experimental research on gas explosion suppression by modified red mud. J. China Coal. Soc. 2014, 39, 1289–1295. [Google Scholar]
- Wang, X.; Zhang, Y.; Liu, B.; Liang, P.; Zhang, Y. Effectiveness and mechanism of carbamide/fly ash cenosphere with bilayer spherical shell structure as explosion suppressant of coal dust. J. Hazard. Mater. 2019, 365, 555–564. [Google Scholar] [CrossRef]
- Cristelo, N.; Segadães, L.; Coelho, J.; Chaves, B.; Sousa, N.R.; Lopes, M.D.L. Recycling municipal solid waste incineration slag and fly ash as precursors in low-range alkaline cements. Waste Manag. 2020, 104, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Long, W.-J.; Peng, J.-K.; Gu, Y.-C.; Li, J.-L.; Dong, B.; Xing, F.; Fang, Y. Recycled use of municipal solid waste incinerator fly ash and ferronickel slag for eco-friendly mortar through geopolymer technology. J. Clean. Prod. 2021, 307, 127281. [Google Scholar] [CrossRef]
- Taki, K.; Choudhary, S.; Gupta, S.; Kumar, M. Enhancement of geotechnical properties of municipal sewage sludge for sustainable utilization as engineering construction material. J. Clean. Prod. 2020, 251, 119723. [Google Scholar] [CrossRef]
- Yan, K.; Meng, X. An investigation on the aluminum dustexplosion suppression efficiency and mechanism of a NaHCO3/DE composite powder. Adv. Powder Technol. 2020, 31, 3246–3255. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Cao, J.; Zheng, L.; Yi, H.; Yu, M. Suppression characteristics of KHCO3 /red-mud composite powders with core-shell structure on methane explosion. J China Coal Soc. 2017, 42, 653–658. [Google Scholar]
- Liu, B.; Zhang, Y.; Zhang, Y.; Liu, E.; Xu, K.; Tian, Z.; Chen, J.; Meng, X.; Yan, K. Study on resource utilization of composite powder suppressor prepared from acrylic fiber waste sludge. J. Clean. Prod. 2021, 291, 125914. [Google Scholar] [CrossRef]
- Ho, H.-C.; Chow, J.-D.; Gau, S.-H. Thermal Mobility of Heavy Metals in Municipal Solid Waste Incinerator Fly Ash (MSWIFA). Environ. Eng. Sci. 2008, 25, 649–656. [Google Scholar] [CrossRef]
- Xiao, H.; Cheng, Q.; Liu, M.; Li, L.; Ru, Y.; Yan, D. Industrial disposal processes for treatment of polychlorinated dibenzo-p-dioxins and dibenzofurans in municipal solid waste incineration fly ash. Chemosphere 2020, 243, 125351. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, G.; Wang, Y.; Liu, X.; Wang, X. Research on synergistically hydrothermal treatment of municipal solid waste incineration fly ash and sewage sludge. Waste Manag. 2019, 100, 182–190. [Google Scholar] [CrossRef]
- Li, J.; Xu, K.; Yao, X.; Chen, S. Prediction and optimization of syngas production from steam gasification: Numerical study of operating conditions and biomass composition. Energy Convers. Manag. 2021, 236, 114077. [Google Scholar] [CrossRef]
- Jiang, H.; Bi, M.; Gao, W.; Gan, B.; Zhang, D.; Zhang, Q. Inhibition of aluminum dust explosion by NaHCO3 with different particle size distributions. J. Hazard. Mater. 2018, 344, 902–912. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, H.; Wang, Q.; Sun, J. Inhibiting effect of Al(OH)3 and Mg(OH)2 dust on the explosions of methane-air mixtures in closed vessel. Sci. China Technol. Sc. 2012, 55, 1371–1375. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Yang, B. Insights into the adsorption/desorption of CO2 and CO on single-atom Fe-nitrogen-graphene catalyst under electrochemical environment. J. Energy Chem. 2021, 53, 20–25. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, D.; Chen, Y.; Gao, Z.; Teng, J. Pore structure of different macroscopically distinguished components within low-rank coals and its methane desorption characteristics. Fuel 2021, 293, 120465. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Zhang, Y.; Chen, J.; Meng, X. Experimental Study on Multiple Explosions during the Development and Utilization of Oil Shale Dust. Shock. Vib. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, Y.; Meng, X.; Chen, J.; Wang, J.; Wang, X.; Zhang, Y. Study on Explosion Characteristics of the Inert Substances at Longkou Oil Shale of China. Process Saf. Environ. 2020, 136, 324–333. [Google Scholar] [CrossRef]
- Liu, B.; Xu, K.; Zhang, Y.; Ge, J.; Zhang, Y. PAN dust explosion inhibition mechanisms of NaHCO3 and Al(OH)3. J. Loss Prev. Process. Ind. 2021, 73, 104619. [Google Scholar] [CrossRef]
- The General Administration of Quality Supervision, Inspection and Quarantine (AQSIQ); Standardization Administration of China (SAC). Powder Extinguishing Agent (GB 4066–2017). 2017. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=E09CC95FFE11E448FB73FB1CED54071F (accessed on 9 July 2021).
- Ma, X.; Meng, X.; Xiao, Q.; Wang, J.; Cui, L. Inhibitory effect of calcium carbonate, diammonium dihydrogen phosphate and silicon dioxide on explosion of flour powder. Fire Sci. Technol. 2020, 39, 31–35. [Google Scholar]
- Huang, Y.; Dai, X.; Cao, W. Experimental Study on Coal Dust Explosion Suppression with Dihydrogen Phosphate Salts and SiO2 Powders. China Saf. Sci. J. 2013, 23, 57–61. [Google Scholar]


| Substance | Fe2O3 | Al2O3 | SiO2 | CaO | TiO2 | Na2O | MgO | Cl |
|---|---|---|---|---|---|---|---|---|
| Red mud | 33.3 | 18.2 | 15.3 | 16.3 | 6.73 | 8.19 | 0.30 | 0 |
| Slag | 12.7 | 26.3 | 41.2 | 7.17 | 6.73 | 1.20 | 2.35 | 3.22 |
| Fly ash | 16.923 | 13.834 | 2.69 | 11.7 | 1.22 | 5.6 | 1.88 | 22.6 |
| Sludge | 36.8 | 12.4 | 12.4 | 2.89 | 0.68 | 5.72 | 1.01 | 0 |
| Solvent Solute | Deionized Water | Ethanol | Decomposition Temperature | Preparation Method | ||
|---|---|---|---|---|---|---|
| WCSC | DCBM | DCAI | ||||
| NaHCO3 | 9.6 | Insoluble | 50 °C | √ | √ | √ |
| KH2PO4 | 22.6 | Insoluble | 252.6 °C | √ | √ | √ |
| Al(OH)3 | Insoluble | Insoluble | 298 °C | — | √ | √ |
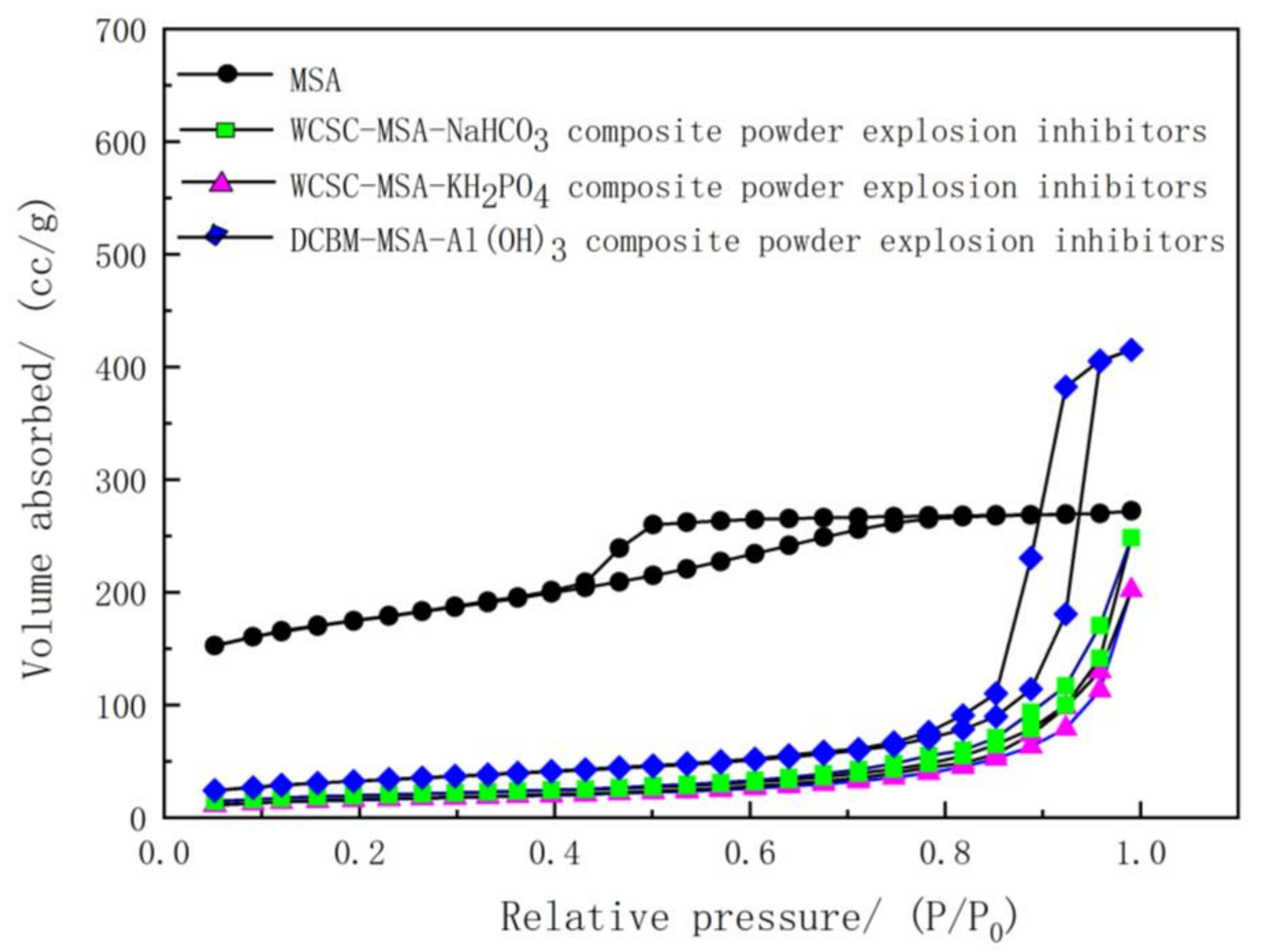
| Sample | BET (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| MSA | 567.6321 | 0.416673 |
| WCSC-MSA-NaHCO3 composite powder explosion inhibitor | 68.4097 | 0.198494 |
| WCSC-MSA-KH2PO4 composite powder explosion inhibitor | 55.9697 | 0.149462 |
| DCBM-MSA-Al(OH)3 composite powder explosion inhibitor | 115.2649 | 0.166799 |
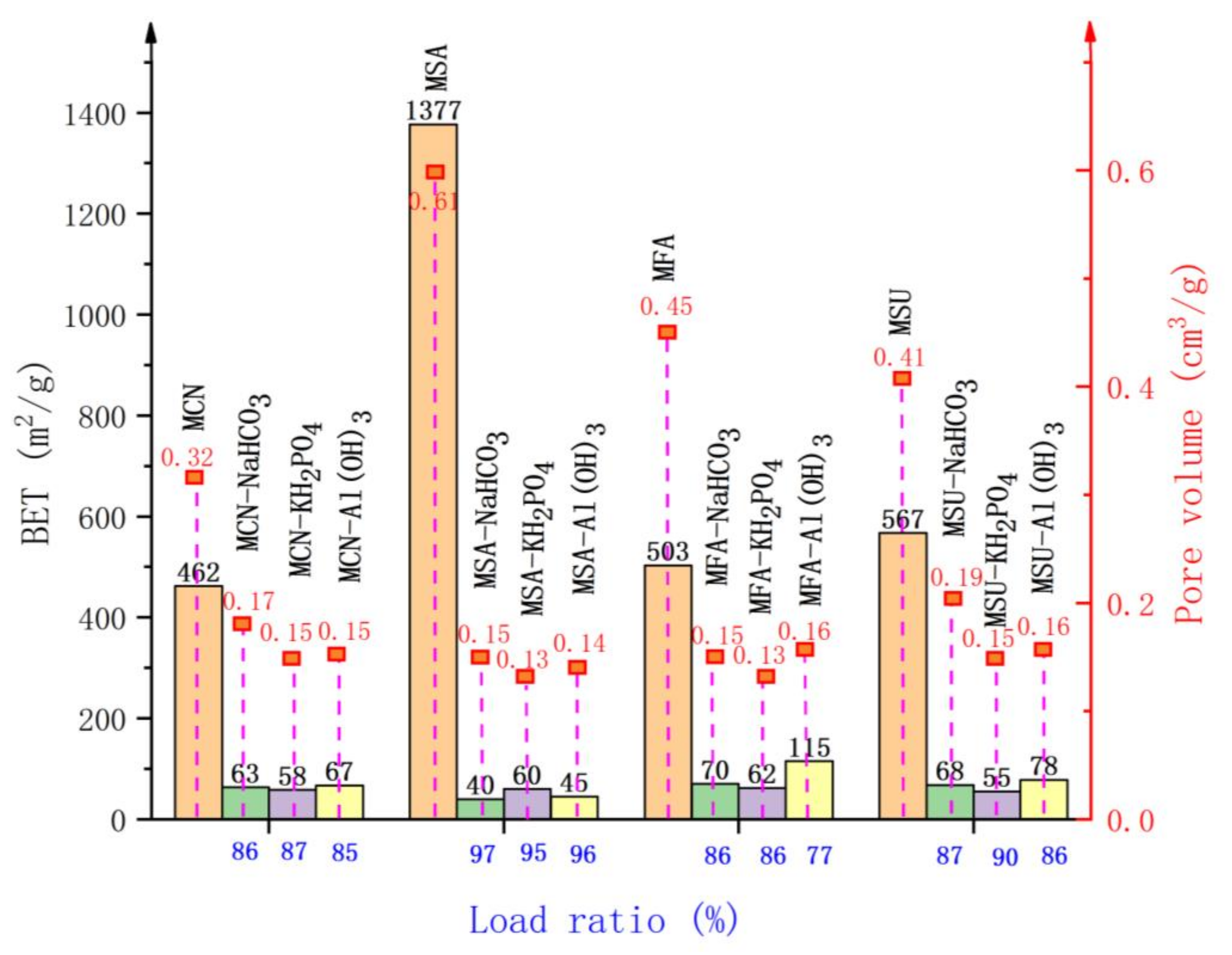
| Carrier | Process Name | Suppressor Name |
|---|---|---|
| Modified red mud | Red mud-based NaHCO3 composite powder explosion inhibitor | MCN-NaHCO3 composite powder explosion inhibitor |
| Red mud-based KH2PO4 composite powder explosion inhibitor | MCN-KH2PO4 composite powder explosion inhibitor | |
| Red mud-based Al(OH)3 composite powder explosion inhibitor | MCN-Al(OH)3 composite powder explosion inhibitor | |
| Modified slag | Slag-based NaHCO3 composite powder explosion inhibitor | MSA-NaHCO3 composite powder explosion inhibitor |
| Slag-based KH2PO4 composite powder explosion inhibitor | MSA-KH2PO4 composite powder explosion inhibitor | |
| Slag-based Al(OH)3 composite powder explosion inhibitor | MSA-Al(OH)3 composite powder explosion inhibitor | |
| Modified fly ash | Fly ash-based NaHCO3 composite powder explosion inhibitor | MFA-NaHCO3 composite powder explosion inhibitor |
| Fly ash-based KH2PO4 composite powder explosion inhibitor | MFA-KH2PO4 composite powder explosion inhibitor | |
| Fly ash-based NaHCO3 composite powder explosion inhibitor | MFA-Al(OH)3 composite powder explosion inhibitor | |
| Modified sludge | Sludge-based NaHCO3 composite powder explosion inhibitor | MSU-NaHCO3 composite powder explosion inhibitor |
| Sludge-based KH2PO4 composite powder explosion inhibitor | MSU-KH2PO4 composite powder explosion inhibitor | |
| Sludge-based NaHCO3 composite powder explosion inhibitor | MSU-Al(OH)3 composite powder explosion inhibitor |
| Consumable | Average Consumption | Unit Price (RMB) | Mass or Volume | |
|---|---|---|---|---|
| Average consumption of 1 kg of modified solid waste | Absolute ethanol (C2H6O) | 0.3 L | 5 | 500 mL |
| Distilled water | 4 L | 10 | 40 L | |
| Ammonia water | 0.4 L | 7 | 500 mL | |
| Hydrochloric acid | 0.1 L | 10 | 500 mL | |
| The average amount of consumables used in the preparation of 1 kg of industrial solid waste based composite powder explosion inhibitors | C2H6O | 0.3 L | 5 | 500 mL |
| Distilled water | 6 L | 10 | 40 L | |
| NaHCO3 | 0.5 kg | 6 | 500 g | |
| KH2PO4 | 0.5 kg | 10 | 500 g | |
| Al(OH)3 | 0.5 kg | 8 | 500 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, Y.; Xu, K.; Zhang, Y.; Hao, Z.; Ma, N. Study on a New Type of Composite Powder Explosion Inhibitor Used to Suppress Underground Coal Dust Explosion. Appl. Sci. 2021, 11, 8512. https://doi.org/10.3390/app11188512
Liu B, Zhang Y, Xu K, Zhang Y, Hao Z, Ma N. Study on a New Type of Composite Powder Explosion Inhibitor Used to Suppress Underground Coal Dust Explosion. Applied Sciences. 2021; 11(18):8512. https://doi.org/10.3390/app11188512
Chicago/Turabian StyleLiu, Bo, Yuyuan Zhang, Kaili Xu, Yansong Zhang, Zheng Hao, and Ning Ma. 2021. "Study on a New Type of Composite Powder Explosion Inhibitor Used to Suppress Underground Coal Dust Explosion" Applied Sciences 11, no. 18: 8512. https://doi.org/10.3390/app11188512
APA StyleLiu, B., Zhang, Y., Xu, K., Zhang, Y., Hao, Z., & Ma, N. (2021). Study on a New Type of Composite Powder Explosion Inhibitor Used to Suppress Underground Coal Dust Explosion. Applied Sciences, 11(18), 8512. https://doi.org/10.3390/app11188512






