Antibacterial Efficacy of Some Medicinal Plants on Multidrug Resistance Bacteria and Their Toxicity on Eukaryotic Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Plant Collection and Preparation
2.3. In Vitro Antibacterial Activities Assay
2.3.1. Disc Diffusion Assay
2.3.2. Minimum Inhibitory Concentration Assay (MIC) and Minimum Bactericidal Concentration Assay (MBC)
2.4. Cytotoxicity Assay
2.4.1. The Erythrocyte Hemolysis
- At: absorbance of test sample
- An: absorbance of the negative control (normal saline)
- Ac: absorbance of the positive control (Triton X-100)
2.4.2. MTT (Cell Proliferation) Assay
Preparation of Cell Lines for MTT Assay and Culture Condition
2.5. In Vivo Assay
2.5.1. Animals and Conditions
2.5.2. Acute Toxicity Test
2.5.3. Mouse Systemic Infection Assay (Challenge Test)
Bacterial Strains and Culture Conditions
Inoculum Preparation
Challenge Procedure
2.6. Statistical Analysis
3. Results
3.1. In Vitro Antibacterial Effect of Plant Extracts
3.1.1. Disc-Diffusion Assay
3.1.2. Minimum Inhibitory Concentration Assay (MIC) and Minimum Bactericidal Concentration Assay (MBC)
3.2. Cytotoxicity Assay
3.2.1. The Erythrocyte Hemolysis
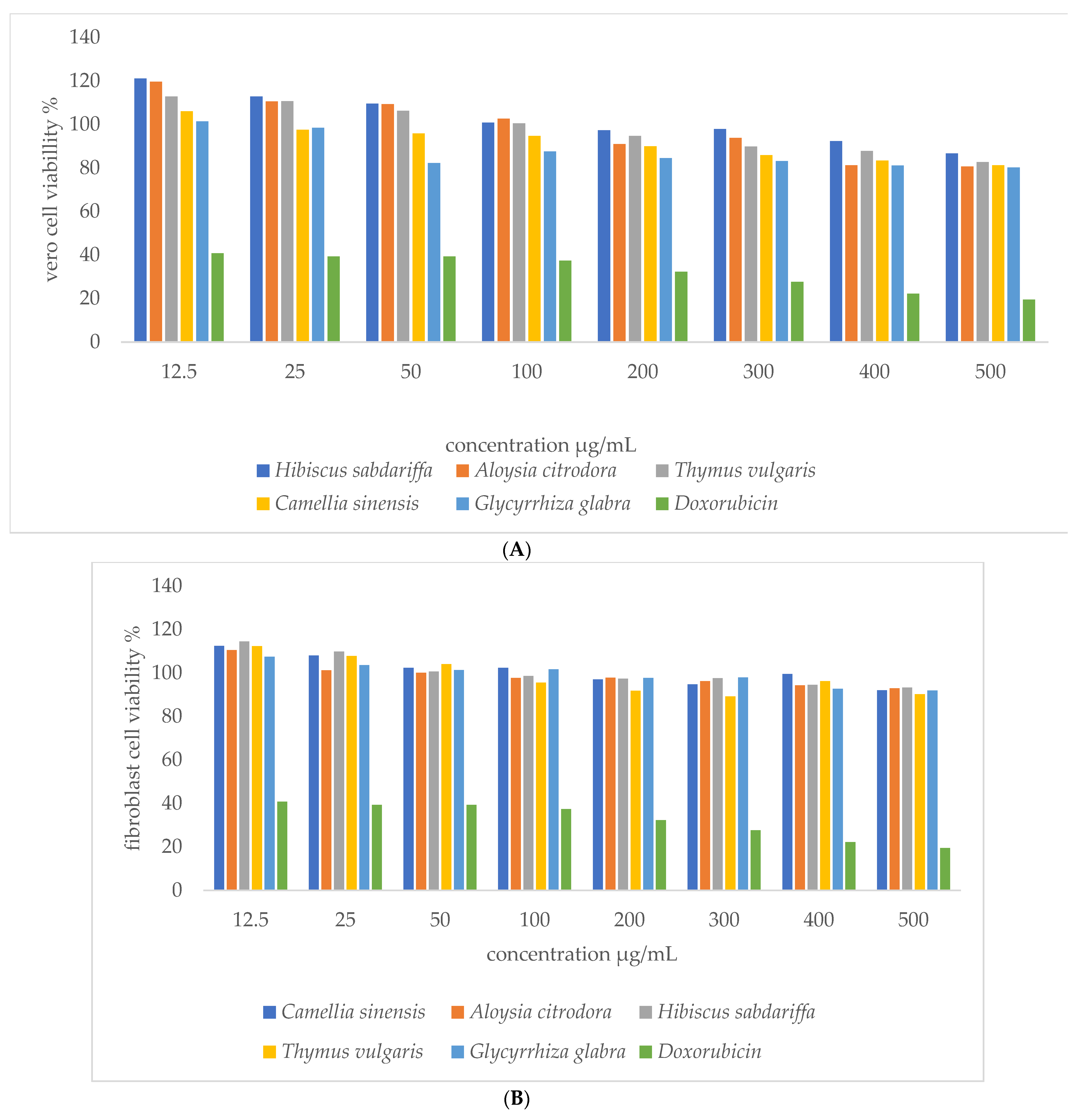
3.2.2. MTT Assay
3.3. In Vivo Assay
3.3.1. Acute Cytotoxicity
3.3.2. Systemic Infection and Treatment Experiment (Challenge Test)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, J.L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol. 2014, 11, 33–39. [Google Scholar] [CrossRef]
- Cilloniz, C.; Martin-Loeches, I.; Garcia-Vidal, C.; San Jose, A.; Torres, A. Microbial Etiology of Pneumonia: Epidemiology, Diagnosis and Resistance Patterns. Int. J. Mol. Sci. 2016, 17, 2120. [Google Scholar] [CrossRef] [Green Version]
- Spellberg, B.; Gilbert, D.N. The future of antibiotics and resistance: A tribute to a career of leadership by John Bartlett. Clin. Infect. Dis. 2014, 59 (Suppl. 2), S71–S75. [Google Scholar] [CrossRef] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; Rex, J.; et al. Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52 (Suppl. 5), S397–S428. [Google Scholar] [PubMed]
- CDC. Antibiotic Resistance Threats in the United States. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 1 July 2021).
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. AYU 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant Natural Products Targeting Bacterial Virulence Factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.D.; Birdi, T.J. Development of botanicals to combat antibiotic resistance. J. Ayurveda Integr. Med. 2017, 8, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Yaghmour, R.M.; Faidi, Y.R.; Salem, K.; Al-Nuri, M.A. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998, 60, 265–271. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and in vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Al-Qura’n, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef] [PubMed]
- WHO Traditional Medicine Strategy 2014–2023. Available online: https://www.who.int/publications/i/item/9789241506096 (accessed on 1 July 2021).
- Vickers, A.; Zollman, C. ABC of complementary medicine: Herbal medicine. BMJ 1999, 319, 1050–1053. [Google Scholar] [CrossRef] [Green Version]
- Fendrihan, S.; Pop, C.E. Biotechnological potential of plant associated microorganisms. Rom. Biotechnol. Lett. 2021, 26, 2700–2706. [Google Scholar] [CrossRef]
- Talib, W.H.; Mahasneh, A.M. Antimicrobial, cytotoxicity and phytochemical screening of Jordanian plants used in traditional medicine. Molecules 2010, 15, 1811–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, H.Y.; Kim, Y.; Park, H.W.; Moon, H.E.; Bae, S.; Kim, J.; Kim, D.G.; Paek, S.H. The Unreliability of MTT Assay in the Cytotoxic Test of Primary Cultured Glioblastoma Cells. Exp. Neurobiol. 2015, 24, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Zohra, A.F.M. Hemolytic activity of different herbal extracts used in Algeria. Int. J. Pharma Sci. Res. 2014, 5, 495–500. [Google Scholar]
- Ibrahim, D.A.; Albadani, R.N. Evaluation of the Potential Nephroprotective and Antimicrobial Effect of Camellia sinensis Leaves versus Hibiscus sabdariffa (in vivo and in vitro Studies). Adv. Pharmacol. Sci. 2014, 2014, 389834. [Google Scholar]
- Al-Mamun, M.A.; Akter, Z.; Uddin, M.J.; Ferdaus, K.M.; Hoque, K.M.; Ferdousi, Z.; Reza, M.A. Characterization and evaluation of antibacterial and antiproliferative activities of crude protein extracts isolated from the seed of Ricinus communis in Bangladesh. BMC Complement. Altern. Med. 2016, 16, 211. [Google Scholar] [CrossRef] [Green Version]
- Mehreen, A.; Waheed, M.; Liaqat, I.; Arshad, N. Phytochemical, Antimicrobial, and Toxicological Evaluation of Traditional Herbs Used to Treat Sore Throat. Biomed. Res. Int. 2016, 2016, 8503426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Oecd Guideline for Testing of Chemicals; OECD: Paris, France, 2001; Volume 12, pp. 1–14. [Google Scholar]
- Arshad, N.; Mehreen, A.; Liaqat, I.; Arshad, M.; Afrasiab, H. In vivo screening and evaluation of four herbs against MRSA infections. BMC Complement. Altern. Med. 2017, 17, 498. [Google Scholar] [CrossRef] [Green Version]
- van Elsas, J.D.; Bailey, M.J. The ecology of transfer of mobile genetic elements. FEMS Microbiol. Ecol. 2002, 42, 187–197. [Google Scholar] [CrossRef]
- Tarawneh, K.A.; Irshaid, F.; Jaran, A.S.; Ezealarab, M.; Khleifat, K.M. Evaluation of Antibacterial and Antioxidant Activities of Methanolic Extracts of Some Medicinal Plants in Northern Part of Jordan. J. Biol. Sci. 2010, 10, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Talib, W.H.; Zarga, M.H.; Mahasneh, A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules 2012, 17, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Koohsari, H.; Ghaemi, E.A.; Sheshpoli, M.S.; Jahedi, M.; Zahiri, M. The investigation of antibacterial activity of selected native plants from North of Iran. J. Med. Life 2015, 8, 38–42. [Google Scholar]
- Fabry, W.; Okemo, P.O.; Ansorg, R. Antibacterial activity of East African medicinal plants. J. Ethnopharmacol. 1998, 60, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Radji, M.; Agustama, R.A.; Elya, B.; Tjampakasari, C.R. Antimicrobial activity of green tea extract against isolates of methicillin-resistant Staphylococcus aureus and multi-drug resistant Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2013, 3, 663–667. [Google Scholar] [CrossRef] [Green Version]
- Oukerrou, M.A.; Tilaoui, M.; Mouse, H.A.; Leouifoudi, I.; Jaafari, A.; Zyad, A. Chemical Composition and Cytotoxic and Antibacterial Activities of the Essential Oil of Aloysia citriodora Palau Grown in Morocco. Adv. Pharmacol. Sci. 2017, 2017, 7801924. [Google Scholar] [CrossRef] [Green Version]
- Teow, S.Y.; Liew, K.; Ali, S.A.; Khoo, A.S.; Peh, S.C. Antibacterial Action of Curcumin against Staphylococcus aureus: A Brief Review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.; Ahhmed, A.; Shin, J.H.; Baek, J.S.; Kim, M.Y.; Kim, J.D. Green Tea Seed Isolated Saponins Exerts Antibacterial Effects against Various Strains of Gram Positive and Gram Negative Bacteria, a Comprehensive Study in vitro and in vivo. Evid. Based Complement. Altern. Med. 2018, 2018, 3486106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramchoun, M.; Sellam, K.; Harnafi, H.; Alem, C.; Benlyas, M.; Khallouki, F.; Amrani, S. Investigation of antioxidant and antihemolytic properties of Thymus satureioides collected from Tafilalet Region, south-east of Morocco. Asian Pac. J. Trop. Biomed. 2015, 5, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Sabeti, B.; Noordin, M.I.; Mohd, S.; Hashim, R.; Dahlan, A.; Javar, H.A. Development and characterization of liposomal doxorubicin hydrochloride with palm oil. Biomed. Res. Int. 2014, 2014, 765426. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Ramot, Y.; Malarkey, D.E.; Blackshear, P.; Kissling, G.E.; Travlos, G.; Nyska, A. Fourteen-week toxicity study of green tea extract in rats and mice. Toxicol. Pathol. 2010, 38, 1070–1084. [Google Scholar] [CrossRef]
- Meena, A.K.; Jain, A.; Pendey, K.; Singh, R.K. Acute Toxicity and Genotoxic Activity of Hibiscus rosa sinensis Flower Extract. Am. J. Phytomed. Clin. Ther. 2014, 2, 524–529. [Google Scholar]
- Howden, B.P.; Davies, J.K.; Johnson, P.D.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010, 23, 99–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuok, C.F.; Hoi, S.O.; Hoi, C.F.; Chan, C.H.; Fong, I.H.; Ngok, C.K.; Meng, L.R.; Fong, P. Synergistic antibacterial effects of herbal extracts and antibiotics on methicillin-resistant Staphylococcus aureus: A computational and experimental study. Exp. Biol. Med. (Maywood) 2017, 242, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Nayim, P.; Mbaveng, A.T.; Wamba, B.E.N.; Fankam, A.G.; Dzotam, J.K.; Kuete, V. Antibacterial and Antibiotic-Potentiating Activities of Thirteen Cameroonian Edible Plants against Gram-Negative Resistant Phenotypes. Sci. World J. 2018, 2018, 4020294. [Google Scholar] [CrossRef]

| Plant Number | Scientific Name | Family | Common Name | Arabic Name | Plant Form |
|---|---|---|---|---|---|
| 1 | Camellia sinensis | Theaceae | Green tea | Shay Akhdur | Herb |
| 2 | Hibiscus sabdariffa | Malvaceae | Roselle | Karkadeh | Flower |
| 3 | Aloysia citrodora | Verbenaceae | lemon verbena | Lemon Aloysia | Herb |
| 4 | Glycyrrhiza glabra | Fabaceae | Liquorice | Erqsus | Root |
| 5 | Thymus vulgaris | Lamiaceae | Thyme | Za’atar | Herb |
| 6 | Urtica pilulifera | Urticaceae | Nettles | Qarass | Herb |
| 7 | Phlomis brachyodon | Lamiaceae | Phlomis | Jerusalem Sage | Shrub |
| 8 | Plantago lanceolata | Plantaginaceae | Plantain | Lamb’s Tongue | Shrub |
| 9 | Anchusa azurea | Boraginaceae | Bugloss | Blue Bull Tongue | Shrub |
| 10 | Pallenis spinosa | Asteraceae | Spiny golden star | Al najmeiah | Flower |
| Antibacterial Activity (Zone of Inhibition, mm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Plants (10 mg/disc) | MRSA ATCC 43300 | MRSA Isolates | MSSA ATCC 25923 | MSSA Isolates | P.A. ATCC 9027 | P.A Isolates | E. coli NTCC O157 | E. coli Isolates |
| Camellia sinensis | 22 | 19.9 | 23 | 19.5 | 9 | 8.5 | 0 | 0 |
| Aloysia citrodora | 21 | 19.3 | 18 | 20 | 9 | 9.3 | 10 | 8.9 |
| Hibiscus sabdariffa | 15 | 14.7 | 15 | 14.6 | 12 | 12 | 11 | 11.5 |
| Glycyrrhiza glabra | 13 | 13.1 | 15 | 15.4 | 0 | 0 | 0 | 0 |
| Phlomis brachyodon | 13 | 14.5 | 14 | 14.2 | 10 | 9.6 | 16 | 15.4 |
| Thymus vulgaris | 15 | 14.5 | 15 | 14.3 | 0 | 0 | 0 | 0 |
| Urtica pilulifera | 8 | 8.7 | 8 | 8.2 | 9 | 9.7 | 13 | 12.4 |
| Anchusa azurea | 13.4 | 13.9 | 14 | 13.1 | 0 | 0 | 12 | 12.4 |
| Plantago lanceolata | 12 | 12.3 | 12 | 12.5 | 0 | 0 | 0 | 0 |
| Pallenis spinosa | 0 | 0 | 0 | 0 | 8 | 8.4 | 13 | 13.9 |
| Vancomycin (30) | 20 (S) | 18 (S) | 21 (S) | 20 (S) | NT | NT | NT | NT |
| Gentamycin (10) | 26 (S) | 25 (S) | 28 (S) | 24 (S) | 20 (S) | 18 (S) | 25 (S) | 22 (S) |
| Plant Extracts | Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| MRSA | MSSA | P. aeruginosa | E. coli | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Aloysia citrodora | 1.3 ± 0.7 | 2.5 ± 1.4 | 1.5 ± 1.0 | 2.8 ± 1.7 | 5.3 ± 1.9 | 9.4 ± 4.0 | 7.5 ± 3.5 | 15.0 ± 7.0 |
| Camellia sinensis | 0.5 ± 0.2 | 0.8 ± 0.4 | 0.5 ± 0.5 | 1.1 ± 0.9 | 3.1 ±1.7 | 5.6 ± 3.6 | NT | NT |
| Hibiscus sabdariffa | 4.0 ± 2.0 | 9.2 ± 4.1 | 5.2 ± 1.5 | 9.9 ± 3.1 | 3.1 ± 0 | 6.3 ± 0 | 9.4 ± 3.1 | 19.9 ± 6.9 |
| Glycyrrhiza glabra | 0.9 ± 0.5 | 1.8 ± 1.0 | 0.7 ± 0.2 | 1.4 ± 0.3 | NT 1 | NT | NT | NT |
| Thymus vulgaris | 5.5 ± 4.9 | 10.9 ± 5.2 | 4.0 ± 1.6 | 8.1 ± 6.25 | NT | NT | NT | NT |
| Vancomycin µg/mL | 1.56 ± 0.50 | 2.2 ± 0.63 | 1.9 ± 0.93 | 2.6 ± 1.11 | NT | NT | NT | NT |
| Gentamycin µg/mL | NT | NT | NT | NT | 5.6 ± 1.96 | 6.4 ± 1.96 | 4.6 ± 0.92 | 4.2 ± 0.86 |
| Groups | Mortality % | Gross Lesion of 10 Mice | Lungs Lesion Scores % | Liver Lesion Scores % | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total No. of Abnormal Lungs | Total No. of Abnormal Liver | Normal | Mild | Moderate | Severe | Normal | Abnormal | ||
| G1: Negative Control | 0 | 00 | 00 | 100 | 0 | 0 | 0 | 100 | 0 |
| G2: Positive Control | 15 | 20 | 20 | 0 | 0 | 0 | 100 | 15 | 85 |
| G3: Camellia sinensis | 0 | 2 | 00 | 80 | 10 | 10 | 0 * | 100 | 0 * |
| G4: Aloysia citrodora | 0 | 5 | 1 | 50 | 30 | 20 | 0 * | 90 | 10 * |
| G5: Hibiscus sabdariffa | 0 | 6 | 3 | 40 | 40 | 20 | 0 * | 70 | 30 * |
| G6: Camellia sinensis and Aloysia citrodora | 0 | 4 | 0 | 60 | 30 | 10 | 0 * | 100 | 0 * |
| G7: Camellia sinensis and Hibiscus sabdariffa | 0 | 6 | 2 | 40 | 40 | 20 | 0 * | 80 | 20 * |
| G8: Hibiscus sabdariffa and Aloysia citrodora | 0 | 6 | 2 | 40 | 30 | 30 | 0 * | 80 | 20 * |
| G9: Vancomycin control | 0 | 7 | 2 | 30 | 30 | 40 | 0 * | 80 | 20 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataineh, S.M.B.; Tarazi, Y.H.; Ahmad, W.A. Antibacterial Efficacy of Some Medicinal Plants on Multidrug Resistance Bacteria and Their Toxicity on Eukaryotic Cells. Appl. Sci. 2021, 11, 8479. https://doi.org/10.3390/app11188479
Bataineh SMB, Tarazi YH, Ahmad WA. Antibacterial Efficacy of Some Medicinal Plants on Multidrug Resistance Bacteria and Their Toxicity on Eukaryotic Cells. Applied Sciences. 2021; 11(18):8479. https://doi.org/10.3390/app11188479
Chicago/Turabian StyleBataineh, Sereen M. B., Yaser H. Tarazi, and Wafá A. Ahmad. 2021. "Antibacterial Efficacy of Some Medicinal Plants on Multidrug Resistance Bacteria and Their Toxicity on Eukaryotic Cells" Applied Sciences 11, no. 18: 8479. https://doi.org/10.3390/app11188479
APA StyleBataineh, S. M. B., Tarazi, Y. H., & Ahmad, W. A. (2021). Antibacterial Efficacy of Some Medicinal Plants on Multidrug Resistance Bacteria and Their Toxicity on Eukaryotic Cells. Applied Sciences, 11(18), 8479. https://doi.org/10.3390/app11188479





